Abstract
OBJECTIVE
To assess whether sensorimotor peripheral nerve function is associated with muscle power in community-dwelling older men.
DESIGN
Longitudinal cohort study with 2.3 ± 0.3 years of follow-up.
SETTING
One clinical site.
PARTICIPANTS
Three hundred seventy-two participants at the Pittsburgh site of the Osteoporotic Fractures in Men (MrOS) Study (N = 5994, age = 77.2 ± 5.1 years, 99.5% white, BMI = 27.9 ± 3.7kg/m2, power = 1.88 ± 0.6watts/kg).
INTERVENTIONS
Not applicable.
MAIN OUTCOME MEASURES
A nerve function ancillary study was performed 4.6 ± 0.4 years after baseline. Muscle power was measured using a power rig. Peroneal motor nerve conduction amplitude, distal motor latency, and mean f-wave latency were measured. Sensory nerve function was assessed using 10-g and 1.4-g monofilaments and sural sensory nerve conduction amplitude and distal latency. Peripheral neuropathy symptoms at the leg and feet were assessed by self-report.
RESULTS
Adjusting for age, height, total body lean and fat mass, one standard deviation lower motor (β = −0.07, p<0.05) and sensory amplitude (β = −0.09, p<0.05) and 1.4-g (β = -0.11, p<0.05) and 10-g monofilament insensitivity (β = −0.17 both p<0.05) were associated with lower muscle power/kg. Compared to the effect of age on muscle power (β per year = −0.05 , p<0.0001), this was equivalent to aging 1.4 years for motor amplitude, 1.8 years for sensory amplitude, 2.2 years for 1.4-g monofilament detection, and 3.4 years for 10-g detection. Baseline 1.4-g monofilament detection predicted greater decline in power/kg. Short-term change in nerve function was not associated with concurrent short-term change in power/kg.
CONCLUSION
Worse sensory and motor nerve function were associated with lower power/kg and are likely important for impaired muscle function in older men. Monofilament sensitivity was associated with greater decline in power/kg and screening may identify early risk for muscle function decline in late-life, which has implications for disability.
Keywords: muscle power, older adults, peripheral nerve function, sensory nerve function, motor nerve function
Lower extremity muscle power is an important determinant of late-life physical function.1,2 Muscle power, a measure of contractile force and shortening speed, has been linked to risk of falls,3 mobility loss measured by physical performance tests such as walking, chair stands, and stair climbing,4-11 and self-reported functional status2,12 in older adults. Compared to strength, muscle power declines more steeply with age4,13 and may be more strongly associated with certain measures of mobility.6,9,11,12 Moreover, training programs designed to improve muscle power and velocity of movement may be more effective at improving physical performance than those that solely incorporate basic resistance training.14-16
Poor muscle power in late-life and its unique relationship with mobility may be, at least in part, due to impairments in peripheral nerve function.17-22 The components of power, force and velocity production, are likely dependent on the number and firing rate of motor units.23 In addition, afferent input and impaired sensory nerve function may play an important role in muscle and physical function;24-26 this is believed to occur through loss of proprioception.24,26-28 Like muscle power, peripheral nerve function declines with age,29-33 and has similarly been linked with physical function limitations and impairments32,34 and increased risk of falls.35-37 The 1999-2000 National Health and Nutrition Examination Survey (NHANES) showed that 35% of adults aged 80 years and older had impaired nerve function measured using simple screening for reduced sensation at the foot.33 Additionally, both poor motor and sensory peripheral nerve function have been related to reduced lower extremity quadriceps strength in the Health Aging and Body Composition (Health ABC) Study; muscle power was not assessed in this study.25
Despite the independent relationship of muscle power and nerve function with mobility-related outcomes, whether peripheral nerve function loss is a determinant of muscle power decline has not been assessed. In a longitudinal cohort study of older men, we evaluated the whether sensory and motor peripheral nerve function measures, commonly used in clinical evaluations and neurologic studies, were related to lower extremity muscle power cross-sectionally and longitudinally with the hypotheses that worse and declining nerve function is associated with poor and declining muscle power.
