Abstract
Microglia, the immune cells of the central nervous system, have long been a subject of study in the Alzheimer’s disease (AD) field due to their dramatic responses to the pathophysiology of the disease. With several large-scale genetic studies in the past year implicating microglial molecules in AD, the potential significance of these cells has become more prominent than ever before. As a disease that is tightly linked to aging, it is perhaps not entirely surprising that microglia of the AD brain share some phenotypes with aging microglia. Yet the relative impacts of both conditions on microglia are less frequently considered in concert. Furthermore, microglial “activation” and “neuroinflammation” are commonly analyzed in studies of neurodegeneration but are somewhat ill-defined concepts that in fact encompass multiple cellular processes. In this review, we have enumerated six distinct functions of microglia and discuss the specific effects of both aging and AD. By calling attention to the commonalities of these two states, we hope to inspire new approaches for dissecting microglial mechanisms.
Keywords: microglia, aging, Alzheimer’s disease, neuroinflammation, neurodegeneration
1. Introduction
In the original 1907 article in which he first described the disease that would come to bear his name, Alois Alzheimer observed a inflammatory component to the diseased brain, writing that the glia had developed “numerous fibers” and “adipose saccules” (translated by ref. [1]). Over 100 years later, it is now well accepted Alzheimer’s disease is marked by neuroinflammatory events involving glia, including astrocytes and, in particular, microglia. A recent PubMed search with the key words “microglia”, “Alzheimer’s disease”, and the Boolean connector “AND” returned 2397 citations. Interestingly, the first of these PubMed articles, published in 1976, is a review article of which the primary focus is not in fact Alzheimer’s disease but rather an assessment of how the human brain responds to aging [2]. Alzheimer’s is, after all, a disease of the aged brain. While aging mechanisms and microglial activity clearly play some role in Alzheimer’s disease, the intersection of these two components has not been thoroughly explored. Microglia also undergo striking morphological and molecular changes with age, yet our understanding of the consequences of these changes within simply normal aging is also limited. In this review, we discuss the significance of microglial aging in Alzheimer’s disease (AD). We begin by first reviewing certain genetic factors that have been of particular interest recently in the AD field, and which specifically implicate microglia. We then examine how specific microglial functions are altered in aging and AD, and whether there may be mechanistic connections between these altered states.
2. What are the typical functions of microglia in the adult brain?
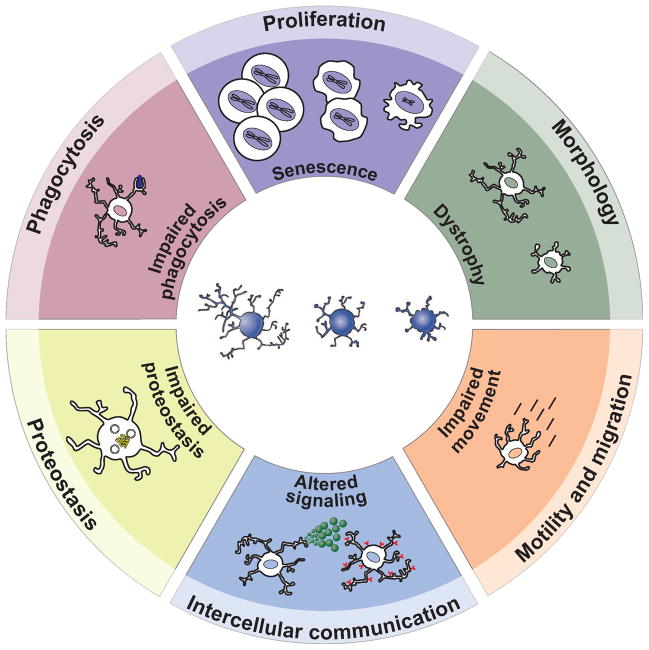
Before we attempt to foray into a discussion of what may go wrong in microglial physiology during aging and AD, it will be helpful to first define what the expected role of microglia should be in the normal, healthy adult brain. Since their discovery nearly 100 years ago, and indeed as suggested in their very definition as the innate immune cells of the central nervous system, it is of general consensus that microglia exist in the adult brain as a protective agent. How do microglia protect and defend the brain from pathological insult? Here, we briefly introduce the major physiological effector functions of microglia, which for the purposes of this review we place into six broad categories: (1) Proliferation; (2) Morphological transformation; (3) Motility and migration (4) Intercellular communication; (5) Phagocytosis; and (6) Proteostasis (Figure 1). In the following sections, we will consider both aging and AD microglial phenotypes within each of these main effector functions. We have termed these changes the “hallmarks of microglia aging”, a concept that we borrow from an influential review by Lopéz-Otín et al. [3] who have attempted to identify and categorize the “hallmarks of aging”. Our Figure 1 is also modeled on a schematic in this review.
Figure 1.
The hallmarks of microglial aging. The six major effector functions/phenotypes of microglia that are the focus of this review are highlighted at the outer ring of this schematic: proliferation, morphology, motility and migration, intercellular communication, proteostasis, and phagocytosis. Within the inner ring of the circle we describe the effects of aging on each of these functions.
The execution of microglial responses to injury or disease via the above effector functions has collectively been termed “microglial activation”, though it has been argued that this terminology incorrectly implies that microglia in the healthy, adult CNS are latent bystanders [4]. In fact, microglia are quite dynamic cells even in an apparently healthy brain, as exemplified through studies of motility and migration functions. In vivo two-photon microscopy studies several years ago revealed that microglia endlessly sample the extracellular space by extending numerous ramified processes, which seems to enable the cells to monitor the brain parenchyma, maintain tissue homeostasis, and react rapidly to brain injury or insult [5, 6].
