Abstract
To establish infection successfully, Staphylococcus aureus must evade clearance by polymorphonuclear neutrophils (PMN). We studied the expression and regulation of the methionine sulfoxide reductases (Msr) that are involved in the repair of oxidized staphylococcal proteins and investigated their influence on the fate of S. aureus exposed to oxidants or PMN. We evaluated a mutant deficient in msrA1 and msrB for susceptibility to hydrogen peroxide, hypochlorous acid and PMN. The expression of msrA1 in wild-type bacteria ingested by human PMN was assessed by real-time PCR. The regulation of msr was studied by screening a library of two-component regulatory system (TCS) mutants for altered msr responses. Relative to the wild-type bacteria, bacteria deficient in Msr were more susceptible to oxidants and PMN. Upregulation of staphylococcal msrA1 occurred within the phagosomes of normal PMN and PMN deficient in NADPH oxidase activity. Furthermore, PMN granule-rich extract stimulated the upregulation of msrA1. Modulation of msrA1 within PMN was shown to be partly dependent on the VraSR TCS. Msr contributes to staphylococcal responses to oxidative attack and PMN. Our study highlights a novel interaction between the oxidative protein repair pathway and the VraSR TCS that is involved in cell wall homeostasis.
Key Words: Methionine sulfoxide reductase, Staphylococcus aureus, Neutrophils, Oxidative stress
Introduction
Staphylococcus aureus is an important human pathogen that is responsible for a broad spectrum of infections, ranging from mild cellulitis to life-threatening endovascular disease. The rising prevalence of antibiotic resistance among staphylococci exacerbates the clinical challenges imposed by staphylococcal disease.
Optimal host defense relies on the capacity of polymorphonuclear neutrophils (PMN) to phagocytose and kill S. aureus with antimicrobial agents delivered to or generated in phagosomes. Especially potent among phagosomal oxidants is hypochlorous acid (HOCl), which is generated by the myeloperoxidase-hydrogen peroxide (MPO-H2O2)-chloride system [1]. PMN-derived oxidants readily damage bacterial proteins, lipids and DNA. Methionine is highly susceptible to oxidation, and oxidation in proteins can disrupt their normal function [2]. Methionine can be oxidized to either of two enantiomers, methionine-S-sulfoxide and methionine-R-sulfoxide, and each can be selectively repaired by a specific class of methionine sulfoxide reductase (Msr); MsrA and MsrB repair the S and R stereoisomers, respectively. The S. aureus genome harbors three msrA genes (msrA1, msrA2 and msrA3) and a single msrB [3]. The adjacent msrA1 and msrB genes are cotranscribed and encode enzymes that provide the majority of the Msr activity in S. aureus [4, 5].
To persist and subsequently establish infection, invading bacteria need to evade, resist or tolerate insults inflicted by PMN. Given the role of Msr in the defense against physiological oxidative stress [6], we speculated that S. aureus might employ these enzymes to repair and thus limit damage caused by PMN-derived oxidants, thereby providing a mechanism to survive within phagosomes.
In this study, we investigated the contribution of Msr to the fate of S. aureus exposed to oxidants and to human PMN. We also explored the regulation of msr in S. aureus ingested by PMN and the role of staphylococcal two-component regulatory systems (TCS) in regulating the msrA1 response.
Methods
Bacterial Strains and Culture Conditions
The S. aureus strains used in this study were constructed in the community-associated S. aureus USA300 LAC strain that is cured of the native plasmid pUSA03 which confers erythromycin resistance [7] unless otherwise indicated. S. aureus was cultured in tryptic soy broth (TSB; BD Biosciences) at 37°C with shaking at 200 rpm. For strains harboring empty vector or complementation plasmids, the media was supplemented with 10 μg/ml of chloramphenicol (Sigma-Aldrich; table 1). To obtain stationary phase organisms, bacteria were inoculated from glycerol stocks into TSB and grown for 16-18 h. For mid-log phase bacteria, the stationary bacteria were subcultured for approximately 2.5 h from a starting optical density at 550 nm (OD550) of 0.05. In experiments involving PMN, 0.01% of human serum albumin (Talecris Biotherapeutics) was added to the subculture media. For experiments measuring msrA1 expression in the presence of both oxidants and vancomycin, an overnight starter culture of S. aureus grown in TSB was washed once with HEPES-buffered Hank's balanced salt solution (HBSS) before being used to innoculate minimal media (7.6 mM NH4SO4, 33 mM KH2PO4, 60 mM K2HPO4, 11 mM NaCl, 3 mM KCl, 0.2% glucose, 1 mM MgSO4, 0.5 µg/ml nicotin, 0.5 µg/ml thiamine, 0.5 µg/ml panthothenate, 3 ng/ml biotin, and 25 µg/ml of each of the following amino acids: Gly, Val, Leu, Thr, Phe, Tyr, Cys, Met, Pro, Arg, and His). The bacteria were then cultured between 4 and 6 h before being treated.
Table 1.
S. aureus strains and plasmids used in this study
| Strain or plasmid ID | Background strain | Plasmid or deletion | Details | Reference |
|---|---|---|---|---|
| Wild-type | USA300-0114 MRSA ΔpUSA03 | erms | [7] | |
| Wild-type pCM28 | USA300-0114 MRSA ΔpUSA03 | pCM28 | erms Cmr | this study |
| Wild-type pEPSA5 | USA300-0114 MRSA ΔpUSA03 | pEPSA5 | erms Cmr | this study |
| ΔmsrA1B | USA300-0114 MRSA ΔpUSA03 | erms | this study | |
| ΔmsrA1B pCM28 | USA300-0114 MRSA ΔpUSA03 | pCM28 | erms | this study |
| ΔmsrA1B pCM28-msrA1B | USA300-0114 MRSA ΔpUSA03 | pCM28-msrA1B | erms Cmr | this study |
| ΔvraSR pEPSA5 | USA300-0114 MRSA ΔpUSA03 | pEPSA5 | erms Cmr | this study |
| ΔvraSR pEPSA5-vraSR | USA300-0114 MRSA ΔpUSA03 | pEPSA5-vraSR | erms Cmr | this study |
| Δ nre | USA300-0114 MRSA ΔpUSA03 | SAUSA300_2337-38Δ | erms | this study |
| Δ 0217-8 | USA300-0114 MRSA ΔpUSA03 | SAUSA300 0217-18Δ | erms | this study |
| Δ 1219-20 | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1219-20Δ | erms | this study |
| Δ kdp | USA300-0114 MRSA ΔpUSA03 | SAUSA300_2035-36Δ | erms | this study |
| Δ 2558-9 | USA300-0114 MRSA ΔpUSA03 | SAUSA300_2558-59Δ | erms | this study |
| Δ 1798-9 | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1798-99Δ | erms | this study |
| Δ lyt | USA300-0114 MRSA ΔpUSA03 | SAUSA300_0254-55Δ | erms | this study |
| Δ pho | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1638-39Δ | erms | this study |
| Δ hss | USA300-0114 MRSA ΔpUSA03 | SAUSA300_2308-09Δ | erms | this study |
| Δ vra | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1865-66Δ | erms | this study |
| Δ arl | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1307-08Δ | erms | this study |
| Δ 1441-2 | USA300-0114 MRSA ΔpUSA03 | SAUSA300_1441-42Δ | erms | this study |
| Δ agr | USA300-0114 MRSA ΔpUSA03 | Δagr:: tetM | tetr | [8] |
| sae:: spec | USA300-0114 MRSA ΔpUSA03 | sae:: spec | specr | [9] |
| graS:: Tn-mariner | USA300-0114 MRSA ΔpUSA03 | graS:: Tn-mariner | ermr | |
| Pcm28 | empty | ampr Cmr | [11] | |
| pEPSA5 | empty | ampr Cmr | [12] | |
| pCM28-msrAlB | pCM28-msrAlB | ampr Cmr | this study | |
| pEPSA5-vraSR | pEPSA5-vraSR | ampr Cmr | this study | |
| pJB38 | ampr Cmr | [10] | ||
| pJMB176 | pJB38ΔmsrA1B | ampr Cmr | this study | |
| pJB204 | pJB38ΔvraSR | ampr Cmr | this study |
For antibiotic sensitivity to ampicillin, erythromycin, spectinomycin and chloramphenicol:s = sensitive;r = resistant.