METHODS
Study Population
We used data from a nerve function ancillary study in which 372 participants had nerve function and power measured during the first visit and 241 participants had these repeated during a second visit. The ancillary study was performed at the Monongahela Valley site 4.6 ± 0.4 years after the 2000-2002 baseline visit. The second visit occurred 2.3 ± 0.3 years later. This ancillary study was part of the Osteoporotic Fractures in Men Study (MrOS), which is a cohort of community dwelling, ambulatory men (N = 5994) aged 65 years and older enrolled between March 2000 and April 2002 at six U.S. clinic sites (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Monongahela Valley near Pittsburgh, PA; Portland, OR; and San Diego, CA; n = 1005 in Pittsburgh at baseline). Eligibility for the main study included ability to walk without assistance of another person or an aide, ability to provide self-reported data, ability to understand and sign an informed consent, absence of bilateral hip replacements, absence of a medical condition that would result in imminent death, and anticipated residence near a clinic site for the duration of the study period. The primary recruitment strategy was mailing invitations to men living in the surrounding communities of clinic sites. Supplementary strategies included community and senior newspaper advertisements and presentations to community groups. The study protocol was approved by the University of Pittsburgh institutional review board and written informed consent was obtained from all participants prior to testing. Out of 662 men with nerve function measured during the first visit of the ancillary study, 372 had muscle power measured and were included in the cross-sectional analyses. Reasons for missing muscle power included temporary equipment failure (n = 205), refusal (n = 11), and inability due to physical limitation (n = 74). Participants with missing cross-sectional muscle power data did not differ by age, but had slightly higher BMI (28.6 vs. 27.9 kg/m2, p = 0.03) and a higher prevalence of diabetes (27.9% vs. 17.0%, p<0.001). Out of the participants included in the cross-sectional analysis, 279 returned for the second visit. The change analysis included data from 241 participants with complete nerve function and muscle power data from the first and second visits of the ancillary study. During the second visit, 1 participant refused muscle power testing and 53 participants were unable due to physical limitations. Participants with missing data for the change analysis were older (78.8 vs. 76.3 years, p<0.0001), but had similar BMI and prevalence of diabetes.
Peripheral Nerve Measures
Nerve conduction was measured bilaterally on the deep peroneal motor and sural sensory nerves using an automated nerve conduction study device (NC-stat®, NeuroMetrix, Inc., Waltham, MA),38 which has been previously validated in healthy older adults with gold standard nerve conduction studies (correlation coefficient > 95%).39 Participants’ feet were warmed to at least 30° C if they were less than 30° C prior to testing. Parameters recorded from the peroneal motor nerve included the compound muscle action potential (CMAP) motor amplitude in millivolts (mV), measured from baseline to the negative peak of the CMAP waveform, the distal motor latency (DML) in milliseconds (ms), the time from the stimulus to the onset of motor activity, and mean F-wave latency (FWL) in ms, the mean value of the time from stimulus to the onset of F-wave activity. Sensory nerve measures included the sural nerve action potential (SNAP) sensory amplitude in microvolts (μV), the difference between negative and positive peak of the SNAP waveform, and the distal sensory latency (DSL) in ms, the time from the stimulus to the negative peak of the SNAP. Light (1.4-g) and standard (10-g) monofilament sensitivity were defined as ability to detect three out of four touches at the dorsum of the great toes. Insensitivity was defined as inability to detect three touches. The standard monofilament was performed only if the participant could not feel the light monofilament. Sensory nerve conduction was performed on the non-dominant side. Motor nerve conduction and monofilament testing were performed on both sides unless technical difficulty occurred. Self-reported peripheral neuropathy symptoms occurring within the past 12 months included: (1) numbness or tingling, (2) sudden stabbing, burning, pain or aches, and (3) an open or persistent sore, or gangrene on either feet or leg. All measures were repeated at the follow-up visit.
Lower Extremity Muscle Power
Muscle power was measured using a single leg press (Nottingham Leg Extensor Power rig, Nottingham, U.K.).40 Participants were seated with their arms crossed over their chest and instructed to push down on a pedal with one foot as hard and as fast as possible through a full range of motion. The maximum power output in watts from five trials was used. Both sides were tested unless the participant had a hip replacement on one side. The ratio of power to body weight in kg was chosen as the outcome of interest since it may better reflect ability to move one's body weight when performing everyday activities.
To correspond with sensory nerve conduction measures, muscle power data on the non-dominant side was used in the analysis unless prohibited by missing data because of inability due to physical limitation. In the case that no sensory nerve conduction data were available, muscle power data was matched to the side in which motor nerve conduction and/or monofilament testing were performed. The number of participants with discordant sides analyzed due to missing data was minimal (n = 5 for motor nerve conduction and n = 1 for monofilament testing).
Additional Covariates
All models were adjusted for age and height, measured using a stadiometer. Weight was measured with a calibrated balance beam scale but was not included as a covariate since power/kg of body weight was the outcome. Since one potential characteristic of overt neuropathy is atrophy of muscle fibers,41 lean mass was included as a potential mediator of the relationship between nerve function and power. Fat mass, was included due to its important metabolic and functional consequences.42,43 Lean and fat mass were measured using dual-energy X-ray absorptiometry (DXA; Hologic 4500A, Hologic, Inc., Bedford, MA). To ensure reproducibility of DXA measurements, standardized measurement and quality-control procedures were used and operators were certified. More localized measures of calf muscle density, which has been positively associated peroneal motor amplitude,44 and muscle area, were added in place of lean and fat mass as potential mediators in subsequent models. Muscle density in mg/cm3, a measure of intermuscular fat, and muscle area (mm2) were measured at 66% of the calf length using peripheral quantitative computed tomography (pQCT - Stratec XCT-2000 scanner, Pforzheim, Germany) as previously described.45 Each of the following covariates was significantly related to muscle power or one of the nerve function predictors at an alpha level of 0.1. Diabetes was defined by self-report, use of hypoglycemic medications or having a baseline fasting glucose ≥126 mg/dl.46 Other chronic health conditions included self-reported hypertension, congestive heart failure, myocardial infarction, stroke, osteoarthritis, and hip pain. Participants self-reported if a doctor or other healthcare provider had ever told them that they had the condition. Ankle-brachial index less than 0.9 was used to define peripheral vascular disease and greater than 1.3 was used to define arterial stiffening. Cognitive function was assessed using the Teng Modified Mini-Mental State Exam (3MSE).47 Lifestyle factors included smoking status (past and current), alcohol consumption (drinks/week) and physical activity measured using the Physical Activity Scale for the Elderly (PASE). 48 Variance inflation factors (VIF) were calculated to assess collinearity. No VIF exceeded 3.