Though microglial activation may protect the brain through much of life, the efficacy of these functions seems to deteriorate with age. However, the effects of age on microglia do not necessarily always present as a loss of these functions. The hallmarks of microglial aging may also sometimes be characterized as dysfunctional and even hyper-reactive responses. The immune system in general undergoes an imbalanced shift toward a proinflammatory status with aging, a concept termed “inflammaging” [7]. If this is also true for microglia, then these cells may shift from a protective role in youth to a state in which some protective mechanisms are lost and others are even detrimental to brain health. Systemic infections and inflammation also activate microglia and drive and exacerbate neurodegeneration [8, 9] (and reviewed in refs. [10, 11]). As such, it has been suggested that microglia may age, in part, as a result of cumulative activation in response to systemic infections over the course of a lifetime (reviewed in refs. [11, 12]). Thus, systemic inflammaging itself could also drive microglia aging, priming the cells to eventually react with exaggerated responses that actually contribute to neurodegeneration (see Graphical Abstract). Accordingly, it may be that AD phenotypes represent an exaggerated state of microglia dysfunction beyond that observed during normal aging. AD-associated genetic variations could also exacerbate these age-related changes, along with other predisposing factors such as cigarette smoking, high blood pressure, and type 2 diabetes [13]. Obesity, for example, has been linked to AD and is promoted by aging (reviewed in ref. [14]). Adipocytes can produce proinflammatory factors and in the obese state adipose tissue may be infiltrated by immune cells (reviewed in ref. [15]). Recent studies have also found that obesity stimulates microglia and enhances the recruitment of peripheral monocytes to the CNS [16, 17]). Obesity could therefore also contribute to low-grade chronic inflammation peripherally and within the brain. Thus while aging and AD microglial phenotypes are by no means equivalent, chronic stimulation over time combined with certain genetic mutations and other risk factors could push aging microglia towards an AD state.
3. Genetic evidence that microglia are important in Alzheimer’s Disease
In the past year, a number of studies have brought more attention to microglia in AD research than ever before by demonstrating strong genetic implications for microglial molecules and the immune system in general. Most recently, a genome-wide association study (GWAS) identified a novel association for late onset Alzheimer’s disease (LOAD) in the HLA-DRB4-DRB1 region (encoding for major histocompatability complex, class II, DRβ4 and DRβ1, respectively) [18]. Earlier this year, Griciuc et al. [19] demonstrated that microglial functions in AD may be modified by CD33, another gene with a role in immune response [20] that has been linked to AD in some (though not all) recent GWAS [18, 21–23]. And perhaps most prominent were the two GWAS published concurrently in the New England Journal of Medicine that identified variants in TREM2 (“triggering receptor expressed on myeloid cells 2”) as risk factors for LOAD [24, 25].
The identification of the TREM2 risk variants garnered great excitement for neuroimmunologists given that this gene encodes an innate immune receptor that is expressed on a subset of myeloid cells that includes immature dendritic cells, osteoclasts, tissue macrophages, and, in the brain, microglia. The excitement was only enhanced when later in the year a study applying integrative network-based approaches to genetic data from the brain tissue of LOAD patients highlighted an immune- and microglia-specific module as the molecular system most strongly associated with the pathophysiology of the disease [26]. Furthermore, a key causal regulator in this module included TYROBP (also known as DAP12), the transmembrane adapter protein through which TREM2 signals. Together, TREM2 and TYROBP mediate phagocytosis of bacteria and apoptotic cells [27, 28]. Additionally, TREM2 suppresses inflammation by inhibiting the production and secretion of cytokines that are initiated by TLR2 and TLR4 signaling [29] (Figure 2). However, it is not yet clear how TREM2 and TYROBP regulate the microglial response in AD. TYROBP is increased in LOAD and TREM2 is upregulated in plaque-associated microglia [26, 30], which, given their roles in phagocytosis, might suggest that this induction occurs as an attempt to cope with amyloid load by enhancing microglia clearance abilities. Still, it is not actually known whether TREM2 even binds Aβ, and this also brings to question why such protective measures would ultimately fail in AD. Since TREM2 also has anti-inflammatory properties, one suggestion is that the mutations identified in these recent studies cause an inability for microglia to suppress inflammation [27, 29]. Impairments in the phagocytic removal of apoptotic cells or bacteria could exacerbate these effects and ultimately lead to neural degeneration.
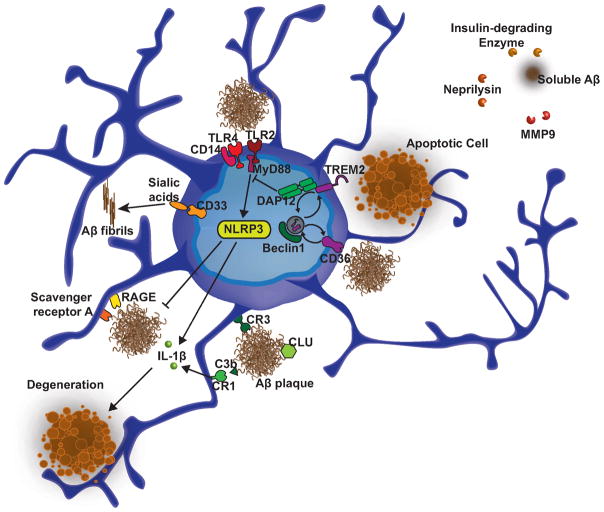
Figure 2.