Construction of ΔmsrA1B, ΔvraSR and TCS Mutants
S. aureus strains with mutations in the graRS [7], agr [8] and saeRS [9]. TCS were available as part of laboratory collections (table 1). Other TCS chromosomal mutations, ΔmsrA1B and ΔvraSR strains were created by homologous recombination using pJB38 [10]. To create the complementing plasmid and strains, the msrA1 and msrB or vraSR genes were amplified by PCR and cloned into the multicopy plasmids pCM28 [11] or pEPSA5 [12]. All clones were passaged through RN4220 [13] and subsequently transduced into the appropriate strains using bacteriophage 80α [14]. For oligonucleotide (Integrated DNA Technologies) sequences, refer to table 2. See online supplementary information for further details (for all online suppl. material, see www.karger.com/doi/10.1159/000355915).
Table 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence 5′ to 3′ | Application |
|---|---|---|
| gapdh primer1 | TACACAAGACGCACCTCACAGA | real-time PCR |
| gapdh primer 2 | ACCTGTTGAGTTAGGGATGATGTTT | real-time PCR |
| msrA1 primer t | TCCAGGCATCAAGTCAGTCG | real-time PCR |
| msrA1 primer 2 | TTGTCAACATGACCGCCACTA | real-time PCR |
| vraS primer t | CGGCAAGTATGATGCTATCTGCTA | real-time PCR |
| vraS primer 2 | TAACTGCGAATCTTGAACCATTTT C | real-time PCR |
| msrA1_5EcoRI | GGGGAATTCGGGCA-ATTGAATCAATTAATTAAAGGTGGCAG | cloning |
| msrA1_up3 NheI | ACGCGTGGTACCGCTAGCGCTAGCATGAATTACCTCCTCTATCTATCTAATTAT | cloning |
| msrB_dwn5mluI | GCTAGCGGTACCACGCGTACGCGTGTGGAAAGTATGTTTAAAAAATTATTCGGAAAAGG | cloning |
| msrB_dwn3BamHI | CCCGGATCCCACATCAATCACTTTTGTTTCACCTTTAATCACAGC | cloning |
| msrA_veri5 | GAGAAAACATTAAATTATTTGTAG | verification |
| msrB_veri3 | CATTGTCACATCAATCAC | verification |
| msrA1B_comp5EcoRI | GGGGAATTCGGCAATTGAATCAATTAATTAAAGGTGGCAG | cloning |
| msrA1B_comp3BamHI | GGGGGATCCCTACTTATCAAAATGTGATATTAAATCGCC | cloning |
| 1865up5EcoRI | GGGGAATTCCGGCGAAACATGACACACAAATATATATCAACGC | cloning |
| 1865up3fuse | ACGCGTGGTACCGCTAGCGCAGCTAGCATGCTATATACTAAGATGAGCATTG | cloning |
| 1865dwn5fuse | GCTAGCGGTACCACGCGTCGCCAATCACAATATAACATCAAATAGACACC | cloning |
| 1865dwn3SalI 1865veri5 | CCCGTCGACGCCGTAATGGAATCGTATTAAAACTACTAAGTGGAGAGCAACAGTAAGAAATAATA TCAAGCACTACC | cloning |
| 1865veri3 | GCACCCGCTGAAACATCTACTCTAATTAAG | verification |
| 1865 comp5BamHI | CCCGGATCCTTAGGAGGATGATTATTTATGAACCACTACATTAGAACAATTGGTTCAATGCTCATC | cloning |
| 1865 comp3SalI | CCCGTCGACGGTGTCTATTTGATGTTATATTGTGATTGGC | cloning |
Neutrophil Isolation
Informed consent was obtained from each individual following a protocol approved by the institutional review board for human subjects at the University of Iowa. Peripheral blood was drawn from normal healthy volunteers or individuals with X-linked chronic granulomatous disease (CGD), and PMN were purified as previously described [15].
Feeding PMN to S. aureus and PMN Killing Assay
Mid-log phase bacteria were pelleted for 5 min and resuspended in 20 mM HEPES-buffered HBSS containing Ca2+ and Mg2+. The OD550 of the suspension was measured and this was converted to colony-forming units (CFU)/milliliter: an OD550 reading of 1.0 was equivalent to 3 × 108 CFU/ml. Bacteria were used immediately or held on ice. Bacteria were opsonized by incubation at 37°C for 20 min in the presence of 10% pooled human serum. Opsonized bacteria were fed to PMN or PMN pretreated with 10 μM diphenylene iodonium (DPI) at the desired multiplicity of infection (MOI) and tumbled end over end at 37°C to allow phagocytosis to proceed. The PMN were then pelleted at 380 g for 5 min, and the extracellular bacteria were aspirated away. The cell pellets were resuspended to the original volume with 20 mM HEPES-buffered HBSS. These samples were processed to quantitate CFU to assess PMN-mediated killing or prepared for gene expression experiments. For PMN killing experiments, a 50-μl sample of cell suspension was removed at the 0 min (time 0). The remaining samples were tumbled at 37°C, and 50-μl aliquots were removed at specified time points. For viability testing, the 50-μl samples were diluted and incubated with 2.5 ml of pH 11 water for 5 min to lyse the PMN. These samples were vortexed and serially diluted into saline. 10 μl of each diluted sample was spotted onto tryptic soy agar plates in at least triplicate. After 16 h of growth at 37°C, the CFU were enumerated. From these, the CFU in the total PMN suspension for each sample were calculated. To assess the viability at each time point, the total calculated CFU for each strain were expressed as a percentage of the starting CFU for that strain. The starting CFU for each strain were taken as the CFU recovered from bacteria fed to DPI-treated PMN at time 0, in which there was minimal bacterial killing (data not shown). For gene expression experiments, the samples were tumbled at 37°C and at the desired time point, and the PMN that had ingested bacteria were pelleted, lysed and processed for real-time PCR analysis.