Statistical Analysis
Jonckheere-Terpstra tests and Generalized Linear Models were used to test for trends in participant characteristics across power/kg tertiles. Pairwise comparisons were made between muscle power tertiles using t-tests and chi-squared statistics. Multivariable linear regression was used to compare: 1) each measure of baseline nerve function to baseline muscle power/kg; 2) each measure of baseline nerve function to change in muscle power/kg; and 3) each measure of change in nerve function to change in muscle power/kg. Separate models for each measure of nerve function were built progressively in order starting with the measure of nerve function. Age and height were added to the first set of minimally adjusted models. Total body lean and fat mass were added to the second set of models. For the third set of models, lean and fat mass were replaced with more localized measures of calf muscle density and cross sectional muscle area. And finally, models were adjusted for lifestyle factors, chronic health conditions, and cognition to assess independent associations.
RESULTS
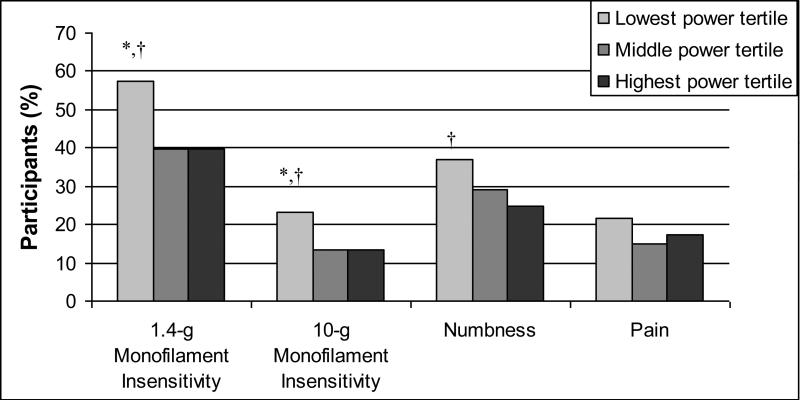
Participant characteristics were compared across muscle power tertiles (Table 1). Those in the lowest power tertile were older, shorter in height, and had a higher BMI, greater fat mass, lower lean mass, and were more likely to have a history of hypertension and worse 3MSE scores. Alcohol consumption frequency (mean = 2.6 ± 1.3 drinks/week), current smoking status (2.7%), and history of stroke (4.6%), congestive heart failure (7.3%), myocardial infarction (17.7%), osteoarthritis (24.2%), and hip pain (15.9%) did not differ across muscle power tertiles (data not shown). Men in the lowest tertile had lower motor and sensory amplitude (Table 2) and were less likely to have 1.4-g and 10-g monofilament sensitivity and more likely to report numbness symptoms in the leg or feet (Figure 1). Distal motor latency (mean = 4.41 ± 0.8 ms), F-wave latency (mean = 60.6 ± 5.9 ms), distal sensory latency (mean = 3.12 ± 0.4 ms), and self-report of open or persistent sores on the feet or leg (2.2%) did not differ across power tertiles (data not shown).
Table 1.
Characteristics of Study Population by Muscle Power (watts/kg) Tertiles
| Lowest tertile ≤1.60 watts/kg (N=122) | Middle tertile >1.60 and ≤2.06 watts/kg (N=129) | Highest tertile >2.06 watts/kg (N=121) | P-value for trend | |
|---|---|---|---|---|
| Muscle power (watts/kg), mean (SD) | 1.27 (0.2)*, † | 1.84 (0.1)‡ | 2.52 (0.4) | <0.0001 |
| Age (years), mean (SD) | 80.0 (5.1)*, † | 76.7 (4.9)‡ | 75.0 (4.1) | <0.0001 |
| White race (%) | 122 (100) | 128 (99.2) | 120 (99.2) | 0.38 |
| Body composition | ||||
| Height (m), mean (SD) | 171.5 (6.8)† | 172.0 (6.7)‡ | 173.9 (6.3) | 0.01 |
| BMI (kg/m2), mean (SD) | 28.1 (3.7)† | 28.2 (3.6) | 27.3 (3.5) | 0.13 |
| Fat mass (kg), mean (SD) | 23.2 (6.6)† | 22.7 (7.1) | 21.3 (6.5) | 0.09 |
| Lean mass (kg), mean (SD) | 55.6 (6.7)† | 56.9 (7.5) | 57.5 (6.6) | 0.09 |
| Muscle density (mg/cm3) | 67.9 (4.6)*, † | 69.4 (3.7) | 70.4 (3.8) | <0.0001 |
| Chronic health conditions | ||||
| Diabetes, n (%) | 25 (20.5) | 20 (15.5) | 16 (13.2) | 0.25 |
| AAI <0.9, n (%) | 46 (42.6) | 41 (34.2) | 36 (30.3) | 0.05 |
| History of hypertension, n (%) | 74 (60.7)† | 66 (51.2) | 50 (41.3) | 0.003 |
| Physical Activity Score (PASE), mean (SD) | 147.4 (65.8) | 146.8 (64.6) | 162.4 (65.2) | 0.11 |
| Cognition 3MSE Score, mean (SD) | 92.6 (6.1) | 94.0 (4.9) | 94.8 (4.4) | 0.003 |
PASE = Physical Activity Scale for the Elderly
p<0.05 for Lowest tertile vs. Middle tertile
p<0.05 for Lowest tertile vs. Highest tertile
p<0.05 for Middle tertile vs. Highest tertile
Table 2.