Microglia molecules genetically implicated in Alzheimer’s disease. Recent studies have identified novel risk factors for AD such as TREM2, which together with previous work, strongly point to a role for microglia and immune mechanisms in the disease. NLRP3 inflammasome activation in microglia mediates a harmful inflammatory response that contributes to AD pathology. On the other hand, microglia express several receptors that bind Aβ (such as scavenger receptor A, RAGE, CD36, CR1, CR3, and toll-like receptors) as well as enzymes that degrade soluble Aβ (i.e., insulin-degrading enzyme, neprilysin, MMP), which may contribute to the clearance of amyloid thus offer protective functions. Likewise, beclin 1, which is decreased in the brains of AD patients, regulates the recycling of receptors such as CD36 and TREM2 and phagocytosis.
Though the past year has been particularly prolific for genetic associations of the immune system and AD, there has been, for some time, evidence for a mechanistic role of certain immune pathways in this disease, with a particular emphasis on the complement system (reviewed in refs. [31, 32]). Complement proteins can be synthesized by diverse CNS cell types, though as the immune cells of the brain microglia are a natural target for studies examining the complement pathway in neurodegeneration. A 2009 GWAS identified variants at CLU and CR1 associated with AD [33], though even prior to this other studies had implicated a role for both genes in AD, specifically in Aβ clearance. Both of these genes encode proteins that participate in the complement system; CLU encodes clusterin (known also as apolipoprotein J), which inhibits hemolysis initiated by the complement protein C5b-6 [34], and CR1 encodes complement receptor 1, the main receptor for the C3b protein. Clusterin, which is secreted by astrocytes, has been found to complex to soluble Aβ [35], is present in plaques [36–38], and directly activates microglia [39]. Given its role as an apolipoprotein, it has been suggested that clusterin functions similarly to APOE in trafficking and clearing Aβ, potentially through interactions with microglia (reviewed in ref. [40]) (Figure 2). CR1 is expressed on microglia[41], but whether signaling through this receptor mediates beneficial or negative effects on microglial functions is AD is not entirely clear. Though there is evidence that CR1 could help clear Aβ by mediating phagocytosis of C3b-opsonized amyloid [41] [42], blocking CR1 can also reduce the production of proinflammatory factors and neurotoxicity by microglia that have been treated with Aβ [41] (Figure 2).
Could age-related changes in immune components contribute to microglial phenotypes in AD? Interestingly, TREM2 expression is increased in the cognitively normal aged brain [43]. Similarly, a fairly recent microarray study found that several components of the complement system are also increased in the brain during aging [44]. Moreover, some of the genes (including CLU) showed a progressive upregulation across normal aging and AD tissue, a concept that the authors term an aging-AD “continuum” [44]. Yet, as further discussed below, these changes do not appear to better equip microglia to combat age-related and AD pathology, and if anything the cells become more proinflammatory and impaired in their normal protective functions. Increases in proteins such as TREM2 could thus represent a compensatory mechanism to combat the harmful, dysfunctional state of aged microglia. We suggest that many such AD-associated microglial factors could be similarly affected during normal aging, and that gaining a greater understanding of changes in microglial functions with age could help shed light on AD mechanisms.
4. Hallmarks of Microglial Dysfunction in Aging and Alzheimer’s Disease
While microglia should have a generally healthy relationship with the rest of the brain under non-pathological conditions, this relationship may deteriorate over time and cause microglia to assume neurotoxic properties. We will discuss here each of the six categories of microglial functions described above and how they may become hallmarks of dysfunction with age and AD. We will put particular emphasis on bringing to light some of the questions that remain unanswered in the field of microglial aging, and ponder how addressing these fundamental questions could reveal insight into AD mechanisms.
4.1. Microglia Proliferation and Senescence
Microglia retain the ability to proliferate and self-renew in adulthood, though this occurs in response to various injury signals and is a relatively infrequent event under normal physiological conditions [45, 46]. Reports from various studies suggest that the microglia proliferative capacity is not lost with age, and in fact may be increased under certain conditions compared to young brains [47]. Moreover, it is consistently reported that the total number and density of microglia increases with age in various brain regions, including the hippocampus [48], visual and auditory cortices [49, 50], and retina [51]. This observed accumulation, combined with the observations that microglia proliferation normally occurs at low levels and that recruitment from circulating bone-marrow derived progenitors occurs very infrequently, if at all [52], have led to the suggestion that microglia are a very long-lived cell population. However, exactly how long microglia live in the adult CNS is a question that has not been addressed. Also, although blood-brain barrier permeability may increase with aging and AD [53], it has not been demonstrated whether this permits increased infiltration of peripheral macrophage precursors. Thus, a number of questions regarding microglia origin in the aging brain currently remain unanswered.