Real-Time PCR
RNA was extracted from bacteria or PMN-ingested bacteria as described previously [11]. cDNA was synthesized with AMV Reverse Transcriptase (Roche) and used as a template for real-time PCR. Reactions were conducted in PerfeCTa SYBR Green fast mix ROX (Quanta Biosciences) with each primer at 200 nM. Expression of gapdh was used to normalize all the gene expression data as described previously [11]. The gapdh primers amplify S. aureus gapdh but do not amplify human gapdh (data not shown). After denaturation at 95°C for 30 s, the samples were cycled at 95°C for 15 s, then at 60°C for 30 s, for 40 cycles using the ABI 7000 system. For primer sequences, refer to table 2.
Bacterial Exposure to Oxidants
In experiments used to determine the susceptibility of bacteria to oxidants, S. aureus strains in the mid-log phase were resuspended in PBS to an OD550 of 0.250. Bacteria (0.5 ml volume) were either left untreated or treated with reagent grade H2O2 or HOCl (Sigma-Aldrich) at predetermined concentrations for 1 h at 37°C. Residual H2O2 was quenched by diluting samples 1:100 in PBS supplemented with catalase (1,300 U/ml; Sigma). Where H2O2-MPO was employed, 75 nmol H2O2 was added to each 0.75-ml sample with 0, 1.5, 3.8, 7.5 or 15 pmol of recombinant MPO. In parallel, samples without S. aureus were assayed for HOCl generation (n = 3), as described in Dypbukt et al. [16]. The average amount of HOCl generated from 3 experiments was used to plot the data. After the 1-hour incubation, samples were diluted and spot-plated onto tryptic soy agar (BD Biosciences). CFU for each strain were enumerated after overnight growth at 37°C and then calculated as a percentage of non-treated bacteria for the same strain at time 0. For gene expression studies, S. aureus was resuspended to an OD550 of 0.25 in minimal media before being challenged with H2O2 or HOCl at the specified concentrations in the presence or absence of 7 µg/ml of vancomycin for 1 h at 37°C.
Bacterial Exposure to PMN Granule-Rich Extract
PMN were resuspended in cold relaxation buffer (RB; 10 mM PIPES, pH 7.3, 100 mM KCl, 3 mM NaCl2, 3.5 mM MgCl2, 1.25 mM EGTA, 1 mM ATP) and sonicated on ice. Unbroken cells and nuclei were pelleted (200 g at 4°C for 5 min) and the supernatant was centrifuged at 10,000 g at 4°C for 20 min. The granule-enriched pellet was washed with ice-cold RB, spun at 10,000 g, resuspended in RB and lysed by freeze thawing 3 times in a methanol-dry ice bath. Granule proteins (107 cell equivalents) and 1.5 × 108 bacteria were incubated together in HBSS with calcium and magnesium for 30 min at 37°C.
Treatment with Cell Wall-Active Antibiotics
In experiments involving TCS mutants, S. aureus were diluted to an OD550 of 0.3 in TSB, in the presence or absence of 7 μg/ml of vancomycin or 25 μg/ml of D-cycloserine (Sigma-Aldrich), and shaken at 200 rpm for 1 h at 37°C.
Msr Activity Assay
Bacteria were washed with assay buffer (50 mM TRIS-HCl, pH 7.5, 145 mM NaCl), pelleted and resuspended into 200 μl of assay buffer. Bacteria were lysed with 25 µg of lysostaphin (Sigma-Aldrich) at 37°C for 10 min. Lysates were sonicated on ice and then incubated with 2 mM dabsyl-methionine sulfoxide (a mixture of the S and R enantiomers; Anaspec) and 20 mM DTT in assay buffer. After 1 h at 37°C, reactions were quenched with acetonitrile. Samples were analyzed on an LC-Dabs HPLC column (Supelco) to separate dabsyl-methionine sulfoxide from dabsyl-methionine. For further details, refer to the online supplementary material.
Statistical Analysis
Statistical analyses were performed using Graphpad Prism 5 software. Data were analyzed using paired t tests or one-way ANOVA with post-tests as indicated in the figure legends. p values <0.05 were considered to be statistically significant.
Results
Validation of the msrA1 msrB Deletion Mutant
To study the role of Msr in S. aureus, we created a mutant in the wild-type background in which both the msrA1 and msrB genes were deleted (ΔmsrA1B). The growth of the ΔmsrA1B double-mutant strain was indistinguishable from that of the parent strain (online suppl. fig. 1).
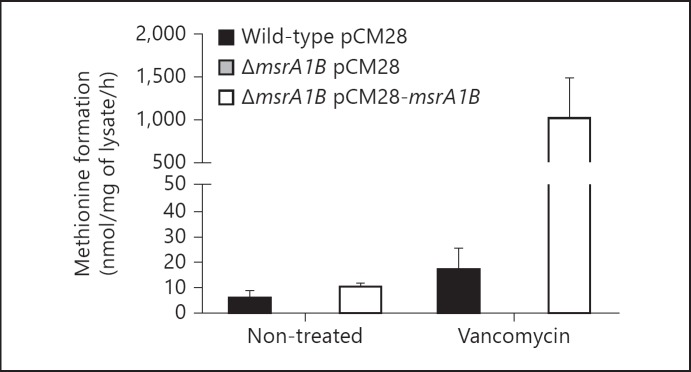
To assess the impact of msrA1B deletion on Msr activity in S. aureus, we studied bacteria in the absence or presence of vancomycin, since Msr expression increases upon exposure to cell wall antibiotics such as vancomycin [17]. In the absence of vancomycin, Msr activity in the wild-type lysates was low, and in ΔmsrA1B, it was below the level of detection (fig. 1). Msr activity was restored when both msrA1 and msrB were reintroduced into the ΔmsrA1B strain via a multicopy plasmid (pCM28). Upon treatment with vancomycin, Msr activity increased in the wild-type and the complemented deletion mutant (ΔmsrA1B with pCM28-msrA1B), but not in ΔmsrA1B. Thus, the data confirm that under these conditions, MsrA1 and MsrB support the majority of Msr activity in S. aureus, which was eliminated in our msrA1B deletion mutant.
Fig. 1.
Msr activity in wild-type and msr mutants. Conversion of dabsyl-methionine sulfoxide to dabsyl-methionine by lysates from wild-type, ΔmsrA1B and complemented ΔmsrA1B mutants were measured. Bacteria were treated with buffer (non-treated) or with vancomycin for 1 h. Data represent the mean ± SEM (n = 3 experiments).
Susceptibility of Wild-Type and msr Mutants to Oxidants and PMN
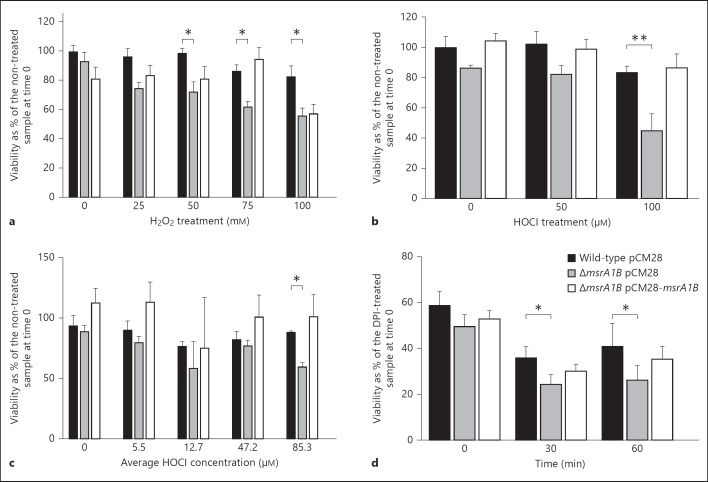
Reasoning that the capacity to repair oxidant-mediated damage by Msr would provide a survival advantage, we compared the abilities of the wild-type, ΔmsrA1B and complemented mutant strains to grow after exposure to H2O2 for 1 h in PBS. The number of culturable bacteria present after treatment with H2O2 was expressed as a percentage of the initial number of bacteria present prior to treatment.
