Abstract
Background
Anaphylaxis is a serious allergic reaction that may cause death; however, the actual risk of death is unclear.
Objective
To estimate the case fatality rate (CFR) among hospitalizations or emergency department (ED) presentations for anaphylaxis and the mortality rate associated with anaphylaxis for the general population.
Methods
This was a population-based epidemiologic study using 3 national databases: Nationwide Inpatient Sample (NIS, 1999-2009), Nationwide Emergency Department Sample (NEDS, 2006-2009), and Multiple Cause of Death Data (MCDD, 1999-2009). Sources for these databases are hospital, ED discharge records and death certificates, respectively.
Results
CFRs were between 0.25% and 0.33% among hospitalizations or ED presentations with anaphylaxis as the principal diagnosis (NIS+NEDS, 2006-2009). These rates represent 63 to 99 deaths per year in the United States, approximately 77% of which occurred in hospitalized patients. Rate of anaphylaxis hospitalizations rose from 21.0 to 25.1 per million population between 1999 and 2009 (annual percent change 2.23%; 95% CI: 1.52%–2.94%), contrasting with a declining CFR among hospitalizations (annual percent change –2.35%; 95% CI: –4.98% to 0.34%). Overall mortality rates ranged from 0.63 to 0.76 per million population (186 to 225 deaths per year, MCDD), and appeared stable in the last decade (annual percent change: –0.31%; 95% CI, –1.54% to 0.93%).
Conclusion
From 2006 to 2009, the overwhelming majority of hospitalizations or ED presentations for anaphylaxis did not result in death, with an average CFR of 0.3%. Anaphylaxis-related hospitalizations rose steadily in the last decade (1999-2009), but this increase was offset by the declining CFR among those hospitalized; both inpatient and overall mortality rates associated with anaphylaxis appeared stable and were well under 1 per million population. Although anaphylactic reactions are potentially life threatening, the probability of dying is actually very low. With the prevalence of anaphylaxis on the rise, practitioners need to stay vigilant and follow the treatment guidelines to further reduce anaphylaxis-related deaths.
Keywords: anaphylaxis, mortality, case fatality, epidemiology, hospitalization, emergency department presentation, death certificate
Introduction
Anaphylaxis is a rapid-onset, potentially life-threatening systemic allergic reaction that can affect people of any age or sex. It usually occurs as a result of an allergen response, which leads to activation of mast cells and basophils, although in many cases, the cause of anaphylaxis is not known. The diagnosis of anaphylaxis is based on recognition of clinical symptoms, and may require a detailed evaluation of the episode, including activities and events occurring within the minutes to hours preceding the event. Patients typically present with a combination of dermatologic, respiratory, cardiovascular, and gastrointestinal symptoms. The most common triggers are medications, insect stings, and foods, with reactions to medications accounting for most of the mortality.1-7
Population-based studies have estimated the anaphylaxis incidence rate in the United States, the United Kingdom, and other developed countries in the range of 40 to 500 per million person-years.6 Lifetime prevalence estimates range from 0.05% to 2% and seem to be increasing.3,6,8 Estimates of anaphylaxis-related mortality have been between 0.5 and 5.5 per million population, with death reportedly occurring in 0.65% to 2% of patients experiencing severe anaphylactic reactions.3,9 Few studies of anaphylaxis in the US population have been conducted, and most studies were limited by small populations or a regional focus.10-15 Among the population-based studies in the United States, the lowest case fatality rate of 0.20% was reported among hospitalized patients younger than 20 years in New York (1990-2006),14 and the highest case fatality rate of 0.86% was reported among hospitalizations for anaphylaxis in Florida (2001).15 The lowest mortality rate of 0.5 per million population was reported by a study using death records from Florida between 1996 and 2005,13 whereas the highest rate of 5.5 per million population (1500 deaths annually) was estimated based on a review of anaphylaxis epidemiologic data.7,16
For the United States, more recent, broader, population-based epidemiologic data are needed to better assess the risk of anaphylaxis. This information will be useful both for informing patients and healthcare providers of the risk, and also for focusing and assessing efforts to reduce the mortality associated with anaphylaxis.3,8,13,17,18 We sought to conduct a large population-based epidemiologic study using current data from 3 US national databases to estimate the case fatality and population mortality associated with anaphylaxis, based on hospital, emergency department (ED) discharge records, and death certificates.
Methods
Data Sources
Three separate databases were used in this study: Nationwide Inpatient Sample (NIS, 1999-2009), Nationwide Emergency Department Sample (NEDS, 2006-2009), and Multiple Cause of Death Data (MCDD, 1999-2009).19-21 These databases are deidentified for public use and were protected through data use agreements.
Nationwide Inpatient Sample, 1999-2009
The NIS is part of the Healthcare Cost and Utilization Project (HCUP), the largest all-payer inpatient care database in the United States, and contains data on ~8 million hospitalizations annually from ~1000 hospitals approximating a 20% stratified sample of US community hospitals. The NIS includes data from up to 45 states that participate in HCUP, covering >96% of the population. The unit of analysis for the NIS is the individual hospitalization rather than individual patient and no patient identifiers or keys are available. Weights are provided to calculate estimates for the entire US population. Diagnostic and procedure codes on the hospital discharge records in the NIS are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The NIS has been widely used to study trends in hospital care and has been validated against the National Hospital Discharge Survey (NHDS) and the Medicare Provider Analysis and Review (MEDPAR).22
Nationwide Emergency Department Sample, 2006-2009
The NEDS, also part of the HCUP, is a database of ED presentations that do not result in an admission (ie, treat-and-discharge presentations) and ED presentations that result in admission to the same hospital. The NEDS contains 25 to 30 million records for ED presentations from ~1000 hospitals approximating a 20% stratified sample of US hospital EDs. Similar to NIS, weights are provided for US projection, and diagnoses and procedures are coded based on the ICD-9-CM. The NEDS was first released in 2006 and has been validated against other national data sources on ED presentations.23
Multiple Cause of Death Data, 1999-2009
The MCDD contains mortality data derived from death certificates for US residents. Each death certificate records an underlying cause of death (disease or injury that initiated the events resulting in death), ≤20 multiple causes leading to death, and demographic data. Causes of death are coded using the International Classification of Diseases, Tenth Revision (ICD-10). Mortality data compiled in MCDD includes death certificates from all 50 states and the District of Columbia. Death certificates for nonresident aliens, US nationals living abroad, and residents of US territories are excluded, as are fetal deaths.