Nerve Conduction Amplitude by Muscle Power (watts/kg) Tertiles
| Lowest tertile ≤1.60 watts/kg (N=122) | Middle tertile >1.60 and ≤2.06 watts/kg (N=129) | Highest tertile >2.06 watts/kg (N=121) | P-value for trend | |
|---|---|---|---|---|
| Motor amplitude (mV), mean (SD) | 2.15 (1.4)† | 2.25 (1.4)‡ | 2.84 (1.5) | 0.0007 |
| Sensory amplitude (μV), mean (SD) | 3.07 (3.2)*, † | 4.01 (3.4) | 4.84 (3.7) | 0.002 |
mV = millivolts; μV = microvolts
p<0.05 for Lowest tertile vs. Middle tertile
p<0.05 for Lowest tertile vs. Highest tertile
p<0.05 for Middle tertile vs. Highest tertile
Figure 1. Monofilament insensitivity and symptoms by muscle power (watt/kg) tertiles.
*p<0.05 for Lowest tertile vs. Middle tertile, †p<0.05 for Lowest tertile vs. Highest tertile, ‡p<0.05 for Middle tertile vs. Highest tertile
One standard deviation lower motor and sensory amplitude were associated with lower power/kg when adjusted for age and height (Table 3). While the associations between motor and sensory amplitude and power remained significant upon further adjustment, fat mass (2nd Models) and muscle density (3rd Models) attenuated the effect sizes by ≥ 10%. Insensitivity with 10-g and 1.4-g (2nd Model only) monofilaments were also associated with lower power/kg, but were attenuated to nonsignificant by muscle density. Fat mass was negatively associated with power/kg (β = −0.02, p < 0.01), whereas muscle density was positively associated with power/kg (β = 0.04, p < 0.0001).
Table 3.
Separate Multivariable Linear Regression Models for each Measure of Nerve Function and Muscle Power (watts/kg)
| 1st Models | 2nd Models | 3rd Models | 4th Models | ||||
|---|---|---|---|---|---|---|---|
| β (SE) | β (SE) | Attenuated by | β (SE) | Attenuated by | β (SE) | Attenuated by | |
| Motor nerve function per SD lower | |||||||
| Motor amplitude | −0.10‡ (0.03) | −0.07* (0.03) | Fat mass | −0.08* (0.03) | Muscle density | −0.07* (0.03) | Fat mass |
| Distal motor latency | −0.05 (0.03) | −0.04 (0.03) | −0.04 (0.03) | −0.05 (0.03) | |||
| Mean F-wave latency | 0.05 (0.03) | 0.05 (0.03) | 0.05 (0.04) | 0.06 (004) | |||
| Sensory nerve function per SD lower | |||||||
| Sensory amplitude | −0.10‡ (0.04) | −0.09* (0.04) | Fat mass | −0.09* (0.04) | Muscle density | −0.09* (0.04) | Fat mass |
| Distal sensory latency | −0.10 (0.34) | −0.06 (0.32) | −0.21 (0.34) | −0.10 (0.32) | |||
| Monofilament insensitivity (yes/no) | |||||||
| 1.4-g | −0.10 (0.06) | −0.11* (0.05) | −0.07 (0.06) | Muscle density | −0.12* (0.03) | ||
| 10-g | −0.16* (0.07) | −0.17* (0.07) | −0.12 (0.07) | Muscle density | −0.16* (0.03) | ||
| Neuropathic symptoms (yes/no) | |||||||
| Numbness | −0.10 (0.06) | −0.09 (0.06) | −0.10 (0.06) | −0.07 (0.06) | |||
| Pain | 0.01 (0.07) | 0.04 (0.07) | 0.07 (0.07) | 0.04 (0.07) | |||
| Open sore | −0.32 (0.18) | −0.36* (0.18) | −0.11 (0.19) | −0.34* (0.21) | |||
1st Models adjusted for age and height; 2nd Models adjusted for variables in 1st Models plus total body lean and fat mass; 3nd Models adjusted for variables in 1st Models plus muscle density and muscle cross-sectional area; 4nd Models adjusted for variables in 2st Models plus diabetes and hypertension; p>0.1 for AAI, CVD, CBVD, osteoarthritis, hip pain, smoking, alcohol use, physical activity, and cognitive function
p<0.05
†p<0.01
p<0.001.
