SHORT SUMMARY
Type 2 innate lymphoid cells (ILC2) produce high levels of Th2 cytokines. Our study demonstrates that cat allergen challenge in allergic rhinitis subjects rapidly induces increased peripheral blood ILC2.
Keywords: type 2 innate lymphoid cells (ILC2), CRTH2, PGD2, allergic rhinitis
To the Editor,
Type 2 innate lymphoid cells (ILC2) have recently been discovered and include nuocytes, natural helper cells, and innate type 2 helper cells1, 2. ILC2 do not express known surface markers for T, B, NK, or NKT cells (lineage-negative) and produce large amounts of Th2 cytokines in response to cytokines IL-33, IL-25, TSLP, and leukotriene D42-4. Studies in animal models have shown that ILC2 contribute to allergen-induced airway hyperresponsiveness and type-2 lung inflammatory responses suggesting that ILC2 may have a role in asthma and allergic disease2. Human ILC2 have been detected in peripheral blood, GI tract, lung, BAL, and nasal polyps and are defined as lineage-negative lymphocytes that express the chemoattractant receptor homologous molecule expressed on TH2 lymphocytes (CRTH2)5. We have previously reported that peripheral blood ILC2 highly express the master Th2 cytokine transcription factor GATA-3, supporting a role for rapid ILC2 Th2 cytokine production6. Despite the potential for ILC2 to contribute to human allergic disease, no studies have yet reported whether allergen exposure in sensitized allergic rhinitis subjects has any effect on peripheral blood ILC2 levels. Thus, we hypothesized that CRTH2-positive ILC2 may be increased in the peripheral blood of cat allergic individuals after nasal cat allergen challenge.
To determine whether changes in the levels of peripheral blood ILC2 occurred after nasal cat allergen challenge, we recruited 7 cat sensitized (skin prick test positive) adults aged 21.9 ± 5.1 yrs (4 female; 3 male) with a current history of rhinitis symptoms on cat exposure to undergo nasal challenge with cat allergen extract and diluent control (Fig E1). Following approval from the UCSD Human Subjects Protection Committee and the FDA (IND # 15185), subjects presented for two study visits. During the initial visit, subjects underwent a nasal diluent control challenge with lactated ringers solution. Subjects then returned 2-12 weeks later for nasal cat allergen challenge, consisting of three consecutively increasing concentrations (4 BAU, 40 BAU and 400 BAU) of Hollister-Stier cat allergen extract administered at 10-minute intervals, in two 100 μl sprays in each nostril (SGD North America bottle, MeadWestVaco nozzle). Peripheral venous blood was collected immediately prior to and four hours following the nasal diluent and allergen challenges. Symptoms were scored using the total nasal symptom score (TNSS) at time points before and after nasal challenges for up to four hours (Fig E2). The TNSS area under the curve was calculated to determine the level of immediate response post diluent or cat challenge (Fig E3).
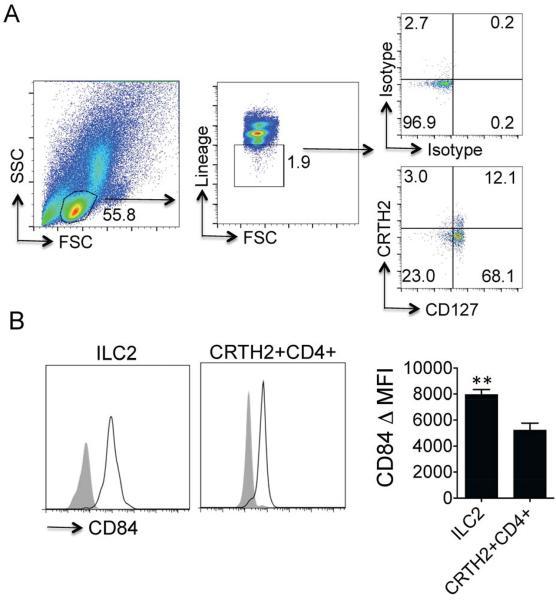
Peripheral blood mononuclear cells (PBMC) from each time point were isolated by density gradient centrifugation in sodium citrate Vacutainer Cell Preparation Tubes (BD, Franklin Lakes, NJ). PBMC were stained with a FITC lineage cocktail (CD3, CD14, CD16, CD19, CD20, CD56; BD, Franklin Lakes NJ), TCRγδ (BD, Franklin Lakes NJ, USA), CD4, CD11b, CD235a, FCeRI, (Ebiosciences San Diego, CA) that allows for exclusion of B, T, NK, and NKT cells as well as mast cells and basophils. Cells were further stained with PE-conjugated CRTH2 or biotin-conjugated CRTH2 (Miltenyi, Auburn CA) followed by streptavidin APC and PE-conjugated CD84 (Biolegend). Flow cytometry was performed using the BD Accuri flow cytometer machine and analyzed with FlowJo software (Tree Star, Inc). The ILC2 population in allergic subjects was defined as lymphocytes that are lineage-negative and express CRTH2 (Fig 1A). ILC2 are also known to express the IL-7 receptor alpha subunit (CD127) and nearly all of the CRTH2 positive cells expressed CD127 as expected (Fig 1A). Peripheral blood ILC2 were also detected in non-allergic individuals (Fig E4).