Case Definition and Study Endpoint
Selection of Anaphylaxis Cases
In previous research, various ICD-9-CM or ICD-10 codes have been used for the selection of anaphylaxis cases.13,14,17,18,24 Some studies included anaphylaxis-nonspecific codes, such as “allergic reaction” or “angioneurotic edema (angioedema),”16,24 whereas others included only anaphylaxis-specific codes, or a subset of those codes.13,17,18 Use of additional anaphylaxis nonspecific codes enhances the sensitivity in case selection but decreases specificity.18
For the analysis of NIS and NEDS, anaphylaxis cases were selected based on the principal diagnosis code on a discharge record using the following ICD-9-CM codes: 995.0 (anaphylactic shock or reaction, unspecified), 995.60 to 995.69 (various food items), and 999.4 (serum). The principal diagnosis is defined as that condition established after study to be chiefly responsible for occasioning the admission of the patient to the hospital for care, and has been used to capture anaphylaxis in most of the epidemiological studies utilizing ICD-9-CM codes.13-15 For MCDD, cases were selected based on 4 ICD-10 codes including the keyword anaphylactic: T78.0 (food), T78.2 (unspecified), T80.5 (serum), and T88.6 (drug). Anaphylaxis may have been entered as the main underlying cause in the original death certificate, but will only show up as one of the multiple causes of deaths in MCDD as it is not permissible as the main underlying cause of death under data processing guidance of CDC.25
ICD-9-CM offers very specific codes capturing food related anaphylaxis cases, but no codes for drug related anaphylaxis cases, which are likely captured under 999.50 (unspecified). On the other hand, ICD-10 provides a specific code for drug related anaphylaxis cases, but no codes for food related anaphylaxis cases.
Primary Outcomes
The primary outcomes were the annual anaphylaxis case fatality rate and population mortality rate. The case fatality rate was defined as the percentage of deaths among hospitalizations or ED presentations for anaphylaxis-associated cases. The overall mortality rate (deaths per million population) was estimated by dividing the total number of deaths by the US resident population from the census data, and inpatient and ED mortality rates were calculated using the same denominators.21 We also estimated the age-adjusted mortality rate based on the 2000 US standard population.26
Statistical Analysis
For MCDD, the number of deaths and mortality rate were obtained via http://wonder.cdc.gov/ (record-level data were also obtained to validate the results for 2007-2009). We assumed that the number of deaths arises from a Poisson distribution27 and calculated the standard error and 95% confidence intervals based on Taylor's expansion.28 Population estimates were used as the denominators for calculations of annual mortality rates and were assumed to be free of sampling error. To assess whether there is any trend in the mortality rate, we used a generalized linear model (GLM) with the Poisson distribution and the log link. To guard against misspecification of the variance, such as the overdispersion commonly observed with Poisson data, we used the Huber-White robust sandwich estimator for variance.29-31 The relative risk for deaths was explored among demographics factors, such as age, gender and race, and it was estimated using a similar generalized linear model with the Poisson distribution and the log link.
For NIS and NEDS, we used SAS SurveyMeans (Version 9.2, SAS Institute, Cary, NC) to obtain estimates for hospitalizations, ED presentations, and deaths, accounting for the sampling design (discharge weight, stratification, and clustering) of these databases.32 To assess trends associated with hospitalization and death rates, we used the same GLM model described earlier as the primary approach. One advantage of the GLM model with log link was that change could be measured as an annual percentage change.
Results
Anaphylaxis-Related Hospitalizations and Inpatient Deaths (NIS)
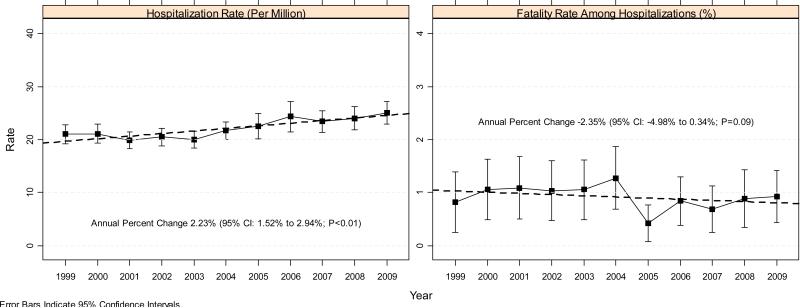
Between 1999 and 2009, the annual number of anaphylaxis-related hospitalizations increased from 5681 to 7708 (Table 1), which corresponds to an increase in hospitalization rates from 21.0 to 25.1 per million population (Figure 1), with an annual increase of approximately 2.23% (95% CI, 1.52%-2.94%; P<0.01). In contrast, case fatality rate among hospitalizations for anaphylaxis appeared to be declining, with an annual change of –2.35% (95% CI, –4.98% to 0.34%; P=0.09). Case fatality rates ranged from 0.42% to 1.27% (average, 0.92%), with the number of inpatient deaths between 28 and 81. The inpatient mortality rates ranged from 0.10 to 0.28 per million population and were stable (annual percentage change of –0.23%; 95% CI, –2.77% to 2.38%; P=0.86). The year 2005 was notably a departure from other years with respect to inpatient anaphylaxis deaths. However, the trend was similar without year 2005; the annual percent change for the case fatality rates was –1.90% (95% CI, –4.30% to 0.56%; P=0.13) and the annual percent change for the inpatient mortality rates 0.25% (95% CI, –1.94% to 2.49%; P=0.83).
Table 1.