If microglia are a long-lived cell, it is interesting to consider what sort of toll a lifetime of chronic or even occasional inflammatory stimulation might take. One group has suggested that the gradual accumulation of microglia with age has evolved as a sort of compensatory mechanism to maintain the necessary level of protection from the overall microglial population as the individual cells become less capable with age [54], citing as support their report that the initial proliferative response to peripheral nerve injury is elevated in aged animals [55]. The mechanisms underlying this difference have not been examined further, however, and could represent a compensatory mechanism but may also be indicative of a loss of regulatory control in aged microglia. And if microglia do indeed continue to proliferate throughout life, what are the potential consequences of continuous cell divisions? Like other somatic cells, microglia seem to be subject to replicative senescence that is associated with telomere shortening [56], two well-known “hallmarks of aging” [3]. Senescent cells are typically subject to strict immune surveillance and are removed by phagocytosis. Being the phagocytes of the brain, however, it is not clear what mechanisms exist (if any) for clearing the senescent microglia themselves. That these cells seem to accumulate at a greater rate then they are cleared suggests that a heterogeneous population of microglia, stemming from different divisions, could occupy the aged brain. Interestingly, microglia also become less evenly distributed throughout the brain with age [50, 57], supporting the concept of divergent subpopulations of these cells throughout the parenchyma. If this is the case, then one might speculate that the intrinsic capabilities of individual microglia may become quite varied over time. It would be interesting to investigate whether the removal of the senescent microglia population would delay age-associated neural pathologies and neurodegenerative diseases. This has already been accomplished by clearing p16Ink4a-positive cells [58], but has not been assessed in the nervous system.
Inspired by Lopéz-Otín et al.’s discussion on stem cell exhaustion in aging, and by our own studies examining how systemic immune factors can influence neural stem cell aging [59], we also wonder if microglia could similarly suffer from an eventual loss in regenerative capacity. It is not known whether a pool of stem-like progenitor cells even exists for microglia (though see ref. [60] for a discussion of potential stem cell-like properties of differentiated macrophages), but if so, then aging could also potentially deplete the brain of healthy microglia. In effect, this would shift the balance towards more senescent and dysfunctional microglia with fewer protective immune cells. The aged brain would thus become less capable of a defensive immune response when confronted with injury or a disease such as AD.
In the AD brain, the accumulation of microglia around plaques along with greatly upregulated levels of various markers have been taken as evidence that microglial proliferation is even further increased with the disease. This is supported by several in vivo studies using markers for proliferating cells in transgenic mice [61–63], though a recent stereological analysis of human AD brains challenged this notion [64]. Clearly, additional studies will be necessary to validate the proliferative potential of microglia in AD and to define the consequences of this expansion, but presumably microglial proliferation around plaques could serve as a line of defense to limit the deposition of amyloid. However, if pools of healthy microglia are exhausted, then expansion of senescent, dysfunctional cells is unlikely to offer much protection. Perhaps by attempting to rejuvenate senescent microglia while also promoting the beneficial functions of relatively younger microglia, we may be able to better address age-related neurodegeneration and disease.
4.2. Morphology
The morphological changes that occur in microglia during different states of activation provided the very first hints of the involvement of these cells in CNS diseases. As ramified microglia with their many thin processes have now become recognizable as surveying cells in the healthy brain [5, 6], the large amoeboid cells surrounding sites of injury were easily recognizable over a century ago as active, phagocytic macrophages. In general, the microglial morphological transformation is characterized by an initial retraction and slight hypertrophy of the branched processes. From there, the cells can acquire a specific morphology that may be dictated by the type of injury [65] (and reviewed in ref. [66]). Microglia in AD have long been known to acquire what is typically considered a “reactive” morphology, with short, thick, and poorly ramified processes [67–69]. Aged-related changes in microglia morphology are less well-characterized, though one study of aged human brains observed “dystrophic” microglia with cytoplasmic abnormalities such as deramified, fragmented, or tortuous processes as well as spheroidal and bulbous swellings [70]. While these structural changes have not necessarily been seen in rodents, it has been noted that the arborizations of aging rodent microglia are significantly reduced in size [50, 51, 57] and complexity [51, 71]. One of these studies also observed that while the average microglia soma size was not different between ages, there was greater variability in soma size in aged animals [50]. We are curious whether this increased variability could be indicative of a more heterogeneous population of microglia with relatively different mitotic ages, as we postulated above. AD mechanisms may also exacerbate the effects of age on microglia morphology, as evidenced by a recent study in which microglia in aged transgenic APP mice were found to have larger cell bodies and shorter processes overall compared to those in younger transgenic mice, with these phenotypes being further exacerbated in brain areas containing plaque pathology [72]. However, the molecular underpinnings of different morphological phenotypes, including dystrophic ones, are unknown, and further studies will be necessary to more conclusively connect microglial morphological characteristics to function.
4.3. Motility and Migration
Microglial processes are highly motile projections that presumably serve to sample the brain parenchyma [6]. In general, microglial process motility is decreased with age [51, 57], which could suggest a decrease in immune surveillance. These studies also found that aged microglia show reduced responses to exogenous ATP and laser-induced focal tissue injuries in terms of process motility, cellular migration, and process thickening. These age-related alterations in microglial dynamics are corroborated by another recent transcriptome characterization that found that young microglia are more enriched for genes involved in motility [73]. Furthermore, the dynamic activity of surveying microglia and their frequent interactions with axon terminals and synaptic elements in the healthy brain have lead to the suggestion that microglia participate in experience-dependent remodeling of neural circuits in the normal mature brain by removing extraneous synaptic elements [6, 74–77]. Thus, impairments in microglia motility and migration with age could impact both normal homeostatic mechanisms as well as responses to injury.