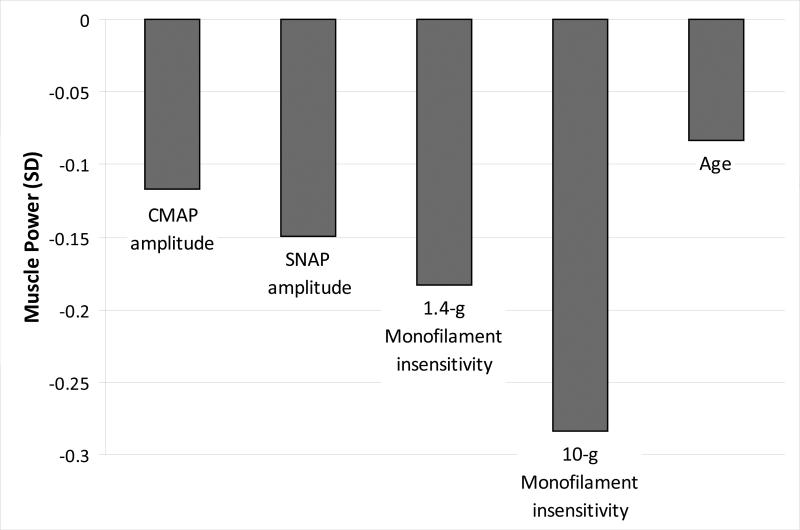
We compared the effect sizes of significant nerve function measures (from 2nd Models) to the effect size of age on muscle power in standard deviations of power/kg (Figure 1). One standard deviation lower motor and sensory amplitude had the effect of aging 1.4 and 1.8 years, respectively and inability to detect 1.4-g and 10-gmonofilament had the effect of aging 2.2 and 3.4 years, respectively. Table 4 shows that inability to detect 1.4-g monofilament at baseline predicted a greater decline in muscle power/kg when adjusted for age and height. Results were consistent when adjusted for lean and fat mass or muscle density and area and additional covariates. Change in nerve function was not associated with a change in power (results not shown).
Table 4.
Separate Multivariable Linear Regression Models for each Measure of Nerve Function and Decline in Muscle Power (watts/kg)
| β (SE) | |
|---|---|
| Motor nerve function per SD lower | |
| Motor amplitude | −0.001 (0.04) |
| Distal motor latency | 0.001 (0.03) |
| Mean F-wave latency | −0.02 (0.04) |
| Sensory nerve function per SD lower | |
| Sensory amplitude | 0.05 (0.04) |
| Distal sensory latency | 0.06 (0.04) |
| Monofilament insensitivity (yes/no) | |
| 1.4-g | −0.15* (0.07) |
| 10-g | −0.11 (0.09) |
| Neuropathic symptoms (yes/no) | |
| Numbness | 0.11 (0.07) |
| Pain | −0.13 (0.09) |
Models adjusted for age and height
p<0.05
DISCUSSION
Although muscle power is known to be dependent on both the nervous and musculoskeletal systems, previous research has not evaluated peripheral nerve function measures commonly used in clinical practice and neurologic studies. Studies have indicated that muscle power declines at an even faster rate with age than strength,4,13 and our findings show that the potential effects of peripheral nerve function are 1.5 to 3.5 times the effect of one year of age. This finding has particularly important consequences, given that neuropathy is a preventable risk factor. Establishing the relationship between muscle power and clinically relevant measures of nerve function in late-life is crucial since older adults experience the highest burden of neuropathy and diminished muscle function and both likely play key roles in the disablement pathway.33,49 Our findings show that poor sensory and motor peripheral nerve function are independently associated with and may be important risk factors for poor muscle power in old age. Risk factors for poor muscle power are understudied in epidemiologic studies of older adults, yet poor muscle power has important consequences in late-life such as impaired mobility,4-12 disability,2,50 and increased risk of falls.3
We found that lower amplitude, but not latency, was associated with poor muscle power. Consistent with our study findings, Strotmeyer and colleagues reported that peroneal motor nerve amplitude, but not conduction velocity, was related to lower extremity muscle strength.25 Latency is the travel time of the response and is measured from the moment of stimulation to the appearance of the action potential. Nerve conduction velocity is typically calculated by dividing the distance between two stimulation sites by the difference between latencies.51 Diminished amplitude may indicate axonal degeneration and motor nerve death, whereas latency or conduction velocity may be a measure of demyelination of the protective sheath surrounding the nerve.52 Amplitude may decline in some individuals, while velocity, driven by the motor units that remain intact, remains normal.44,53 In participants in the lowest muscle power tertile, we observed lower amplitude but no difference in latency, compared to those in higher muscle power tertiles (Table 2). These two aspects of motor nerve decline may occur separately, with muscle power relating to axonal degeneration and nerve death.