At the lowest concentration of H2O2 tested (25 mM), there was no significant difference in the growth of the wild-type or ΔmsrA1B mutant after exposure to the oxidant. At higher concentrations of H2O2 (50, 75 and 100 mM), the viability of the wild-type mutant was greater than that of ΔmsrA1B mutant (p < 0.05; fig. 2a). Viability of the complemented mutant treated with 25-75 mM of H2O2 was likewise better than that of the ΔmsrA1B strain, suggesting that msrA1B contributed to better survival of bacteria in the presence of H2O2 at these concentrations. At 100 mM H2O2, ΔmsrA1B and its complement were equally susceptible to killing by H2O2, suggesting that expression of msrA1B driven from the complementation plasmid where gene dosage is not precisely controlled may be limited.
Fig. 2.
Susceptibility of wild-type and msr mutants to oxidants and PMN. Wild-type, ΔmsrA1B and complemented ΔmsrA1B strains were grown to the mid-log phase, resuspended in PBS and treated with H2O2 (a), HOCl (b) or HOCl generated by the MPO-H2O2 system (c) at the stated concentrations for 1 h. After 1 h at 37°C, the bacteria were plated and CFU enumerated after overnight growth. Data represent CFU/ml as a percentage of non-treated control for each strain at time 0. Data are the mean ± SEM (n = 6 experiments for a and b, and n = 3 experiments for c). At each concentration of the oxidant, a paired t test was used to compare the wild-type and ΔmsrA1B strains (* p < 0.05, ** p < 0.01). d Each bacterial strain was opsonized in pooled human serum and then fed to normal or DPI-treated PMN (MOI of 5:1). Ingested bacteria were recovered at the indicated time points and CFU were enumerated. At each time point, the viability of a strain is expressed as bacteria recovered from normal PMN as a percent of the same strain recovered from DPI-treated PMN at time 0. A paired t test was used to compare the wild-type and ΔmsrA1B strains at each time point (* p < 0.05). Data represent the mean ± SEM (n = 6 experiments).
Although H2O2 contributes to oxidative stress imposed on bacteria within PMN phagosomes, most of the oxygen consumed by activated PMN is converted into HOCl [18, 19, 20], a more potent antimicrobial agent [21]. To determine whether Msr-deficient S. aureus was more vulnerable to damage by the physiologically relevant oxidant HOCl, we subjected S. aureus to sublethal amounts of HOCl or a HOCl-generating system (MPO-H2O2-chloride). The latter system generates toxic oxidants continuously and better mirrors conditions that exist within PMN phagosomes.
The number of bacteria recovered after treatment with HOCl was expressed as a percentage of the initial number of bacteria prior to treatment (fig. 2b). Wild-type bacteria were more resistant to killing by 100 µM HOCl than was ΔmsrA1B (p < 0.01; fig. 2b). In the presence of the HOCl-generating system, the wild-type and ΔmsrA1B strains also exhibited a significant difference in survival at the high end of concentrations of HOCl produced (p < 0.05; fig. 2c).
Given that Msr can influence the susceptibility of wild-type S. aureus to the antimicrobial effects of H2O2 and HOCl, oxidants that are generated by activated PMN, we reasoned that Msr-deficient bacteria would be more vulnerable to PMN killing. We fed opsonized wild-type, ΔmsrA1B and complemented mutants to PMN for 10 min to allow maximum ingestion. Subsequently, extracellular bacteria were removed and the fate of ingested S. aureus assessed immediately (time 0) and after 30 and 60 min (fig. 2d). Because PMN begin to kill bacteria soon after the ingestion of bacteria and during the 10 min that are allowed for phagocytosis to occur (data not shown), we measured in parallel the viability of bacteria fed to DPI-treated PMN. The pharmacological inhibition of the NADPH oxidase by DPI [22] ensured that minimal killing of bacteria was achieved by time 0, thus providing us with the total number of ingested bacteria at the start of the time course. To quantitate PMN killing of ingested S. aureus over time, we enumerated viable bacteria recovered from normal PMN at each time point relative to the number of bacteria that was recovered at time 0 from the same strain fed to DPI-treated PMN. There was a reduction in the viability of all strains over time, but viable bacteria persisted even 1 h after ingestion. The ΔmsrA1B mutant was more susceptible to PMN killing than was the wild-type mutant at 30 and 60 min (p < 0.05).
Together, these data indicate that msrA1B contributed to the recovery of bacteria, not only after exposure to oxidants in isolation but also in the more complicated context of the PMN phagosome, where oxidants and granule proteins synergize to damage ingested targets.
msrA1 Induction in S. aureus Exposed to PMN
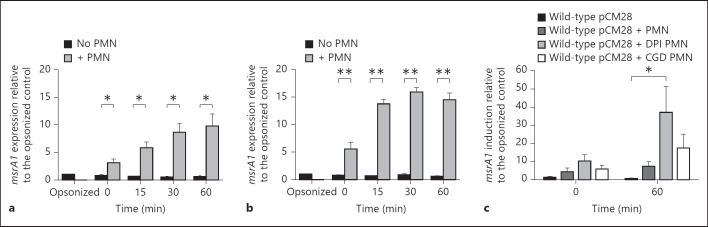
To examine the expression of msr in PMN-ingested bacteria, we recovered S. aureus from PMN that had been fed bacteria. After 10 min of phagocytosis by PMN, extracellular bacteria were aspirated away and the time course initiated. At time 0, the level of msrA1 in the ingested bacteria was higher in organisms grown to the stationary phase relative to those grown to the mid-log phase (fig. 3a, b), with levels in both increasing further at 15 min and remaining high at 30 and 60 min. Incubation in the absence of PMN did not promote the expression of msrA1.
Fig. 3.
msrA1 expression in wild-type mutant ingested by normal and oxidase-deficient PMN. Serum-opsonized wild-type S. aureus in the mid-log (a) and stationary phase (b) were incubated alone or fed to PMN at an MOI of 1:1. At 0, 15, 30 and 60 min after phagocytosis, both ingested bacteria had recovered from PMN and bacteria incubated alone were prepared for real-time PCR. The expression of msrA1 was normalized to the expression of gapdh, and fold change in gene expression was relative to that in opsonized S. aureus prior to exposure to PMN. Data represent the mean ± SEM (n = 3 experiments). At each time point, a paired t test was used to analyze the difference between the ingested and non-ingested bacteria (* p < 0.05, ** p < 0.01). c Opsonized mid-log phase wild-type S. aureus was incubated alone or fed at an MOI of 1:1 to suspended normal PMN, DPI-treated PMN or PMN derived from 1 of 4 unrelated individuals with X-linked CGD. Data represent the mean ± SEM (n = 4 experiments). Data after 60 min were analyzed by 1-way ANOVA followed by a Tukey post-test (* p < 0.05).