In vivo two-photon imaging has also been used to study dynamic microglia behavior in the brains of double-transgenic APP reporter mice (PDAPP+/−;CX3CR1/GFP+/−). Microglia appear rapidly (within one day) at the site of newly formed plaques [78]. However, cells that are localized around amyloid pathology in older transgenic mice exhibit less process movement compared to those in young transgenic mice [72]. While these imaging studies do not necessarily determine whether the recruited microglia arrive as a result of proliferation or chemotaxis, in vitro studies using Aβ peptides [79, 80] and ex vivo experiments with AD postmortem cortical sections [81] demonstrate that Aβ can direct stimulate microglial chemotaxis. Furthermore, increased levels of chemokines and chemokine receptors in AD brains that are known to regulate microglial migration do support the notion that Aβ deposits attract and activate microglia. MCP-1, MIP-1α, MIP-1β, IL-8, and M-CSF, in particular, may mediate the microglial attraction to plaques [82–85]. The growth factor VEGF, along with its receptor Flt-1, which is expressed on microglia, has also been implicated in the microglial chemotactic response to Aβ deposits through both in vitro and in vivo experiments [86]. Finally, Aβ-associated microglia are induced to produce chemokines themselves, which is thought to feed back to recruit and activate more cells to localize the inflammatory reaction [87–89]. The microglial production of other inflammatory mediators is further discussed in the next section.
4.4. Intercellular Communication: Expression of Activation Markers and Cytokine Production
Microglia can be visualized in brain tissue or cultures by various surface-associated or intracellular molecules, and the levels of these proteins are often expressed to different degrees depending upon the activation level of the cell. While the functions of some markers are well established, others are less understood, though their expression may be found to be associated with a proinflammatory or anti-inflammatory microglial phenotype. For example, to better understand their activation states based on such immunophenotyping, a number of studies have attempted to define microglia in terms of classical activation (M1, “proinflammatory”) and alternative activation (M2, “anti-inflammatory”), as has been used to classify peripheral macrophage polarization [90]. The relative secretion levels of pro- and anti-inflammatory factors is perhaps more functionally relevant in terms of intercellular signaling, and thus can also provide some indication of activation state.
It is well established that microglial aging is associated with a general upregulation of certain markers, particularly MHC II antigens (particularly HLA-DR) and CD68, as well as CD11b/CR3, CD14, and pattern recognition receptors [59, 91–94] (and reviewed in refs. [95, 96]). HLA-DR [67–69, 97], CD11b/CR3 [98], CD68 [99, 100], and Toll-like receptor expression (reviewed in ref. [101]) is even more prominent in microglia of AD patients and mouse models, with expression more localized around compact amyloid deposits rather than diffuse deposits (reviewed in ref. [102]). In general the expression of these sorts of markers, which are associated with antigen presentation, lysosomal functioning, and the recognition of various pathogens, complement proteins, and other “danger” signals, is associated with a more reactive microglia phenotype. Thus one might expect that the acquisition of these phenotypes indicates that microglia in the aged brain, and even more so in the AD brain, are more readily responsive (and hence better armed) to defend against potential insults to the brain. However, we have already seen that AD-associated microglia are apparently incapable of mounting a sufficient protective response, at least in late stages of the disease. And the majority of studies and reviews on microglia in aging suggest that the neuroprotective functions of these cells become impaired with age while neurotoxic responses are heightened (see, for example, refs. [103, 104]). As such, categorizing microglial activation under aging and AD conditions has been particularly challenging.
A very general summary of the research examining M1 and M2 phenotypes in the aging CNS might also give the impression that aging shifts microglial polarization away from the alternative M2 state to the classical, proinflammatory M1 state. However, the conclusions to be drawn from this research are more nuanced. Many studies in fact find that both M1 markers (such as IL-1β, TNF-α, iNOS, and IL-6) and M2 markers (notably YM1, Arg-1, Mrc and IL-10) are increased in aged mice compared to young mice [71, 105–108]. Importantly, the results appear to be highly dependent on the pathological context in which the microglia were activated. For example, in a traumatic brain injury (TBI) model, mRNA levels of IL-4Rα, an M2 marker, were lower in aged mice relative to young TBI mice [106], but were increased when studied in the context of immune challenge using peripheral LPS injections [107]. Others have found, using cocktails of polarizing cytokines or intracerebral hemorrhage induction, that while aged microglia are capable of adopting alternative activation states, the M2 genes are induced to a lesser degree or more slowly in aged animals compared to young [109, 110]. And still others have seen no significant age-related differences in microglial activation phenotypes when studied in the absence of any pathological insults [105, 111]. Cytokine secretion from aged microglia is also a complex story; while production and release of proinflammatory factors is generally increased, this function is again dependent upon treatment with various stimulants [112–116]. Thus for future studies it would be of value to consider not only how the basal state of microglial activation might be changed with age, but also how microglial responses to various pathological insults are altered.
How are microglial activation states altered in AD? For mouse models, there have also been reported increases in both M1 and M2 markers compared to age-matched wildtype controls [117]. Correspondingly, a recent study found that the NLRP3 inflammasome, which mediates IL-1β production, is activated in AD and that inhibition ofNLRP3 can induce microglial phagocytosis and an M2 phenotype [118]. Treating cultured microglia with Aβ also fairly reliably induces the secretion of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [87, 88, 119–122]. Interestingly, however, there is also evidence for an aging and disease interaction that contributes to the microglial state, as microglia in young APP/PS1 mice present an alternative activation phenotype, while in older animals the cells are more classically activated [105] (see also ref. [123] for review).