Sensory nerve function measured by monofilament detection and average vibration perception threshold was associated with muscle strength in the previous study as well.25 In our study, sensory amplitude was related to muscle power. While motor nerves directly innervate muscle, it is less clear how the sensory nerves are involved. Blocking afferent input in healthy individuals has led to impaired maximal voluntary contractions,24 which may occur through loss of proprioceptive feedback.27 Since severe sensory neuropathy is associated with poor ankle proprioception,54,55 sensory input may be necessary to achieve proper placement, timing, and movement of the leg and foot during testing. An additional explanation could be that large fiber neuropathies, which are the most common type of neuropathies in older adults,56 may affect both sensory and motor nerves, with most deficits first presenting as sensory loss. This can progress to reduced position sense, muscle weakness and wasting, and depressed tendon reflexes.56 Impaired sensory nerve function may thereby represent or be a surrogate measure for a more generalized loss of peripheral nerve function.
Our results suggest that insensitivity to the 1.4-g monofiliment test may be an early indicator of those at future risk for muscle power decline. In contrast to the 10-g monofilament, which is generally associated with clinical disease and predictive of foot ulceration,57 the 1.4-g monofilament may be a more sensitive measure, detecting sensory nerve function loss at an early stage.58,59 Since risk factors for declining muscle power in older adults are understudied, this test could potentially be used to help develop prevention strategies to preserve muscle function.
Fat mass and muscle density attenuated the relationships of amplitude and monofilament insensitivity with muscle power. While data from the Health ABC study, show that older adults with greater fat mass had greater strength but lower muscle quality,60 few have focused on the relationship between fat mass and muscle power or its additional component of velocity, which could pose an additional challenge for individuals with greater fat mass. Interestingly, diminished nerve function in older adults has been previously associated with lower muscle density, a measure of intermuscular fat, but not with cross-sectional muscle area,44 which could suggest that age-related changes in nerve function lead to changes in muscle tissue structure over macroscopic changes in muscle mass.
A major strength of this study is the inclusion of both motor and sensory peripheral nerve function measures. We also used reproducible, sensitive, and specific measures of nerve conduction for both motor and sensory nerves.38,61 Our measure of muscle power has been previously validated and is commonly used.40 Models were adjusted for a number of potential confounders, including body composition, lifestyle factors, and comorbidities. And finally, we were able to examine longitudinal relationships between nerve function and muscle power change.
Study Limitations
One limitation of this study is that some of our null finding are likely attributable to the short time period (2.3 years) between measures. Our results may not apply to other populations such as non-whites, women, the “young-old” and institutionalized individuals. Future studies should assess the relationship between muscle power and clinical measures of motor and sensory nerve function in a larger more diverse population with a broader range of function.
Conclusions
We showed that sensory and motor nerve function are independently associated with muscle power, which is associated with poor outcomes in older adults such as falls,3 impaired mobility,4-12 and disability.2,50 Future work should investigate whether there is a direct relationship between poor nerve function and these poor health outcomes. Since monofilament insensitivity was predictive of greater muscle power decline, future studies should also test whether simple screening for monofilament detection may identify individuals at risk for muscle power decline. Detecting poor or declining muscle power early on could lead to more effective disability prevention and treatment efforts, such as training programs targeted at increasing muscle power.14-16 Importantly, understanding risk factors in the disablement pathway such as poor nerve function and impaired muscle power can help identify multiple points of intervention. Future studies should characterize the effects of known and novel risk factors of poor nerve function on muscle power.
Figure 2. Effect size of nerve conduction amplitudes and monofilament insensitivity compared to one year of age.
Motor and sensory amplitude per standard deviation (SD) lower; 1.4-g and 10-g monofilament insensitivity (yes/no); age per year older; separate models adjusted for age, height, total body lean and fat mass; age adjusted for height, total body lean and fat mass; SD = Standard Deviation.
Acknowledgements of presentation
Ward RE, Caserotti P, Faulkner K, Boudreau RM, Cawthon PM, Newman AB, Cauley JA, Strotmeyer ES. Peripheral nerve function and lower extremity muscle power in older men. Gerontologist. 2011. 51:S112. The Gerontological Society of America 64th Annual Scientific Meeting, Boston, MA, USA.