Given that Msr participates in cellular oxidant defense, we sought to determine whether oxidants generated within PMN phagosomes could drive msrA1 expression in ingested S. aureus. To that end, we compared msrA1 expression in S. aureus fed to normal PMN, PMN pretreated with DPI and PMN isolated from individuals with CGD, thereby providing PMN with pharmacologic or genetic absence of a functional phagocyte NADPH oxidase as a source of reactive oxygen species [23]. We detected high levels of msrA1 expression in S. aureus ingested by DPI-treated and CGD PMN, despite the absence of PMN-generated oxidants in those PMN. The magnitude of the induction was highest in bacteria ingested by DPI-treated PMN, followed by bacteria ingested by CGD PMN at 60 min (fig. 3c).
The Role of Oxidants in the Expression of msrA1
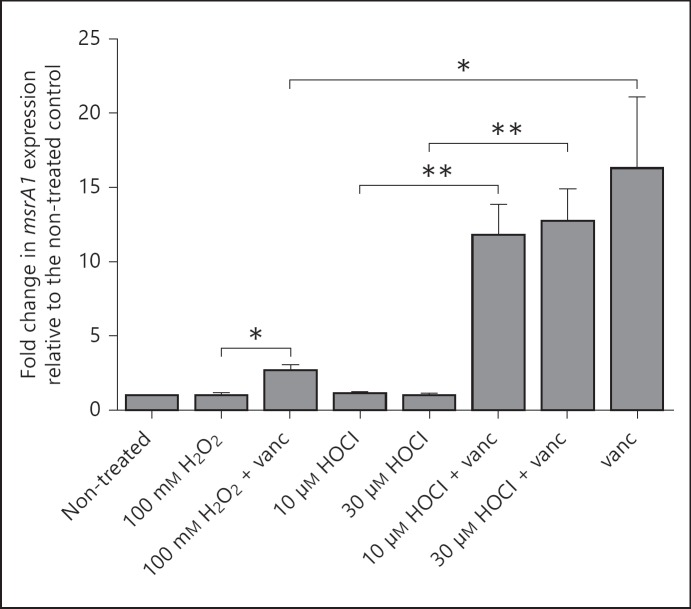
Given that the induction of msrA1 was more marked in PMN that were unable to produce oxidants, we studied whether oxidants may have a role in dampening the msrA1 response. We treated bacteria grown in minimal media with vancomycin to induce msrA1 expression in the presence or absence of H2O2 or HOCl. Since HOCl is such a potent antimicrobial agent [1], much lower concentrations of HOCl (10 and 30 µM) were used relative to H2O2 (100 mM). At the tested concentrations, neither oxidant induced msrA1 expression when used alone, but vancomycin alone induced msrA1 more than 15-fold (fig. 4). In the presence of 10 or 30 µM of HOCl, the level of msrA1 induced by vancomycin was similar to that observed with vancomycin treatment alone. However, in the presence of 100 mM H2O2, the effect of vancomycin on msrA1 induction was markedly reduced (fig. 4). These data suggest that although oxidants did not directly induce msrA1, exposure to H2O2 but not to HOCl may limit the msrA1 response to stimuli arising from disruption of the bacterial cell wall. The observed decrease in vancomycin-induced msrA1 response in the presence of H2O2 did not reflect a global inhibition of transcription by oxidant treatment, as the expression of the H2O2-responsive gene alkyl hydroperoxide reductase was increased as expected [24] (online suppl. fig. 2).
Fig. 4.
msrA1 expression in S. aureus treated with oxidants and vancomycin (vanc). Wild-type S. aureus cultured in minimal media was treated with H2O2 or HOCl in the presence or absence of vancomycin (7 µg/ml) or with vancomycin alone for 1 h at 37°C. Bacteria were then assessed for msrA1 expression by real-time PCR and the data were normalized to gapdh expression. The data represent the fold change in msrA1 expression relative to the non-treated control (mean ± SEM, n = 6). A paired t test was used to compare sample conditions (* p < 0.05, ** p < 0.01).
Modulation of msrA1 by Staphylococcal TCS
We sought to determine which pathways in S. aureus participate in the detection and relay of signals that modulate the induction of msrA1. To sense and respond to the environment, prokaryotes utilize TCS [25], which typically consist of a cell surface histidine kinase as sensor and a cytoplasmic regulator to coordinate a transcriptional response. Only a few of the 16 TCS encoded in the S. aureus genome have been thoroughly characterized.
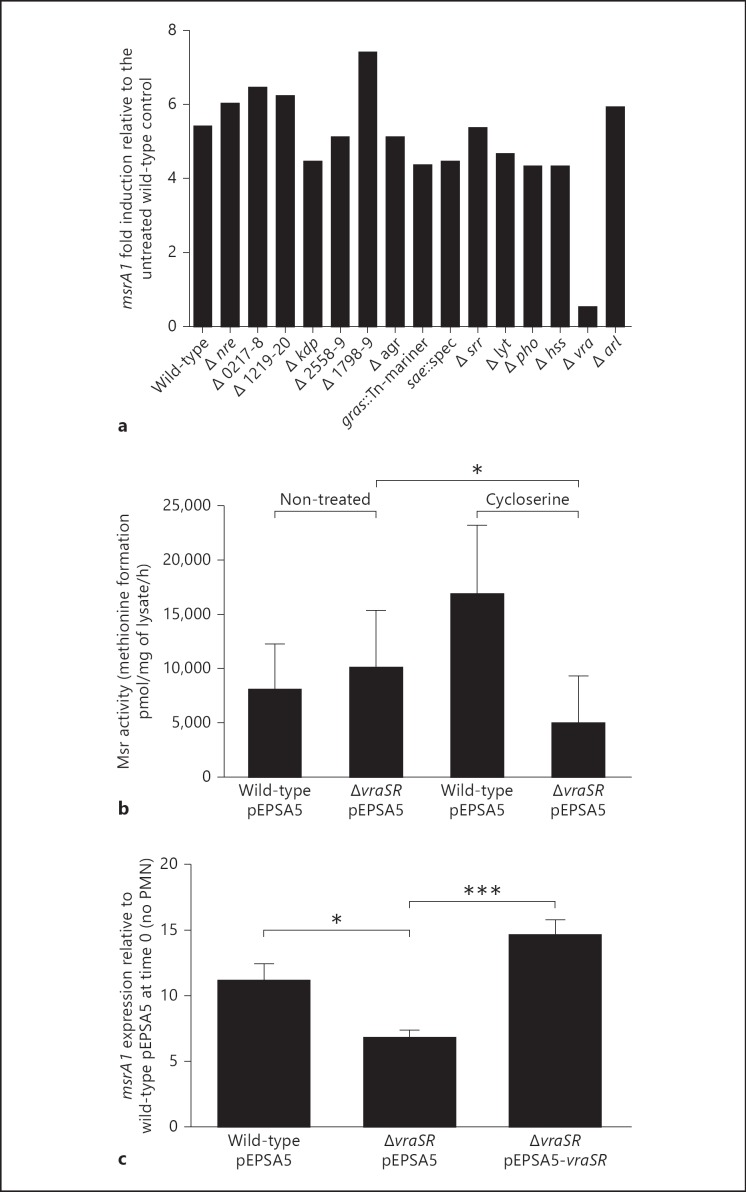
In a screen of a library of 15 nonessential TCS mutants for impaired msrA1 induction in response to vancomycin, deletion of vraSR resulted in loss of msrA1 induction under our experimental conditions (fig. 5a). To confirm the observed relationship between VraSR and Msr, we quantitated Msr activity in the vraSR deletion mutant (ΔvraSR) and its isogenic wild-type mutant after treatment with a cell wall-active antibiotic structurally unrelated to vancomycin. We chose D-cycloserine for its ability to induce msrA1 and, more importantly, because its antistaphylococcal activity, in contrast to that of vancomycin, is unchanged in the absence of a functional VraSR system [26, 27]. At a concentration of D-cycloserine that modulated Msr activity with the least impact on viability (data not shown), D-cycloserine-treated wild-type mutants showed approximately 2-fold more Msr activity than did the non-treated controls. In contrast, treatment of ΔvraSR resulted in less Msr activity relative to the treated wild-type strain (p < 0.05; fig. 5b). These data suggested that VraSR contributed to the regulation of msrA1 in response to cell wall-active antibiotics.