Anaphylaxis Hospitalizations, ED Presentations, Case Fatalities, and Population Mortality
| Anaphylaxis as the Principal Diagnosis |
|||||
|---|---|---|---|---|---|
| Year | Hospitalizations or ED Visits |
Deaths in the Hospital or ED |
Case Fatality Rate Among Hospitalizations or ED Visits (95% CI)b | ||
| Count | Rate - Per Million (95% CI)a | Count | Mortality Rate - Per Million (95% CI)a | ||
| NIS (Hospitalizations) | |||||
| 1999 | 5,863 | 21.0 (19.2, 22.8) | 48 | 0.17 (0.05, 0.29) | 0.82% (0.25%, 1.39%) |
| 2000 | 5,938 | 21.1 (19.3, 22.9) | 63 | 0.22 (0.10, 0.34) | 1.06% (0.49%, 1.63%) |
| 2001 | 5,681 | 19.9 (18.3, 21.5) | 62 | 0.22 (0.10, 0.34) | 1.09% (0.50%, 1.68%) |
| 2002 | 5,907 | 20.5 (18.8, 22.1) | 61 | 0.21 (0.10, 0.33) | 1.04% (0.47%, 1.60%) |
| 2003 | 5,807 | 20.0 (18.4, 21.5) | 61 | 0.21 (0.10, 0.32) | 1.05% (0.48%, 1.62%) |
| 2004 | 6,365 | 21.7 (20.0, 23.4) | 81 | 0.28 (0.15, 0.41) | 1.27% (0.68%, 1.87%) |
| 2005 | 6,670 | 22.5 (20.1, 24.9) | 28 | 0.10 (0.02, 0.17) | 0.42% (0.08%, 0.77%) |
| 2006 | 7,302 | 24.4 (21.5, 27.2) | 61 | 0.20 (0.09, 0.32) | 0.84% (0.38%, 1.30%) |
| 2007 | 7,062 | 23.4 (21.4, 25.4) | 49 | 0.16 (0.06, 0.26) | 0.69% (0.25%, 1.13%) |
| 2008 | 7,310 | 24.0 (21.9, 26.2) | 64 | 0.21 (0.08, 0.34) | 0.88% (0.34%, 1.42%) |
| 2009 | 7,708 | 25.1 (23.0, 27.2) | 71 | 0.23 (0.11, 0.36) | 0.93% (0.43%, 1.42%) |
| NEDS (ED Presentations, without admission) | |||||
| 2006 | 18,027 | 60.2 (54.5, 66.0) | 22 | 0.07 (0.00, 0.15) | 0.13% (0.00%, 0.25%) |
| 2007 | 17,735 | 58.8 (53.5, 64.1) | 14 | 0.05 (0.00, 0.10) | 0.08% (0.00%, 0.17%) |
| 2008 | 21,039 | 69.2 (63.0, 75.4) | 13 | 0.04 (0.00, 0.09) | 0.06% (0.00%, 0.13%) |
| 2009 | 21,822 | 71.1 (64.6, 77.6) | 27 | 0.09 (0.02, 0.16) | 0.12% (0.02%, 0.23%) |
| NIS+NEDS (Hospitalizations and ED Presentations without admission) | |||||
| 2006 | 25,329 | 84.6 (78.2, 91.0) | 83 | 0.28 (0.14, 0.41) | 0.33% (0.00%, 0.81%) |
| 2007 | 24,797 | 82.2 (76.5, 87.9) | 63 | 0.21 (0.09, 0.32) | 0.25% (0.00%, 0.70%) |
| 2008 | 28,349 | 93.2 (86.7, 99.8) | 77 | 0.25 (0.12, 0.39) | 0.27% (0.00%, 0.82%) |
| 2009 | 29,530 | 96.2 (89.4, 103.0) | 99 | 0.32 (0.18, 0.46) | 0.33% (0.00%, 0.84%) |
Note: All counts derived from NIS and NEDS are projected national estimates based on the discharge weights, and are subject to rounding error.
The denominator was the US population.
Deaths among hospitalizations or ED presentations (without admission) with a principal diagnosis of anaphylaxis.
Figure 1.
Trend in the anaphylaxis hospitalization and deaths among hospitalizations for anaphylaxis (NIS).
The discharge diagnosis most frequently linked to anaphylaxis-related hospitalizations was 995.0 (unspecified) and accounted for 63% to 71% of all anaphylaxis hospitalizations (Table 2). Peanut-related anaphylaxis (995.61) hospitalizations almost doubled in the last decade, from 256 (4%) in 1999 to 512 (7%) in 2009. The breakdown of deaths by ICD-9-CM code could not be reported because most of the counts were ≤10.
Table 2.
Anaphylaxis-Related Hospitalizations and ED Presentations by ICD-9-CM Diagnosis Code
| Year | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 |
| NIS (Hospitalizations) | 5,863 | 5,938 | 5,681 | 5,907 | 5,807 | 6,365 | 6,670 | 7,302 | 7,062 | 7,310 | 7,708 |
| Anaphylaxis ICD-9-CM Code, n (%) | |||||||||||
| 995.0, Unspecified | 4,037 (69%) | 4,197 (71%) | 3,997 (70%) | 4,065 (69%) | 4,118 (71%) | 4,254 (67%) | 4,255 (64%) | 4,587 (63%) | 4,553 (64%) | 4,955 (68%) | 5,008 (65%) |
| 995.60, Unspecified Food | 240 (4%) | 158 (3%) | 158 (3%) | 99 (2%) | 148 (3%) | 169 (3%) | 196 (3%) | 262 (4%) | 246 (3%) | 251 (3%) | 194 (3%) |
| 995.61, Peanuts | 256 (4%) | 284 (5%) | 248 (4%) | 354 (6%) | 301 (5%) | 375 (6%) | 540 (8%) | 562 (8%) | 578 (8%) | 470 (6%) | 512 (7%) |
| 995.62, Crustaceans | 202 (3%) | 179 (3%) | 165 (3%) | 89 (2%) | 156 (3%) | 207 (3%) | 202 (3%) | 176 (2%) | 255 (4%) | 202 (3%) | 258 (3%) |
| 995.63, Fruits, Vegetables | 134 (2%) | 124 (2%) | 108 (2%) | 156 (3%) | 84 (1%) | 132 (2%) | 198 (3%) | 182 (2%) | 118 (2%) | 178 (2%) | 206 (3%) |
| 995.64, Tree Nuts, Seeds | 182 (3%) | 218 (4%) | 167 (3%) | 220 (4%) | 254 (4%) | 313 (5%) | 290 (4%) | 345 (5%) | 268 (4%) | 255 (3%) | 344 (4%) |