In addition, Aβ stimulates the production of reactive oxygen species (ROS) and reactive nitrogen intermediates such as nitric oxide (NO) [120–122]. Oxidative stress caused by these species is a well-recognized contributor to neuronal loss and is observed early in AD, and activation of microglial NADPH oxidase is believed to be the primary source of ROS (reviewed in ref. [124]). Oxidative stress is also increased in aging (reviewed in ref. [103]); this is supported by gene-expression profiling of aged brains [125] and age-related increases in the NADPH oxidase enzyme NOX2 [126]. NOX2 contributed to microglial ROS production in an LPS-induced model of systemic inflammation, and the authors of this study suggested that neuroimmune activation and age act synergistically to increase oxidative stress and neurodegeneration [126]. This again highlights the idea that aging is an important factor to consider when studying microglial activation states and functions in AD, and in other disease models. However, while microglial activation phenotypes are clearly altered with aging and in Alzheimer’s disease patients and mouse models, we would argue that it is difficult, at this time, to generalize how these states are defined.
Why do the majority of cytokines, receptors, and other molecules tend to increase with age, regardless in some cases of their pro- or anti-inflammatory properties? These collective findings could suggest that aged, and AD, microglia exist in a state of uncontrolled activation and stimulation, though some of their normal protective output functions may actually be hindered. In support of this, it has also been shown that a few molecules that curtail microglial activation and dampen proinflammatory events are actually decreased with age. This includes fractalkine and the receptor through which it signals on microglia, CX3CR1 [44, 127, 128] and CD200, which signals through CD200R [129, 130]. It is important to remember that in young and healthy brains, the production of inflammatory mediators does serve an initial protective function by enabling intercellular communication as well as helping to rapidly destroy harmful material such as invading microorganisms. It is the disruption of this careful balance between the stimulation and restraint of microglia that can lead to excessive activity. Neurons and other cells are then subject to microglia-induced damage via proinflammatory cytokines and oxidative stress (Figure 3). Thus, while there is unequivocal data demonstrating that aging and AD are accompanied by changes in intracellular signaling and marker expression, the explanations for the causes, and functional consequences, of these phenotypic changes remain somewhat speculative at this point in time.
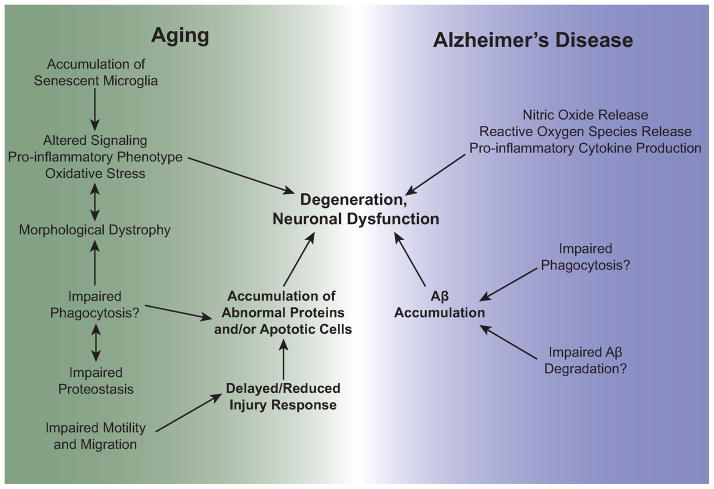
Figure 3.
Understanding the intersection of microglial aging and Alzheimer’s disease. The hallmarks of microglial aging may be further exacerbated in AD as a result of disease-associated genetic variants and environmental influences. Potential connections between these phenotypes are shown in the figure. Such impairments in microglial functions may contribute to both aging and disease phenotypes and neuronal degeneration and dysfunction.
4.5. Phagocytosis
Phagocytosis, the process of recognizing, engulfing, and degrading extracellular material, is carried out by professional phagocytes of the innate immune system: macrophages, dendritic cells, and neutrophils. In the CNS, this function is mainly orchestrated by microglia (although there are reports of other CNS cell types, notably astrocytes and neural progenitor cells performing phagocytosis [131, 132]). In this subsection, we focus mainly on the initial uptake phase of phagocytosis and then discuss intracellular mechanisms and degradation in the following Proteostasis subsection. This is because studies of phagocytosis have often focused on engulfment and clearance of material from the extracellular space but do not necessarily demonstrate the final outcome of the ingested particle. Loss of proteostasis itself is also considered another hallmark of aging [3], and entire fields are devoted to the study of the decline of chaperone-mediated protein folding, the ubiquitin-proteasome system, and the autophagy-lysosomal system in aging. Yet surprisingly, phagocytosis is rarely considered in discussions on the effects of aging on protein homeostasis.
Of all the microglial effector functions discussed here, the effects of aging on phagocytosis are perhaps the least defined. Data from two ultrastructural studies in rodent brains indicate that aged (up to 24 months of age in mice and 29.5 months in rats) microglia show an accumulation of various types of noncellular inclusions (vacuoles, large vesicles, and lysosomal inclusions) and condensed debris (suggested to be the pigment granule lipofuscin) [49, 50]. Vaughan and Peters, in as early as 1974 [49], suggested that the intracellular material was not of recent acquisition, thus perhaps indicating that aged microglia are impaired in their ability to degrade and turnover ingested material. In contrast, one recent study concluded that the capacity for microglia to phagocytose apoptotic cells in the subgranular zone (SGZ) is preserved with age [133]. However, it is worth noting that the age of the mice examined in this study extended only as far as 12 months (equivalent to maybe 40–50 years-of-age in humans), and furthermore that the total number of apoptotic cells in the SGZ, on which the phagocytic measurement index was based, was extremely low in 12-month-old animals (approximately 6 cells in total). While this study demonstrates that aging microglia are capable of clearing small numbers of dead cells in a healthy brain, it would be interesting to examine the efficiency of phagocytosis in young versus aged microglia that have been challenged with a pathological insult. As demonstrated by the studies reviewed above examining immunophenotyping, age-associated effects on microglia are clearly dependent on the injury model in which they are studied. Additionally, aging is likely to differentially affect phagocytosis of various targets such as myelin, Aβ, and cellular debris.