Fig. 5.
Regulation of msrA1 by TCS. a Wild-type and mutant strains of S. aureus that were each deficient in one TCS were grown to the mid-log phase and treated with vancomycin for 1 h (table 1). TCS mutants are labeled in the graph with the gene locus or TCS name where known. The expression of msrA1 measured by real-time PCR was normalized to the expression of gapdh. Fold change in msrA1 is expressed relative to the untreated wild-type strain. The data represent the mean of two independent experiments. b Wild-type and ΔvraSR grown to the stationary phase were diluted to an OD550 of 0.3 and then treated with buffer or with D-cycloserine. Msr activity in bacterial lysates was measured. Data shown represent the mean ± SEM (n = 4). A paired t test was performed between the cycloserine-treated wild-type and mutant samples (* p < 0.05). c Serum-opsonized wild-type, ΔvraSR and complemented ΔvraSR grown to the mid-log phase were fed to PMN (MOI of 1:1), and msrA1 expression in the ingested bacteria was measured and normalized to gapdh expression. Fold change is expressed relative to opsonized wild-type bacteria at time 0. Data represent the mean ± SEM (n = 9) and are analyzed by 1-way ANOVA with a Tukey post-test (* p < 0.05, *** p < 0.001).
To determine whether VraSR participated in the regulation of msrA1 in PMN-ingested bacteria, we measured msrA1 expression in wild-type, ΔvraSR and ΔvraSR mutants complemented with a multicopy plasmid (ΔvraSR with pEPSA5-vraSR) after phagocytosis by PMN. Phagocytosis of S. aureus by PMN was the same for all the strains (data not shown). Both wild-type and complemented mutants significantly upregulated msrA1 in response to ingestion by approximately 11- and 15-fold, respectively. In contrast, ΔvraSR exhibited a relatively muted response, with only an approximately 7-fold induction in msrA1 (p < 0.05; fig. 5c). Similar to the trend observed in msrA1, vraS expression in S. aureus ingested by DPI-treated PMN and CGD PMN was higher than that in normal PMN (online suppl. fig. 3). Taken together, these data provide support for a link between VraSR, a TCS that can be activated by perturbations to the cell wall, and Msr, one of the oxidant repair systems. However, they also highlight the complexity of msrA1 regulation in a biological setting composed of multiple interacting antimicrobial toxins, as occurs in PMN phagosomes, and implicate factors other than VraSR.
PMN Granules Induce the Expression of msrA1
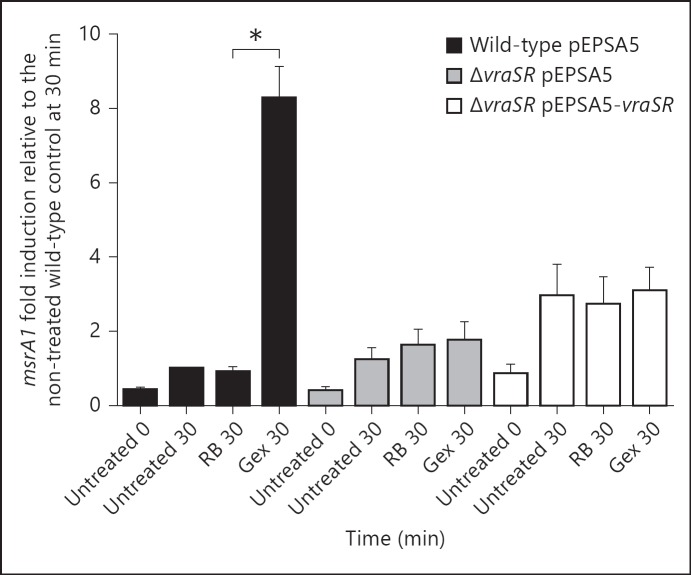
Optimal PMN antimicrobial action relies not only on oxidants from the NADPH oxidase but also on granule proteins released into phagosomes during degranulation [28]. Granule proteases (e.g., elastase, cathepsin G) [29, 30, 31] and cationic antimicrobial proteins (e.g., defensins and cathelicidins) [32] can compromise the stability of bacterial membranes that in turn may disrupt the cell wall [33, 34, 35]. Because our data demonstrate that upregulation of msrA1 in ingested S. aureus could be mediated by factors other than PMN-derived oxidants (fig. 3c) and could be induced by cell wall stress, we hypothesized that granule proteins could trigger a response that was relayed through VraSR. To test this hypothesis, we measured msrA1 in bacteria exposed to PMN granule-rich extracts in the absence of exogenous reactive oxygen species. The level of msrA1 expression was lowest at the start of the experiment prior to any treatment in all the strains. After a 30-min incubation period, msrA1 expression was slightly higher in bacteria that were not treated or in the RB control. This increase in msrA1 expression may reflect changes in the growth status of the bacteria, since msrA1 expression varies during different growth phases [3]. In bacteria treated with granule extract, msrA1 expression was significantly higher in the wild-type when compared to the RB-treated bacteria at 30 min (p < 0.05; fig. 6). In contrast, the level of msrA1 in granule extract-treated samples in ΔvraSR was not significantly elevated compared to its RB control. These data suggest that granule proteins alone promoted increased msrA1 expression and that VraSR contributed to the modulation of msrA1 in response to granule proteins.
Fig. 6.
msrA1 expression in S. aureus treated with PMN granule proteins. Wild-type, ΔvraSR and complemented ΔvraSR strains were treated with PMN granule extracts (Gex), PBS (negative control) or RB (vehicle control). The levels of msrA1 in the samples at 0 and 30 min were assessed by real-time PCR. The expression levels were normalized to the expression of gapdh. Data represent the mean ± SEM (n = 3 experiments). To compare the RB sample with the Gex sample at 30 min, a paired t test was used for each strain (* p < 0.05).