Figure 1.
(A) Lymphocytes were identified from whole PBMCs (left) and lineage-negative cells gated (middle). Lineage-negative lymphocytes were further assessed for expression of CD127 and CRTH2 or isotype control staining (right). ILC2 were identified as lineage-negative CRTH2+ lymphocytes. Example shown is 4 hours after cat allergen exposure in cat allergic individual (B) ILC2 (left) and CRTH2+ CD4+ cells (middle) were analyzed for baseline CD84 expression in cat allergic individuals and change in MFI compared with respective isotype determined (right). **P < 0.005, t-test, n= 5 individual subjects.
Human ILC2 are a recently discovered population of cells that are known to express CRTH2 and CD1615, but reported expression of other markers is very limited and we thus sought to identify expression of novel human ILC2 surface molecules. As mouse ILC2 express molecules involved in T cell-B cell interactions including Inducible T-cell Costimulator (ICOS)2, we assessed ILC2 for levels of CD84, a CD2 signaling lymphocyte activation molecule (SLAM) family member with a role in T cell-B cell contact7. Interestingly, we detected high levels of CD84 on peripheral blood ILC2 from cat allergic individuals at baseline (Fig 1B). CD84 expression has been identified on human T cells and we compared levels of ILC2 CD84 expression with peripheral blood Th2 cells (CD4+ CRTH2+ cells, Fig E5). ILC2 expressed CD84 at significantly higher levels in allergic subjects compared with Th2 cells based on change in median fluorescence intensity (ΔMFI) (Fig 1B). ILC2 from non-allergic individuals also showed increased CD84 expression compared with Th2 cells (Fig E6). Thus, we demonstrate the novel finding that human ILC2 highly express the SLAM family member CD84, although this expression is not selective for ILC2. Allergen challenge did not increase levels of CD84 expression on ILC2 or CD4 cells.
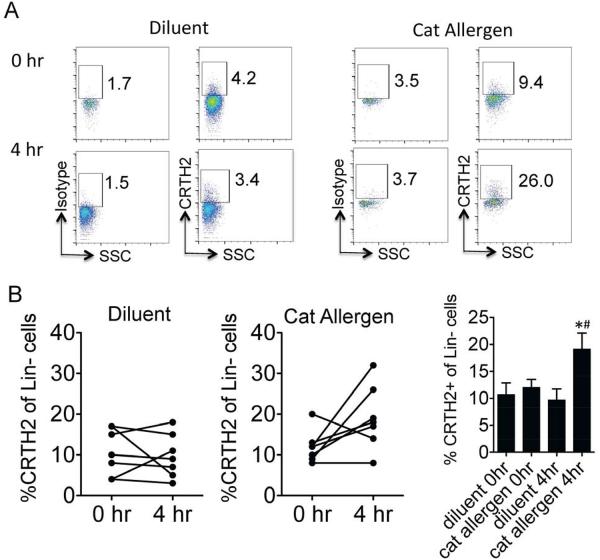
We next assessed the percent of CRTH2+ ILC2 in the peripheral blood of cat allergic subjects before and four hours after nasal cat allergen or diluent challenges (Fig 2). The baseline percent of CRTH2+ cells within the lineage negative population was 10.7 ± 1.9 and 12.0 ± 1.3 at the diluent and cat allergen challenge visit respectively (Fig 2B). Four hours after diluent challenge, the % CRTH2+ cells did not change significantly (9.7 ± 1.8) compared with time zero. However, after cat allergen challenge, the % CRTH2+ cells nearly doubled to 19.1 ± 2.6 compared to baseline (p = 0.05) and compared to diluent challenge at four hours (p < 0.05) (Fig 2B). Thus, nasal cat allergen challenge induced an increased percent of peripheral blood CRTH2-positive ILC2 when measured four hours after challenge.