Certainly the ability of microglia to at least recognize and uptake Aβ is well establishedand is strongly supported by the presence of multiple receptors that bind Aβ as a ligand. These receptors include TLR2 [134], TLR4 and the TLR4-interacting molecule CD14 [135, 136], RAGE [137], the scavenger receptors CD36 and scavenger receptor A [138, 139], and the formyl peptide receptor FPRL-1 [140] (see also ref. [141] for review) (Figure 2). And indeed, the regulation and effectiveness of microglial clearance mechanisms in AD appears to be dependent on age and the stage of the disease, with microglia being more efficient at removing Aβ in early stages but less efficient in later stages [141]. A recent study showed that microglial phagocytic capacity is impaired in late stages of cerebral amyloidosis [142], and in vitro studies using microglia cultured from adult animals have shown that aged microglia internalize less Aβ peptide than young cells [113, 143]. Microglia from old APP/PS1 mice have also been found to have decreased expression of Aβ-binding receptors and Aβ-degrading enzymes [138]. This again suggests that the interaction between AD mechanisms and aging is an important concern when assessing the role of microglia in the disease.
Phagocytosis is a particularly challenging effector function to study, especially in vivo, in part because debris clearance is the culmination of several cellular events. Reductions in phagocytic efficiency could arise as a result of impairments in the detection of targets (i.e., through a downregulation of certain receptors) or in chemotactic functions, leading to a delayed migration to the site of injury. If these initial steps are overcome, any other subsequent phagocytic mechanisms could be impaired in aging or AD, from the engulfment of particles, to the fusion of internal phagosome and lysosome compartments, to the degradation and expulsion of the ingested material. Thus, if future therapeutic studies will seek to improve microglia clearance mechanisms in AD, the distinct stages of phagocytosis and proteostasis mechanisms will need to be dissected in order to clarify how this function is affected by aging and AD.
4.6. Proteostasis
Protein homeostasis (proteostasis) mechanisms are crucial for cellular and organismal health as they ensure protein quality control and prevent the accumulation of aberrantly formed proteins. Loss of proteostasis is a common theme in studies of general aging that encompasses many pathways, including impairments in chaperone-mediate protein folding and stability, protein trafficking, protein degradation, and autophagy. A major consequence of declining proteostasis is the aggregation of proteins, which is particularly prominent in age-associated neurodegenerative diseases such as Huntington’s, Parkinson’s, and Alzheimer’s disease [144]. In this final section, we apply the idea of impaired proteostasis somewhat loosely to microglia functions and focus mainly on phagocytosis-mediated mechanisms, but in doing so hope to draw some particular attention to the importance of microglial protein clearance and degradation. Separating this idea also helps to highlight other microglia clearance functions that are not phagocytosis-related. For example, it has been shown that microglia are capable of clearing soluble forms of Aβ via macropinocytosis [145] and through the production of various Aβ degrading enzymes, such as insulin-degrading enzyme (IDE), neprilysin (NEP), and MMP9, which may be secreted to degrade Aβ extracellularly [146–148] (Figure 2). While these “proteostasis” mechanisms have been studied in the context of AD, the effects of aging on microglial proteostasis specifically have not been addressed. It has generally been assumed that microglial uptake in AD is a protective measure that can delay disease progression by promoting clearance of neurotoxic Aβ. But in point of fact, is has been argued that there has yet to be sufficient evidence that microglia are capable of true phagocytosis of Aβ [149]; although microglia do appear to engulf amyloid, it is unclear whether the cells can degrade the protein. Without subsequent degradation, amyloid-containing microglia are less likely to be capable of clearing additional Aβ, and the intracellular presence of the protein could also render the cells toxic and proinflammatory.
The assumption that microglia can exert such protective effects through Aβ clearance was also called into question by a prominent study that showed that ablation of microglia via HSVTK did not influence plaque deposition nor amyloid-associated neuritic dystrophy in APP transgenic mouse models [150]. This has been cited as evidence that Aβ clearance and the progression of amyloidosis are not in fact dependent on microglia (and, for that matter, that microglia-mediated neuroinflammatory responses do not mediate AD neurodegeneration). Though this work certainly demonstrates that microglial functions alone are insufficient to limit Aβ-associated pathology, the study utilized APP/PS1 and APP23 transgenic mouse models, both of which were generated through expression of mutated PS1 and/or APP under the neuron-specific Thy1 promoter element [151, 152]. Thus, these models are not necessarily representative of AD-associated genetic alterations that target microglia, a sentiment that is also supported by the recent studies that genetically implicate microglia in AD, as described above. In support of the importance of examining microglia-specific genetic alterations in the context of AD, a study from our laboratory recently demonstrated that genetic-reduction of beclin 1, a protein classically associated with the autophagy degradation pathway, disrupted phagocytosis and retromer-mediated recycling of the phagocytic receptors CD36 and TREM2 in microglia, and that this protein is decreased in microglia from AD brains [153].