Acknowledgements of financial support: The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
List of abbreviations
- NHANES
National Health and Nutrition Examination Survey
- Health ABC Study
Health Aging and Body Composition Study
- MrOS
Osteoporotic Fractures in men study
- CMAP
compound muscle action potential
- mV
millivolts
- DML
distal motor latency
- ms
milliseconds
- FWL
F-wave latency
- SNAP
sural nerve action potential
- μV
microvolts
- DSL
distal sensory latency
- DXA
dual-energy X-ray absorptiometry
- pQCT
peripheral quantitative computed tomography
- 3MSE
Teng Modified Mini-Mental State Exam
- PASE
Physical Activity Scale for the Elderly
- VIF
Variance inflation factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? J Am Geriatr Soc. 2010 Dec;58(12):2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuo HK, Leveille SG, Yen CJ, Chai HM, Chang CH, Yeh YC, Yu YH, Bean JF. Exploring how peak leg power and usual gait speed are linked to late-life disability: data from the National Health and Nutrition Examination Survey (NHANES), 1999-2002. Am J Phys Med Rehabil. 2006 Aug;85(8):650–658. doi: 10.1097/01.phm.0000228527.34158.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002 Mar;31(2):119–125. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 4.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994 Sep;23(5):371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001 Sep;49(9):1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 6.Bean JF, Kiely DK, Herman S, Leveille SG, Mizer K, Frontera WR, Fielding RA. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002 Mar;50(3):461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 7.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007 May;88(5):604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Bean JF, Kiely DK, Leveille SG, Herman S, Huynh C, Fielding R, Frontera W. The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol A Biol Sci Med Sci. 2002 Nov;57(11):M751–756. doi: 10.1093/gerona/57.11.m751. [DOI] [PubMed] [Google Scholar]
- 9.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003 Aug;58(8):728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 10.Lindemann U, Claus H, Stuber M, Augat P, Muche R, Nikolaus T, Becker C. Measuring power during the sit-to-stand transfer. Eur J Appl Physiol. 2003 Jun;89(5):466–470. doi: 10.1007/s00421-003-0837-z. [DOI] [PubMed] [Google Scholar]
- 11.Marsh AP, Miller ME, Saikin AM, Rejeski WJ, Hu N, Lauretani F, Bandinelli S, Guralnik JM, Ferrucci L. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1186–1193. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, Pu CT, Hausdorff JM, Fielding RA, Singh MA. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000 Apr;55(4):M192–199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 13.Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med. 1994 Apr;15(3):149–151. doi: 10.1055/s-2007-1021037. [DOI] [PubMed] [Google Scholar]
- 14.Bean JF, Herman S, Kiely DK, Frey IC, Leveille SG, Fielding RA, Frontera WR. Increased Velocity Exercise Specific to Task (InVEST) training: a pilot study exploring effects on leg power, balance, and mobility in community-dwelling older women. J Am Geriatr Soc. 2004 May;52(5):799–804. doi: 10.1111/j.1532-5415.2004.52222.x. [DOI] [PubMed] [Google Scholar]
- 15.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002 Apr;50(4):655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 16.Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008 Dec;18(6):773–782. doi: 10.1111/j.1600-0838.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 17.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979 Mar;46(3):451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 18.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003 Feb;13(1):40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 19.Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004 Apr;91(4):450–472. doi: 10.1007/s00421-003-0991-3. [DOI] [PubMed] [Google Scholar]
- 20.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci. 2004 Dec;59(12):1334–1338. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- 21.Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci Med Sci. 2005 Sep;60(9):1111–1117. doi: 10.1093/gerona/60.9.1111. [DOI] [PubMed] [Google Scholar]
- 22.Clemencon M, Hautier CA, Rahmani A, Cornu C, Bonnefoy M. Potential role of optimal velocity as a qualitative factor of physical functional performance in women aged 72 to 96 years. Arch Phys Med Rehabil. 2008 Aug;89(8):1594–1599. doi: 10.1016/j.apmr.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 23.Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2010 May;65(5):495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. The control of the deafferented hand. Brain. 1990 Oct;113(Pt 5):1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- 25.Strotmeyer ES, de Rekeneire N, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, Shorr RI, Vinik AI, Harris TB, Newman AB. Sensory and Motor Peripheral Nerve Function and Lower-Extremity Quadriceps Strength: The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2009 Sep 28; doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strotmeyer ES, de Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, Shorr RI, Vinik AI, Harris TB, Newman AB. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: the Health, Aging, and Body Composition (Health ABC) study. Diabetes Care. 2008 Sep;31(9):1767–1772. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Luca CJ, Gonzalez-Cueto JA, Bonato P, Adam A. Motor unit recruitment and proprioceptive feedback decrease the common drive. Journal of neurophysiology. 2009 Mar;101(3):1620–1628. doi: 10.1152/jn.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, Vellas B, Harris TB. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care. 2003 Dec;26(12):3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 29.Buschbacher RM. Peroneal nerve motor conduction to the extensor digitorum brevis. Am J Phys Med Rehabil. 1999;78(6 Suppl):S26–31. doi: 10.1097/00002060-199911001-00006. 1999. [DOI] [PubMed] [Google Scholar]