| 995.65, Fish | 227 (4%) | 224 (4%) | 233 (4%) | 278 (5%) | 272 (5%) | 251 (4%) | 207 (3%) | 359 (5%) | 255 (4%) | 300 (4%) | 285 (4%) |
| 995.66, Food Additives | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 995.67, Milk Products | 85 (1%) | 67 (1%) | NR | NR | 58 (1%) | 113 (2%) | 111 (2%) | 117 (2%) | 140 (2%) | 55 (1%) | 113 (1%) |
| 995.68, Eggs | NR | NR | NR | NR | NR | NR | 67 (1%) | NR | NR | NR | NR |
| 995.69, Other Food | 369 (6%) | 364 (6%) | 371 (7%) | 389 (7%) | 313 (5%) | 390 (6%) | 487 (7%) | 428 (6%) | 465 (7%) | 484 (7%) | 631 (8%) |
| 999.4, Serum | 77 (1%) | 68 (1%) | 101 (2%) | 84 (1%) | 64 (1%) | 83 (1%) | 99 (1%) | 130 (2%) | 136 (2%) | 103 (1%) | 117 (2%) |
| NEDS (ED Presentations, without admission) | 18,027 | 17,735 | 21,039 | 21,822 | |||||||
| Anaphylaxis ICD-9-CM Code, n (%) | |||||||||||
| 995.0, Unspecified | 9,196 (51%) | 8,709 (49%) | 10,236 (49%) | 10,559 (48%) | |||||||
| 995.60, Unspecified Food | 985 (5%) | 1,118 (6%) | 1,329 (6%) | 1,404 (6%) | |||||||
| 995.61, Peanuts | 2,158 (12%) | 1,899 (11%) | 2,633 (13%) | 2,540 (12%) | |||||||
| 995.62, Crustaceans | 704 (4%) | 742 (4%) | 827 (4%) | 883 (4%) | |||||||
| 995.63, Fruits, Vegetables | 443 (2%) | 464 (3%) | 544 (3%) | 611 (3%) | |||||||
| 995.64, Tree Nuts, Seeds | 1,153 (6%) | 1,256 (7%) | 1,372 (7%) | 1,425 (7%) | |||||||
| 995.65, Fish | 1,078 (6%) | 1,097 (6%) | 1,230 (6%) | 1,169 (5%) | |||||||
| 995.66, Food Additives | 77 (0%) | 74 (0%) | 69 (0%) | 98 (0%) | |||||||
| 995.67, Milk Products | 244 (1%) | 254 (1%) | 287 (1%) | 360 (2%) | |||||||
| 995.68, Eggs | 143 (1%) | 136 (1%) | 219 (1%) | 266 (1%) | |||||||
| 995.69, Other Food | 1,576 (9%) | 1,658 (9%) | 1,916 (9%) | 2,217 (10%) | |||||||
| 999.4, Serum | 271 (2%) | 328 (2%) | 379 (2%) | 289 (1%) | |||||||
Note: All counts derived from NIS and NEDS are projected national estimates based on the discharge weights, and are subject to rounding error.
Abbreviation: NR Not reported for count <=10 per data user agreement.
Anaphylaxis-Related ED Presentations and Deaths (NEDS)
From 2006 to 2009, the number of anaphylaxis-related ED presentations (without admission) ranged from 17,735 to 21,822, and the number of deaths ranged from 13 to 27 (Table 1). The case fatality rates were between 0.06% and 0.13% (average, 0.10%) among ED presentations (without admission). The mortality rates were between 0.04 and 0.09 per million population. A trend was not assessed, because only 4 years of data were available from NEDS. As with hospitalizations, anaphylaxis-related ED presentations were most frequently coded with 995.0 (unspecified; 48%-51%) or 995.61 (peanut-related; 11%-13%) (Table 2).
Anaphylaxis-Related Hospitalizations, ED Presentations, and Deaths (NIS+NEDS)
Between 2006 and 2009, the total hospitalizations and ED presentations (without admission) with anaphylaxis-related principal diagnosis ranged from 24,797 to 29,530, and the total deaths were between 63 and 99 (Table 1). Of these deaths, approximately 77% happened in the hospital (range, 72%-84%). The case fatality rates were between 0.25% and 0.33% (average, 0.30%). The anaphylaxis mortality rates for deaths in a hospital or ED were between 0.21 and 0.32 per million population. Although 75% to 89% (1999–2009) of hospitalizations for anaphylaxis were admitted from the ED, 74% to 78% of the anaphylaxis ED visits were not considered severe enough to warrant hospital admission from 2006 to 2009.
Anaphylaxis-Related Deaths (MCDD)
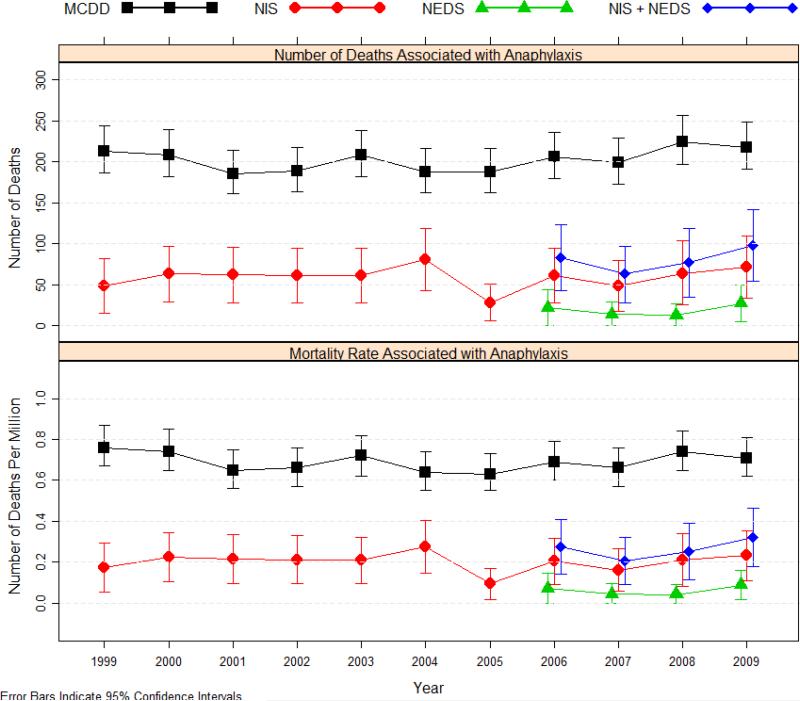
There were 2229 anaphylaxis-related deaths between 1999 and 2009 (0.69 per million population). The annual number of deaths related to anaphylaxis ranged from 186 to 225, corresponding to mortality rates between 0.63 and 0.76 per million population (Table 3). Mortality rates associated with anaphylaxis remained stable in the last decade, with an estimated annual decrease of –0.31% (95% CI, –1.54% to 0.93%; P=0.62). Age-adjusted rates based on the 2000 US standard population were similar to crude rates (annual percentage change –1.02%; 95% CI, –2.26% to 0.24%; P=0.11).