Figure 2.
(A) ILC2 were analyzed in peripheral blood before (0hr) and 4 hours after nasal challenge with diluent (left) or cat allergen (right). Lineage-negative lymphocytes gated and CRTH2 expression or isotype staining shown from one subject. (B) % CRTH2 positive cells of lineage-negative population measured at 0 and 4 hours after diluent (left) and cat allergen challenges (middle). Total % CRTH2 positive cells at each time point enumerated (right). *P < 0.05 compared with diluent at 4 hours, #P = 0.05 compared with cat allergen at 0 hours, t-test, n= 7 individual subjects.
ILC2 produce large amounts of IL-5 and IL-13 in response to IL-25, IL-33, TSLP and LTD4 and could initiate and/or propagate allergic airway inflammation. Our studies demonstrate that the % of CRTH2+ ILC2 in the peripheral blood is rapidly increased (within 4 hours) after allergen challenge. Potential mechanisms for the ILC2 increase in the peripheral blood may be due to enhanced ILC2 recruitment from the bone marrow triggered by either humoral (cytokine, chemokine or mediator production in the nose) and/or cellular mechanisms (cells released from the nasal mucosa trafficking to the bone marrow). The human ILC2 marker CRTH2 is the receptor for prostaglandin D2 (PGD2), a lipid mediator that has a known role in chemotaxis and activation of immune cells. Importantly, a previous study demonstrated that high levels of serum 9α,11β-PGF2, the major PGD2 metabolite, is induced within 5 minutes after airway allergen challenge suggesting that PGD2 is rapidly available systemically for recruitment of CRTH2+ cells after allergen exposure8. We have also recently determined that PGD2 induces chemotaxis of CRTH2 positive human blood ILC2 in-vitro suggesting that PGD2 may directly regulate migration of human ILC2 into tissues9. The role of increased peripheral blood ILC2 numbers after allergen challenge is unclear. One hypothesis is that greater ILC2 availability in the blood (within 4 hours after challenge) may result in greater numbers of cytokine-producing nasal mucosa ILC2 at later time points, but this would need to be investigated in future studies. Strategies to inhibit ILC2 recruitment in allergic individuals may reduce tissue Th2 cytokine levels that contribute to allergic inflammation.
Supplementary Material
Acknowledgments
*Grant support: This study was supported by UCSD Allergy/Immunology and Scripps Clinic Allergy/Immunology Divisional funds, as well as NIH grant ULRR031980 and UL1TR000100 of CTSA funding to UCSD CTRI. T.A.D. is supported by NIH grant K08 AI080938 and ALA/AAAI Allergic Respiratory Diseases Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–42. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 3.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132:205–13. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 6.Doherty TA, Khorram N, Chang JE, Kim HK, Rosenthal P, Croft M, et al. STAT6 regulates natural helper cell proliferation during lung inflammation initiated by Alternaria. Am J Physiol Lung Cell Mol Physiol. 2012;303:L577–88. doi: 10.1152/ajplung.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–65. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochenek G, Nizankowska E, Gielicz A, Swierczynska M, Szczeklik A. Plasma 9alpha,11beta-PGF2, a PGD2 metabolite, as a sensitive marker of mast cell activation by allergen in bronchial asthma. Thorax. 2004;59:459–64. doi: 10.1136/thx.2003.013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.