- 30.Baldereschi M, Inzitari M, Di Carlo A, Farchi G, Scafato E, Inzitari D. Epidemiology of distal symmetrical neuropathies in the Italian elderly. Neurology. 2007 May 1;68(18):1460–1467. doi: 10.1212/01.wnl.0000260606.36443.29. [DOI] [PubMed] [Google Scholar]
- 31.Rivner MH, Swift TR, Malik K. Influence of age and height on nerve conduction. Muscle Nerve. 2001 Sep;24(9):1134–1141. doi: 10.1002/mus.1124. [DOI] [PubMed] [Google Scholar]
- 32.Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002 Jan;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 33.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004 Jul;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 34.Resnick HE, Vinik AI, Schwartz AV, Leveille SG, Brancati FL, Balfour J, Guralnik JM. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: the Women's Health and Aging Study. Diabetes Care. 2000 Nov;23(11):1642–1647. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 35.Ferrucci L, Bandinelli S, Cavazzini C, Lauretani F, Corsi A, Bartali B, Cherubini A, Launer L, Guralnik JM. Neurological examination findings to predict limitations in mobility and falls in older persons without a history of neurological disease. Am J Med. 2004 Jun 15;116(12):807–815. doi: 10.1016/j.amjmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992 Jun;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 37.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992 Oct;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 38.Kong X, Lesser EA, Megerian JT, Gozani SN. Repeatability of nerve conduction measurements using automation. Journal of clinical monitoring and computing. 2006 Dec;20(6):405–410. doi: 10.1007/s10877-006-9046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher MA. Comparison of automated and manual F-wave latency measurements. Clin Neurophysiol. 2005 Feb;116(2):264–269. doi: 10.1016/j.clinph.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60(5):385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 41.Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997 May;127(5 Suppl):1011S–1013S. doi: 10.1093/jn/127.5.1011S. [DOI] [PubMed] [Google Scholar]
- 42.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997 May;127(5 Suppl):998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 43.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, Caserotti P, Kritchevsky SB, Newman AB, Goodpaster BH, Satterfield S, Cummings SR, Harris TB. Health A, Body Composition S. Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc. 2011 May;59(5):781–787. doi: 10.1111/j.1532-5415.2011.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006 Aug;27(8):1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Faulkner KA, Zmuda JM. Osteoporotic Fractures in Men Research G, Tobago Health Studies Research G. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009 Aug;94(8):2735–2742. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003 Jan;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 47.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. The Journal of clinical psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 48.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993 Feb;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 49.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 50.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004 Nov;59(11):1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 51.Ward RE, Boudreau RM, Vinik AI, Zivkovic SA, Njajou OT, Satterfield S, Harris TB, Newman AB, Strotmeyer ES. Reproducibility of peroneal motor nerve conduction measurement in older adults. Clin Neurophysiol. 2013 Mar;124(3):603–609. doi: 10.1016/j.clinph.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arezzo JC, Zotova E. Electrophysiologic measures of diabetic neuropathy: mechanism and meaning. International review of neurobiology. 2002;50:229–255. doi: 10.1016/s0074-7742(02)50079-9. [DOI] [PubMed] [Google Scholar]
- 53.Falck B, Stalberg E. Motor nerve conduction studies: measurement principles and interpretation of findings. J Clin Neurophysiol. 1995 May;12(3):254–279. [PubMed] [Google Scholar]
- 54.Simoneau GG, Derr JA, Ulbrecht JS, Becker MB, Cavanagh PR. Diabetic sensory neuropathy effect on ankle joint movement perception. Arch Phys Med Rehabil. 1996 May;77(5):453–460. doi: 10.1016/s0003-9993(96)90033-7. [DOI] [PubMed] [Google Scholar]
- 55.Van den Bosch CG, Gilsing MG, Lee SG, Richardson JK, Ashton-Miller JA. Peripheral neuropathy effect on ankle inversion and eversion detection thresholds. Arch Phys Med Rehabil. 1995 Sep;76(9):850–856. doi: 10.1016/s0003-9993(95)80551-6. [DOI] [PubMed] [Google Scholar]
- 56.Vinik AI, Strotmeyer ES, Nakave AA, Patel CV. Diabetic neuropathy in older adults. Clin Geriatr Med. 2008 Aug;24(3):407–435, v. doi: 10.1016/j.cger.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005 Apr;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 58.Bourcier ME, Ullal J, Parson HK, Dublin CB, Witherspoon CA, Ward SA, Vinik AI. Diabetic peripheral neuropathy: how reliable is a homemade 1-g monofilament for screening? The Journal of family practice. 2006 Jun;55(6):505–508. [PubMed] [Google Scholar]
- 59.Thomson MP, Potter J, Finch PM, Paisey RB. Threshold for detection of diabetic peripheral sensory neuropathy using a range of research grade monofilaments in persons with Type 2 diabetes mellitus. Journal of foot and ankle research. 2008;1(1):9. doi: 10.1186/1757-1146-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koster A, Ding J, Stenholm S, Caserotti P, Houston DK, Nicklas BJ, You T, Lee JS, Visser M, Newman AB, Schwartz AV, Cauley JA, Tylavsky FA, Goodpaster BH, Kritchevsky SB, Harris TB. Health ABCs. Does the amount of fat mass predict age-related loss of lean mass, muscle strength, and muscle quality in older adults? J Gerontol A Biol Sci Med Sci. 2011 Aug;66(8):888–895. doi: 10.1093/gerona/glr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, Cohen JA, Fisher MA, Howard JF, Kinsella LJ, Latov N, Lewis RA, Low PA, Sumner AJ. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005 Jan 25;64(2):199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]