Table 3.
Anaphylaxis-Related Deaths From the Multiple Cause of Death Data (MCDD)
| Year | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Total |
| US Population (Million) | 279.0 | 281.4 | 285.3 | 288.4 | 290.8 | 293.7 | 296.4 | 299.4 | 301.6 | 304.1 | 307.0 | 3227.1 |
| No. of Anaphylaxis-Related Deaths | 213 | 209 | 186 | 189 | 208 | 188 | 188 | 206 | 199 | 225 | 218 | 2229 |
| Mortality Rate (Per Million) | 0.76 | 0.74 | 0.65 | 0.66 | 0.72 | 0.64 | 0.63 | 0.69 | 0.66 | 0.74 | 0.71 | 0.69 |
| 95% CI | (0.67,0.87) | (0.65,0.85) | (0.56,0.75) | (0.57,0.76) | (0.62,0.82) | (0.55,0.74) | (0.55,0.73) | (0.6,0.79) | (0.57,0.76) | (0.65,0.84) | (0.62,0.81) | (0.66,0.72) |
| Age-Adjusted Rate (Per Million)a | 0.78 | 0.76 | 0.66 | 0.65 | 0.71 | 0.64 | 0.64 | 0.65 | 0.64 | 0.69 | 0.70 | 0.68 |
| Anaphylaxis Cause of Death (%) | ||||||||||||
| T78.0 (food reaction) | 4% | 5% | 6% | 4% | 5% | 6% | 6% | 5% | 6% | 7% | 6% | 5% |
| T78.2 (unspecified) | 79% | 85% | 71% | 77% | 73% | 66% | 78% | 74% | 73% | 73% | 77% | 75% |
| T80.5 (serum) | 0% | 0% | 0% | 1% | 1% | 1% | 1% | 0% | 1% | 0% | 0% | 0% |
| T88.6 (drug) | 17% | 11% | 23% | 20% | 22% | 27% | 17% | 21% | 22% | 21% | 18% | 20% |
| Underlying Cause of Death, Top 4 (%)b,c | ||||||||||||
| Y57.9 (Drug or medicament, unspecified) | 13% | 7% | 11% | 13% | 36% | 35% | 41% | 38% | 34% | 40% | 35% | 28% |
| X23 (Contact with hornets, wasps and bees) | 12% | 19% | 15% | 16% | 13% | 9% | 14% | 13% | 11% | 12% | 12% | 13% |
| X57 (Unspecified privation) | 15% | 23% | 20% | 19% | NA | NA | NA | NA | NA | NA | NA | 7% |
| X59 (Exposure to unspecified factor) | 6% | 10% | 8% | 8% | 6% | 7% | 7% | 3% | NA | NA | NA | 5% |
| Place of Death, Top 4 (%) | ||||||||||||
| Medical Facility - Inpatient | 55% | 59% | 52% | 48% | 49% | 54% | 48% | 49% | 51% | 50% | 51% | 52% |
| Medical Facility - Outpatient or ED | 27% | 27% | 32% | 37% | 35% | 32% | 37% | 35% | 37% | 33% | 32% | 33% |
| Medical Facility - Dead on Arrival | 3% | 3% | 2% | 3% | 2% | 3% | 2% | 2% | 2% | 4% | 4% | 3% |
| Decedent's home | 8% | 6% | 6% | 7% | 9% | 9% | 6% | 7% | 6% | 6% | 5% | 7% |
| Mortality Rate by Select Subgroup (Per Million) | ||||||||||||
| 0-17 years | UR | UR | UR | UR | UR | UR | UR | UR | UR | UR | UR | 0.10 |
| 18-34 years | 0.45 | 0.36 | NR | NR | 0.35 | 0.32 | 0.29 | 0.29 | 0.33 | 0.30 | 0.42 | 0.32 |
| 35-44 years | 0.58 | 0.58 | 0.55 | 0.58 | 0.52 | 0.50 | UR | 0.55 | UR | 0.49 | 0.58 | 0.52 |
| 45-54 years | 1.01 | 0.90 | 1.20 | 0.97 | 0.86 | 0.77 | 0.89 | 1.20 | 1.07 | 1.10 | 0.79 | 0.98 |
| 55-64 years | 1.39 | 2.18 | 0.91 | 1.24 | 1.51 | 1.38 | 1.29 | 1.30 | 0.95 | 1.81 | 1.64 | 1.42 |
| 65-74 years | 1.85 | 1.58 | 1.47 | 1.92 | 1.80 | 1.57 | 1.88 | 2.22 | 1.86 | 1.69 | 1.83 | 1.79 |
| 75-84 years | 2.37 | 2.18 | 2.38 | 1.96 | 2.25 | 1.62 | 2.30 | UR | 2.53 | 1.84 | 2.05 | 2.04 |
| Female | 0.75 | 0.67 | 0.63 | 0.57 | 0.67 | 0.59 | 0.59 | 0.65 | 0.45 | 0.73 | 0.60 | 0.63 |
| Male | 0.78 | 0.82 | 0.68 | 0.75 | 0.76 | 0.69 | 0.68 | 0.73 | 0.88 | 0.75 | 0.82 | 0.76 |
| Black or African American | 0.77 | 0.57 | 0.97 | 0.74 | 1.05 | 0.75 | 0.64 | 1.14 | 0.75 | 0.97 | 0.88 | 0.84 |
| White | 0.79 | 0.80 | 0.61 | 0.67 | 0.68 | 0.65 | 0.65 | 0.64 | 0.67 | 0.73 | 0.72 | 0.69 |
Abbreviation: UR, Unreliable for Deaths <20; NA, Not Available.
Rate was adjusted based on year 2000 standard population. Not all death certificates reported age information. As a result, year 2000 age-adjusted rate was slightly different from the unadjusted rate.
X57 was no longer reported as underlying cause of death after 2002, which might explain the increase in Y57.9 after 2002.
A single individual could have multiple causes of death listed on their death certificates.