Discussion
Optimal antimicrobial action in PMN relies heavily on phagocyte-generated oxidants. In such an inhospitable environment, ingested S. aureus rapidly initiates a program of transcriptional responses that promote its survival and dissemination [11, 36], making it likely that the net balance between sustained damage and repair dictates the fate of ingested bacteria. Defensive strategies employed by bacteria in response to oxidants involve both genes encoding proteins that target oxidants, such as catalase, superoxide dismutase, thioredoxin, and alkyl hydroperoxide reductase [36], as well as those that repair oxidant-mediated damage and restore normal function. Msr are key enzymes in the defense against damage mediated by reactive oxygen species that are produced as a byproduct of aerobic respiration, and their evolutionary conservation across nearly all species underscores their importance [37, 38]. HOCl oxidizes protein methionines in Escherichia coli in a dose-dependent fashion, and methionine oxidation closely correlates with loss of bacterial viability [39]. Conversely, the capacity of Msr to repair damaged methionines parallels the increased resistance of E. coli to killing by HOCl [39] and likewise contributes to the defenses of Mycobacterium tuberculosis[40] and Helicobacter pylori against HOCl [41]. Thus, we reasoned that oxidation of key proteins in S. aureus may also result in reduced viability when the Msr-mediated repair system is inadequate to meet demands.
We demonstrate that the absence of Msr activity increased the susceptibility of S. aureus to the oxidants H2O2 and HOCl. We found that the ability of the Msr system to temper the effects of oxidant damage was less marked when S. aureus was treated with low doses of H2O2 or HOCl, which may indicate that the role of Msr in oxidant defense becomes more important when S. aureus is subjected to higher levels of oxidant stress and greater levels of protein damage (fig. 2b). In addition, we observed that even in the absence of any oxidant treatment, the percentage of recovered ΔmsrA1B was sometimes slightly lower than that of the wild-type and complemented strain (fig. 2a-c). Although this difference did not reach statistical significance, it suggested that ΔmsrA1B may be slightly more sensitive to the aerobic environment during the 1-hour incubation in PBS than the other strains. This behavior contrasts with our observation that all the strains showed similar growth in TSB. Since TSB is a rich source of substrates that could serve as oxidant sinks, it is likely that the enriched media both promotes microbial growth directly and neutralizes some of the environmental oxidants, thus limiting their deleterious effects on the bacteria.
The absence of Msr activity increased the susceptibility of S. aureus not only to reagent H2O2 and HOCl but also to the antimicrobial effects of PMN. In contrast to the immediate exposure to high concentrations of oxidants imposed by adding H2O2 or HOCl to suspensions of S. aureus, oxidant generation within phagosomes occurs gradually, following the kinetics of NADPH oxidase assembly and activation. As a result, oxidants within PMN may be generated sufficiently slowly to allow reparative responses such as the induction of msr to be engaged. Given the role of Msr in oxidant repair, we speculated that PMN-derived oxidants may drive the upregulation of msrA1 that we observed in S. aureus ingested by PMN. Our data show that bacteria ingested by normal PMN upregulated msrA1 rapidly after phagocytosis. S. aureus ingested by PMN that have impaired NADPH oxidase activity and were not exposed to phagosomal oxidants also upregulated msrA1 after phagocytosis and to higher levels. These data suggest that the msrA1 response to ingested S. aureus may be muted by the presence of oxidants in the phagosome, either directly by oxidant attack on bacteria or indirectly by oxidative modifications of the host-derived phagosomal contents. In the absence of PMN, S. aureus exposed simultaneously to H2O2 and a cell wall-active antibiotic (vancomycin) had a blunted msrA1 transcriptional response relative to that seen in vancomycin alone. Unexpectedly, HOCl, a much more powerful oxidant than H2O2, did not influence vancomycin-induced msrA1. The mechanistic basis for the differential responses to the two oxidants is unknown and merits further study. These findings illustrate how oxidants and granule proteins present in phagosomes can act synergistically on target bacteria.
The S. aureus genome encodes three other msr genes besides msrA1, including msrB, msrA2 and msrA3. Since msrB is cotranscribed and regulated by the same promoter, the pattern of expression follows that of msrA1 and it is responsive to the same stimuli. The other two msr genes are located at different genomic loci and under the regulation of promoters that are responsive to differing stresses than that of msrA1 and msrB. Not only do msrA2 and msrA3 show a different pattern of expression to msrA1 and msrB during varied phases of growth, they also do not respond to the same stress signals [3]. For these reasons and also because MsrA1 and MsrB account for almost all the Msr activity in S. aureus (fig. 1), the expression of msrA2 and msrA3 was not evaluated in this study.
The regulation of msrA1 in S. aureus is not yet fully elucidated, although msr is one of a set of genes known as the cell wall stress stimulon, which is regulated by stresses inflicted by cell wall-active antibiotics such as β-lactams, vancomycin and D-cycloserine [42]. Genes in the cell wall stress stimulon overlap with those regulated by VraSR, a TCS that influences susceptibility of S. aureus to vancomycin by modulating cell wall biosynthesis [26]. The VraSR system senses disruptions of cell wall biosynthesis in target bacteria, which may explain why both cell wall-active antibiotics and antimicrobial peptides induce the VraSR system [43]. In our study, PMN granule proteins alone stimulated msr expression in a VraSR-dependent fashion. The mechanism by which VraSR is activated and how it subsequently modulates msrA1 remain to be elucidated, including whether VraR directly or indirectly regulates msr. We noted that the introduction of the complementation plasmid into ΔvraSR increased msrA1 expression to higher levels than that observed in ΔvraSR both in the presence and absence of granule extracts but did not restore it to wild-type levels. The levels of msrA1 were also similar in the RB and granule extract-treated samples for this strain. Complementation of a mutation can be challenging and, given that the restoration of vraSR expression was driven from a multicopy plasmid and from an inducible promoter rather than from its native promoter, the gene dosage and regulation of vraSR may not accurately emulate the wild-type strain under these conditions. Nonetheless, the levels of vraSR in ΔvraSR with pEPSA5-vraSR were higher than in ΔvraSR and allowed for greater msrA1 expression.
The transcriptional changes that occur in phagocytosed bacteria reflect a coordinated response to the multitude of stresses experienced within the host environment. Given the complexity of this environment, multiple signaling pathways underpin the net global response, and their cross-talk can contribute to the modulation of stress response genes such as msr. Indeed, our data show that there are factors other than VraSR that can modulate the expression of msrA1 in S. aureus within the PMN environment. The identification and characterization of signaling systems in addition to VraSR in the regulation of msrA1 modulation remain an area of interest.
Our study highlights Msr as one of the mechanisms by which S. aureus may resist the consequences of the multifaceted antimicrobial attack that occurs in PMN phagosomes and reemphasizes that the relationships between stress and repair pathways are not necessarily straightforward. We speculate that Msr and functionally related proteins in the antioxidant pathway serve important roles in cell wall biosynthesis and homeostasis. Further understanding of the relationship between oxidant stress, both exogenous and endogenous, and cell wall damage in the context of elucidating how S. aureus survives within PMN may provide insights into the pathogenesis of staphylococcal disease and into the development of novel therapeutic approaches.