Anaphylaxis-related mortality was less frequent in women (1026 deaths; 0.63 per million population) than in men (1203 deaths; 0.76 per million population). By age group, mortality rate was highest in people aged 75 to 84 years (287 deaths; 2.04 per million population), and lowest in children 17 years or younger (84 deaths; 0.10 per million population). The relative risk was 19.5 (95% CI, 15.3-24.9; P<0.01) between the 2 age groups. Seventy-five percent (n=1675) of all anaphylaxis-related deaths were coded with T78.2 (anaphylactic shock). Overall, 87% (n=1943) of the deaths occurred in a medical facility (inpatient, outpatient/ED, dead on arrival), and about 7% (148) occurred at the decedent's home. In cases in which the etiology was reported, most anaphylaxis-related deaths were coded with Y57.9 (drug; n=617 [28%]) or X23 (contact with hornets, wasps, and bees; n=295 [13%]); etiology was not specified for most cases.
Discussion
There are many aspects of an anaphylactic reaction that make them frightening to both patients and healthcare providers, including their rapidity of onset and their potential to be fatal. Because the onset of symptoms can occur rapidly and outside the healthcare setting, patients who have had anaphylactic reactions need to remain vigilant to try to prevent re-exposure to the causative allergen. Sometimes avoidance is not possible because the patient cannot prevent the exposure (eg, insect sting), the patient is not aware that the allergen is present (eg, ingredients in food), or the relevant allergen cannot be discerned or does not exist. There has been a concerted effort in recent years to clearly label foods that contain common allergens and make food handlers more aware of the risks of food allergies and how to minimize these risks. In addition to reducing the risk of allergen exposure, guidelines universally recommend that patients who have had anaphylactic reaction be supplied with an epinephrine autoinjector with instructions regarding when and how to self-treat.4,33-35 Epidemiologic data on the risk of fatal reactions may also be helpful for patients to put the risk into some perspective.
To effectively counsel patients about the potential that an anaphylactic reaction will be fatal, it is necessary to have accurate data regarding the risk of a fatal outcome from an anaphylactic reaction. This is particularly important when recommending a therapeutic intervention that poses a risk of anaphylaxis, such as allergy immunotherapy or vaccination. The Epidemiology of Anaphylaxis Working Group (sponsored by the American College of Allergy, Asthma and Immunology) concluded that data on anaphylaxis incidence and prevalence are sparse and often imprecise.8 Case fatality and mortality data related to anaphylaxis are even more limited, with no robust US national estimates currently available.7,13,16
The present study was designed to address these information gaps using 3 national databases. Mortality rates ranged from 0.63 to 0.76 per million population (average, 0.69) based on the MCDD, and from 0.21 to 0.32 per million population (average, 0.26) with anaphylaxis as the primary cause of death occurring in the hospital or ED based on NIS and NEDS (Figure 2). The higher mortality rates from the MCDD may reflect that anaphylaxis is captured as one of up to 20 causes leading to death, whereas mortality rates based on hospital or ED discharge coding only captured cases in which an anaphylaxis-rated code was used as the primary diagnosis. According to death certificate data, 87% of all anaphylaxis-related deaths occur in the hospital or ED setting, so deaths outside the hospital cannot fully explain the difference. Despite the increased prevalence of anaphylaxis3,6,8, our study showed that mortality rates remained stable in the 11-year period between 1999 and 2009, and were considerably less than 1 per million population. A number of factors could have contributed to this, such as patients being diagnosed early and treated properly; the wide availability of epinephrine autoinjectors; or increased awareness of anaphylaxis, resulting in more mild cases being presented.
Figure 2.
Mortality associated with anaphylaxis in the United States, 1999-2009.
The mortality rate is important from a population perspective, but most patients and their healthcare providers are more interested in understanding the likelihood of a fatal outcome if a severe anaphylactic reaction occurs. This is especially important when the precipitating causes cannot be identified (idiopathic anaphylaxis) or avoided (bee stings or prescribed therapies). The present analysis demonstrates that – reflecting in part the quality of care provided in the urgent care setting – the vast majority hospital and/or ED presentations with anaphylaxis did not result in death. Among hospitalizations for anaphylaxis, an average of 0.92% of the cases resulted in death (range, 0.42%-1.27%). NEDS data showed an even lower case fatality rate (average, 0.10%; range, 0.06%-0.12%) among those who presented to an ED but were not admitted, although this might just reflect that patients with more severe anaphylaxis were rapidly admitted to the hospital or that anaphylactic reactions that occur in the hospital—those likely to be related to medications or contrast agents—have a higher case fatality rate. When the cases that present for clinical care are combined (NIS+NEDS), the fatality rates ranged between 0.25% and 0.33% (average, 0.30%). Given that 87% of all anaphylaxis-related deaths occur in the hospital or ED setting, the combined NIS and NEDS data are a good estimate of the case fatality rates for severe anaphylactic reactions. Of course many, indeed, most anaphylactic reactions are mild or moderate1, and, while there is no mechanism to discern this information, it seems likely that many patients having milder reactions (e.g., isolated pruritus and flushing or acute urticaria) either do not seek medical attention or seek care outside hospitals or EDs. Therefore, the case fatality rate from our study could be a vast overestimate of the case fatality rate when all anaphylactic reactions are considered.
Of the various demographic factors, age was most significantly associated with anaphylaxis deaths. From MCDD, the mortality rate was highest in people aged 75 to 84 years (2.04 per million population) and lowest in children 17 years or younger (0.10 per million population; relative risk 19.5), and increased with advancing age. The relative risk of case fatality (NIS+NEDS) was 19.9 for the same age-group comparison (case fatality rate, 1.19% for age 75-84 years; 0.06% for ≤17 years). The higher risk associated with advancing age is likely explained by recognition that elderly are less able to tolerate complications of anaphylaxis (hypotension, hypoxia, arrhythmias) or the treatment for anaphylaxis (epinephrine) than children or younger healthy adults due to the underlying comorbidities, such as cerebrovascular or cardiovascular diseases. However, we cannot rule out additional or alternative explanations including, for example, the increasing risk that comes with age for exposure to causative agents, such as drugs.
It is also noteworthy that our data demonstrate an increase in anaphylaxis rates of ~2.23% per year from 1999-2009. This increase parallels the rise in prevalence of allergic sensitization that has occurred during this same period, including sensitization to food allergens.6,36 This is difficult to categorically assess as few of the putative causes of anaphylaxis have specific codes and, even when available, their validity cannot be discerned. However, support for increased allergic sensitization driving the increased rate of anaphylaxis is certainly consistent with our specific observation that peanut-related anaphylaxis (995.61) hospitalizations appeared to be on the rise during this same period, from 256 (4%) in 1999 to 512 (7%) in 2009.
A number of authors have reported anaphylaxis-related case fatality rates and population mortality rates. Yocum et al published the first population-based study on anaphylaxis in the United States, and identified a total of 154 anaphylaxis cases (1 death) among 1255 residents in Olmsted County, MN (1983-1987), with a case fatality rate of 0.65% and a mortality rate of 1.36 per million population.11 However, the study was limited to the predominantly white, middle-class population of Olmsted County. In what is probably the largest US population-based study to date, Simon and Mulla reported a mortality rate of 0.5 per million population based on death certificates in Florida (1996-2005).13 Using hospital discharge data, the same authors reported a case fatality rate of 0.86% among hospitalizations in Florida (2001).15 In Switzerland, Helbling et al reported a case fatality rate of 1.2% (3 deaths out of 249 cases) with a mortality rate of 1 per million population for Canton Bern, with a population of approximately 1 million people (1996-1998).37 The highest case fatality rate of 2% (4 deaths out of 229 cases) was reported by Moneret-Vautrin in the French Allergy Vigilance Network study involving food anaphylaxis.9 The study focused on a specific etiology for anaphylactic reactions, which may explain the higher case fatality rate than what was determined in the current study.
In contrast to previous studies, ours was based on 3 US national databases spanning the last decade. Not only do we report the case fatality rates separately for hospitalizations and ED presentations, but this is the first study to report the case fatality rates among combined cases. Because deaths can occur outside medical facilities, we also obtained estimates from MCDD to validate and supplement results from the NIS and NEDS. In comparison, our results were at the lower end of the previously published results for both case fatality and population mortality. Furthermore, MCDD showed among known etiologies, drug was the leading cause of anaphylaxis deaths, followed by contact with hornets, wasps, and bees, with food reactions being a distant third.
Despite these methodological improvements, our study has several limitations. First, it relies on discharge records and death certificates for identification of anaphylaxis cases. The accuracy and completeness of the coding for anaphylaxis may significantly affect our results. Schneider et al systematically reviewed validated methods for identifying anaphylaxis using administrative and claims data, and reported mixed positive predictive value based on use of ICD-9-CM codes.24 Simon et al compared anaphylaxis deaths reported based on death certificates and hospital discharges and concluded that anaphylaxis may be underreported by both sources.13 Second, the analysis for NIS and NEDS was based on hospital and ED discharge records with the principal diagnoses for anaphylaxis, whereas the analysis for MCDD was based on having anaphylaxis as one of the multiple causes of death, but not necessarily the underlying cause. The results from NIS and NEDS may underestimate the number of anaphylaxis cases and are not directly comparable to the results from MCDD. Third, it is unclear how much the rise in anaphylaxis-related hospitalizations is attributable to the increased prevalence as opposed to increased awareness of the condition. Fourth, because there were no patient identifiers in NIS and NEDS, patients may appear more than once in hospitalizations or ED visits, but this limitation does not apply to death.
Nonetheless, to our knowledge, this is the largest and most comprehensive epidemiologic study to date on anaphylaxis mortality, and the results from 3 separate databases appear to corroborate and complement each other. With anaphylaxis being the most feared consequence for patients with allergic reactions,36 these results may help healthcare professionals to better inform their patients as to the risk and outcome of anaphylactic reactions. For example, there were 84 anaphylaxis-related deaths occurred based on the death certificate data (MCDD) for children 17 years or younger over the 11-year study period between 1999 and 2009, as compared with 115 children who died from influenza from September 2010 to August 2011, with most of them not vaccinated.38
Conclusion
From 2006 to 2009, the overwhelming majority of hospitalizations or ED presentations for anaphylaxis did not result in death, with an average case fatality rate of 0.3%, probably reflecting in part the quality of care provided in the urgent care setting. Anaphylaxis-related hospitalizations rose steadily in the last decade, but this increase was offset by the declining case fatality rate among those hospitalized; both inpatient and overall mortality rates associated with anaphylaxis appeared stable in the last decade (1999-2009) and were well under 1 per million population. Mortality rates varied greatly among different age groups, with people 65 years or older having the highest rates and children 17 years or younger having the lowest rates. Although anaphylactic reactions are potentially life threatening, the probability of dying is actually very low for those cases that require ED or hospital attention, and is likely much lower when all anaphylactic reactions are considered. With the prevalence of anaphylaxis on the rise, practitioners need to stay vigilant and follow the treatment guidelines to further reduce anaphylaxis-related deaths, and continued effort is also needed to monitor the mortality trend associated with anaphylaxis.33-35
Acknowledgment
Funding/Support: This research was supported by Endo Pharmaceuticals Inc. (Malvern, PA). Dr. Borish receives his salary from the National Institutes of Health (RO1 AI1057438 and UO1 AI100799).
Role of the Sponsor: The funding sources did have a role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript.
Research funding:
This research was supported by Endo Pharmaceuticals Inc. (Malvern, PA). Dr. Borish receives his salary from the National Institutes of Health (RO1 AI1057438 and UO1 AI100799).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Conflicts of Interest:
Dr. Ma is an employee and Dr. Danoff is a former employee of Endo Pharmaceuticals Inc.
Dr. Borish has served as an advisor or consultant for Endo Pharmaceuticals Inc.
Clinical Implications: Although anaphylactic reactions are potentially life threatening, the probability of dying is actually very low. However, practitioners need to stay vigilant and follow the treatment guidelines to further reduce anaphylaxis-related deaths.
Additional Contributions: We would like to thank Dr. Paul Allison from University of Pennsylvania for his statistical consultation; Betzaida Tejada Vera, Arialdi Miniño and Sigrid Economou from Centers for Disease Control and Prevention for their support on the Multiple Cause of Death Data; HCUP User Support from the Healthcare Cost and Utilization Project for their support on the NIS and NEDS databases; Kris Schuler and Jeffery Coleman from Complete Healthcare Communications Inc. for their editorial support. Dr. Allison, Kris Schuler and Jeffery Coleman received funding support from Endo Pharmaceuticals Inc.
Conflict of Interest Disclosures: Dr. Ma is an employee and Dr. Danoff is a former employee of Endo Pharmaceuticals Inc. Dr. Borish has served as an advisor or consultant for Endo Pharmaceuticals Inc.