Disclosure Statement
None of the authors have conflicts of interests.
Supplementary Material
Supplementary data
Acknowledgements
The authors express special thanks to the members of the Nauseef and Horswill laboratories for their help in this project. This work was supported by National Institutes of Health grants R01 AI078921 and R01 AI07958 to A.R.H. and W.M.N., respectively, and by a Merit Review Grant (to W.M.N.) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, with facilities and resources of the Veterans Administration in Iowa City, Iowa, USA.
References
- 1.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Singh VK. Expression of four methionine sulfoxide reductases in Staphylococcus aureus. Int J Microbiol. 2012;2012:719594. doi: 10.1155/2012/719594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh VK, Moskovitz J, Wilkinson BJ, Jayaswal RK. Molecular characterization of a chromosomal locus in Staphylococcus aureus that contributes to oxidative defence and is highly induced by the cell-wall-active antibiotic oxacillin. Microbiology. 2001;147:3037–3045. doi: 10.1099/00221287-147-11-3037. [DOI] [PubMed] [Google Scholar]
- 5.Singh VK, Moskovitz J. Multiple methionine sulfoxide reductase genes in Staphylococcus aureus: expression of activity and roles in tolerance of oxidative stress. Microbiology. 2003;149:2739–2747. doi: 10.1099/mic.0.26442-0. [DOI] [PubMed] [Google Scholar]
- 6.Luo S, Levine RL. Methionine in proteins defends against oxidative stress. FASEB J. 2009;23:464–472. doi: 10.1096/fj.08-118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boles BR, Thoendel M, Roth AJ, Horswill AR. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nygaard TK, Pallister KB, Ruzevich P, Griffith S, Vuong C, Voyich JM. SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J Infect Dis. 2010;201:241–254. doi: 10.1086/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose JL, Fey PD, Bayles KW. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol. 2013;79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. agr-dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, Wall D, Wang L, Brown-Driver V, Froelich JM, KG C, King P, McCarthy M, Malone C, Misiner B, Robbins D, Tan Z, Zhu Zy ZY, Carr G, Mosca DA, Zamudio C, Foulkes JG, Zyskind JW. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol. 2002;43:1387–1400. doi: 10.1046/j.1365-2958.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 13.Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 14.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 15.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 16.Dypbukt JM, Bishop C, Brooks WM, Thong B, Eriksson H, Kettle AJ. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic Biol Med. 2005;39:1468–1477. doi: 10.1016/j.freeradbiomed.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Singh VK, Jayaswal RK, Wilkinson BJ. Cell wall-active antibiotic induced proteins of Staphylococcus aureus identified using a proteomic approach. FEMS Microbiol Lett. 2001;199:79–84. doi: 10.1111/j.1574-6968.2001.tb10654.x. [DOI] [PubMed] [Google Scholar]
- 18.DeRusso PA, Philpott CC, Iwai K, Mostowski HS, Klausner RD, Rouault TA. Expression of a constitutive mutant of iron regulatory protein 1 abolishes iron homeostasis in mammalian cells. J Biol Chem. 1995;270:15451–15454. doi: 10.1074/jbc.270.26.15451. [DOI] [PubMed] [Google Scholar]
- 19.Saleem A, Yuan ZM, Taneja N, Rubin E, Kufe DW, Kharbanda SM. Activation of serine/threonine protein kinases and early growth response 1 gene expression by tumor necrosis factor in human myeloid leukemia cells. J Immunol. 1995;154:4150–4156. [PubMed] [Google Scholar]
- 20.Chapman AL, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J Biol Chem. 2002;277:9757–9762. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 22.Cross AR, Jones OT. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baehner RL, Nathan DG. Leukocyte oxidase: defective activity in chronic granulomatous disease. Science. 1967;155:835–836. doi: 10.1126/science.155.3764.835. [DOI] [PubMed] [Google Scholar]
- 24.Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol. 2007;189:1025–1035. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno T. His-Asp phosphotransfer signal transduction. J Biochem. 1998;123:555–563. doi: 10.1093/oxfordjournals.jbchem.a021972. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49:807–821. doi: 10.1046/j.1365-2958.2003.03599.x. [DOI] [PubMed] [Google Scholar]
- 27.Dengler V, Meier PS, Heusser R, Berger-Bachi B, McCallum N. Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics. BMC Microbiol. 2011;11:16. doi: 10.1186/1471-2180-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hager M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med. 2010;268:25–34. doi: 10.1111/j.1365-2796.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 29.Odeberg H, Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975;56:1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odeberg H, Olsson I. Microbicidal mechanisms of human granulocytes: synergistic effects of granulocyte elastase and myeloperoxidase or chymotrypsin-like cationic protein. Infect Immun. 1976;14:1276–1283. doi: 10.1128/iai.14.6.1276-1283.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorne KJ, Oliver RC, Barrett AJ. Lysis and killing of bacteria by lysosomal proteinases. Infect Immun. 1976;14:555–563. doi: 10.1128/iai.14.2.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 33.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 34.Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185. [DOI] [PubMed] [Google Scholar]
- 35.Belaaouaj A. Neutrophil elastase-mediated killing of bacteria: lessons from targeted mutagenesis. Microbes Infect. 2002;4:1259–1264. doi: 10.1016/s1286-4579(02)01654-4. [DOI] [PubMed] [Google Scholar]
- 36.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 37.Kryukov GV, Kumar RA, Koc A, Sun Z, Gladyshev VN. Selenoprotein R is a zinc-containing stereo-specific methionine sulfoxide reductase. Proc Natl Acad Sci USA. 2002;99:4245–4250. doi: 10.1073/pnas.072603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarrago L, Gladyshev VN. Recharging oxidative protein repair: catalysis by methionine sulfoxide reductases towards their amino acid, protein, and model substrates. Biochemistry (Mosc) 2012;77:1097–1107. doi: 10.1134/S0006297912100021. [DOI] [PubMed] [Google Scholar]
- 39.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc Natl Acad Sci USA. 2009;106:18686–18691. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee WL, Gold B, Darby C, Brot N, Jiang X, de Carvalho LP, Wellner D, St John G, Jacobs WR, Jr, Nathan C. Mycobacterium tuberculosis expresses methionine sulphoxide reductases A and B that protect from killing by nitrite and hypochlorite. Mol Microbiol. 2009;71:583–593. doi: 10.1111/j.1365-2958.2008.06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahawar M, Tran V, Sharp JS, Maier RJ. Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J Biol Chem. 2011;286:19159–19169. doi: 10.1074/jbc.M111.223677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pechous R, Ledala N, Wilkinson BJ, Jayaswal RK. Regulation of the expression of cell wall stress stimulon member gene msrA1 in methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:3057–3063. doi: 10.1128/AAC.48.8.3057-3063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belcheva A, Golemi-Kotra D. A close-up view of the VraSR two-component system. A mediator of Staphylococcus aureus response to cell wall damage. J Biol Chem. 2008;283:12354–12364. doi: 10.1074/jbc.M710010200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data