References
- 1.Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Johansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shoshan M, Clarke AE. Anaphylaxis: past, present and future. Allergy. 2011;66(1):1–14. doi: 10.1111/j.1398-9995.2010.02422.x. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S161–181. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 5.Kim H, Fischer D. Anaphylaxis. Allergy Asthma Clin Immunol. 2011;7(suppl 1):S6. doi: 10.1186/1710-1492-7-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JK, Vadas P. Anaphylaxis: mechanisms and management. Clin Exp Allergy. 2011;41(7):923–938. doi: 10.1111/j.1365-2222.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 7.Matasar MJ, Neugut AI. Epidemiology of anaphylaxis in the United States. Curr Allergy Asthma Rep. 2003;3(1):30–35. doi: 10.1007/s11882-003-0007-8. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman P, Camargo CA, Jr., Bohlke K, et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol. 2006;97(5):596–602. doi: 10.1016/S1081-1206(10)61086-1. [DOI] [PubMed] [Google Scholar]
- 9.Moneret-Vautrin DA, Morisset M, Flabbee J, Beaudouin E, Kanny G. Epidemiology of life-threatening and lethal anaphylaxis: a review. Allergy. 2005;60(4):443–451. doi: 10.1111/j.1398-9995.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 10.Dibs SD, Baker MD. Anaphylaxis in children: a 5-year experience. Pediatrics. 1997;99(1):E7. doi: 10.1542/peds.99.1.e7. [DOI] [PubMed] [Google Scholar]
- 11.Yocum MW, Butterfield JH, Klein JS, et al. Epidemiology of anaphylaxis in Olmsted County: A population-based study. J Allergy Clin Immunol. 1999;104(2 Pt 1):452–456. doi: 10.1016/s0091-6749(99)70392-1. [DOI] [PubMed] [Google Scholar]
- 12.Bohlke K, Davis RL, DeStefano F, et al. Epidemiology of anaphylaxis among children and adolescents enrolled in a health maintenance organization. J Allergy Clin Immunol. 2004;113(3):536–542. doi: 10.1016/j.jaci.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Simon MR, Mulla ZD. A population-based epidemiologic analysis of deaths from anaphylaxis in Florida. Allergy. 2008;63(8):1077–1083. doi: 10.1111/j.1398-9995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin RY, Anderson AS, Shah SN, Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990 -2006. Ann Allergy Asthma Immunol. 2008;101(4):387–393. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- 15.Mulla ZD, Simon MR. Hospitalizations for anaphylaxis in Florida: epidemiologic analysis of a population-based dataset. Int Arch Allergy Immunol. 2007;144(2):128–136. doi: 10.1159/000103224. [DOI] [PubMed] [Google Scholar]
- 16.Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001;161(1):15–21. doi: 10.1001/archinte.161.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol. 2008;101(2):185–192. doi: 10.1016/S1081-1206(10)60208-6. [DOI] [PubMed] [Google Scholar]
- 18.Mulla ZD, Lin RY, Simon MR. Perspectives on anaphylaxis epidemiology in the United States with new data and analyses. Curr Allergy Asthma Rep. 2011;11(1):37–44. doi: 10.1007/s11882-010-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agency for Healthcare Research and Quality HCUP nationwide inpatient sample (NIS). [December 3, 2012];Healthcare cost and utilization project (HCUP) 1999-2009 Available at: www.hcupus.ahrq.gov/nisoverview.jsp.
- 20.Agency for Healthcare Research and Quality HCUP nationwide emergency department sample (NEDS). [December 3, 2012];Healthcare cost and utilization project (HCUP) 2006-2009 Available at: http://www.hcup-us.ahrq.gov/nedsoverview.jsp. [PubMed]
- 21.Centers for Disease Control and Prevention National Center for Health Statistics. [March 5, 2013];Multiple cause of death on CDC WONDER online database. 1999-2009 Available at: http://wonder.cdc.gov/wonder/help/mcd.html.
- 22.Agency for Healthcare Research and Quality HCUP Methods Series 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/reports/2007niscomparisonrpt.jsp.
- 23.Owens PL, Barrett ML, Gibson TB, et al. Emergency department care in the United States: a profile of national data sources. Ann Emerg Med. 2010;56(2):150–165. doi: 10.1016/j.annemergmed.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Schneider G, Kachroo S, Jones N, et al. A systematic review of validated methods for identifying anaphylaxis, including anaphylactic shock and angioneurotic edema, using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):240–247. doi: 10.1002/pds.2327. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention National Center for Health Statistics. [March 5, 2013];Instructions for Classifying the Underlying Cause-of-Death, ICD-10. Available at: http://www.cdc.gov/nchs/nvss/instruction_manuals.htm.
- 26.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep. 1998;47(3):1–16. 20. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention National Center for Health Statistics. [March 5, 2013];Vital statistics of the United States: mortality, 1999 technical appendix. Available at: http://wonder.cdc.gov/wonder/sci_data/mort/mcmort/type_txt/mcmort05/techap99.pdf.
- 28.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 29.McCullagh P, Nelder JA. Generalized linear models. Chapman & Hall/CRC; 1989. [Google Scholar]
- 30.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 31.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 32.Ma L, El Khoury AC, Itzler RF. The burden of rotavirus hospitalizations among Medicaid and non-Medicaid children younger than 5 years old. Am J Public Health. 2009;99(suppl 2):S398–404. doi: 10.2105/AJPH.2008.148494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemp SF, Lockey RF, Simons FE. World Allergy Organization ad hoc Committee on Epinephrine in A. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63(8):1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477–480. e471–442. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Simons FE, Ardusso LR, Bilo MB, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12(4):389–399. doi: 10.1097/ACI.0b013e328355b7e4. [DOI] [PubMed] [Google Scholar]
- 36.Tang ML, Osborne N, Allen K. Epidemiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2009;9(4):351–356. doi: 10.1097/ACI.0b013e32832db95a. [DOI] [PubMed] [Google Scholar]
- 37.Helbling A, Hurni T, Mueller UR, Pichler WJ. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin Exp Allergy. 2004;34(2):285–290. doi: 10.1111/j.1365-2222.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Influenza-Associated Pediatric Deaths — United States, September 2010–August 2011. MMWR. Morbidity and Mortality Weekly Reports. 2011;60(36) [PubMed] [Google Scholar]