We find it noteworthy that while phagocytosis has generally received little attention in the field of aging, proteostasis mechanisms beyond phagocytic engulfment are rarely studied in the field of microglia. It is our hope that by demonstrating the parallels between normal aging denominators and changes in microglial functions that these processes may come to be viewed from a novel angle.
5. Conclusion
The six “hallmarks of microglial aging” that we described in this review senescence, dystrophy, impaired movement, altered signaling, impaired phagocytosis, and impaired proteostasis – have been proposed to delineate specific processes that may be studied and compared in the context of aging and AD. In separating these hallmarks, we do not mean to suggest that these microglial functions are isolated events; it is certainly important to remember that all of these functions are inherently linked, and that the profile of one response is likely to influence the outcome of another. Phagocytosis of a particular protein, for example, may be accompanied by the secretion of a certain subset of cytokines that then further induce proliferation and chemotaxis. However, it is our hope that defining these hallmarks may contribute to developing a framework for studying microglial aging and its underlying mechanisms. In drawing parallels to AD microglial phenotypes, we also hope that viewing microglial aging in this context will additionally offer novel insight into the role of neuroinflammation in this disease. We have attempted to illustrate the intersection of aging and AD microglial dysfunction in Figure 3.
The observations described here are mainly descriptive in nature; strictly speaking, most of the data on the subject indicate what microglial aging looks like but not why it happens. Understanding the mechanisms that underlie the hallmarks will be crucial to designing future interventions to halt, or even reverse, their progression and ameliorate the impact of microglial aging on surrounding neural tissue. As mentioned at the beginning of this review, a general proinflammatory phenotype accompanies aging in mammals at system-wide level, and this systemic inflammaging may not only mirror microglial aging phenotypes but could have a causal role as well. Since systemic immune challenges trigger microglial activation, chronic inflammation could eventually drive microglia towards a proinflammatory, dysfunctional state. Aged microglia may thus be primed such that additional stimuli cause them to become over-reactive, eventually leading to neurodegeneration and, in some cases, progression to AD.
For a number of the hallmarks, the microglial aging phenotypes seem to be exacerbations of the normal microglial functions, wherein at lower levels of activation they offer some benefit to brain health but at higher levels are more damaging than helpful. Though the reaction to age- and AD-associated neuroinflammation has often been to treat these conditions with anti-inflammatory therapies, this generally assumes that much of the microglial activity has detrimental outcomes. But microglial activation is a delicately balanced process that is not so easily understood or contained. While more and more studies have generally begun to take into account both the harmful and protective effects of microglia, whether microglia activation is a causative factor (hence, “bad for”) or reactive to (perhaps, “good for”) neurodegeneration is unclear. Understanding these two sides at both a functional and molecular level will be necessary in combating AD and the ill effects of aging.
Acknowledgments
The authors thank Joseph Castellano and Eva Czirr for insightful comments and suggestions on this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stelzmann R, Norman Schnitzlein H, Reed Murtagh F. An English translation of Alzheimer’s 1907 paper, “Über eine eigenartige Erkankung der Hirnrinde”. Clinical anatomy. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 2.Samorajski T. How the human brain responds to aging. J Am Geriatr Soc. 1976;24(1):4–11. doi: 10.1111/j.1532-5415.1976.tb03246.x. [DOI] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 5.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behavior and Immunity. 2007;21(4):490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9(1):1–1. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta neuropathologica. 2010;120(3):277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 11.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–7. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 12.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2012:1–10. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 13.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–28. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee EB. Obesity, leptin, and Alzheimer’s disease. Annals of the New York Academy of Sciences. 2011;1243(1):15–29. doi: 10.1111/j.1749-6632.2011.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease—The emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89(3–4):144–149. doi: 10.1016/j.brainresbull.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid Precursor Protein and Proinflammatory Changes Are Regulated in Brain and Adipose Tissue in a Murine Model of High Fat Diet-Induced Obesity. PLoS ONE. 2012;7(1):e30378. doi: 10.1371/journal.pone.0030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behavior and Immunity. 2013;35(C):33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert J-C, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013 doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griciuc A, Serrano-Pozo A, Parrado Antonio R, Lesinski Andrea N, Asselin Caroline N, Mullin K, et al. Alzheimer’s Disease Risk Gene CD33 Inhibits Microglial Uptake of Amyloid Beta. Neuron. 2013:1–13. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crocker PR, Mcmillan SJ, Richards HE. CD33-related siglecs as potential modulators of inflammatory responses. Annals of the New York Academy of Sciences. 2012;1253(1):102–111. doi: 10.1111/j.1749-6632.2011.06449.x. [DOI] [PubMed] [Google Scholar]
- 21.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83(5):623–32. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naj AC, Jun G, Beecham GW, Wang L-S, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 Variants in Alzheimer’s Disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Gaiteri C, Bodea L-G, Wang Z, Mcelwee J, Podtelezhnikov Alexei A, et al. Integrated Systems Approach Identifies Genetic Nodes and Networks in Late-Onset Alzheimer’s Disease. Cell. 2013;153(3):707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann H, Daly MJ. Variant TREM2 as risk factor for Alzheimer’s disease. N Engl J Med. 2013;368(2):182–4. doi: 10.1056/NEJMe1213157. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109(4):1144–56. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutajangout A, Wisniewski T. The Innate Immune System in Alzheimer’s Disease. Int J Cell Biol. 2013;2013:576383. doi: 10.1155/2013/576383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56(13):1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- 31.Crehan H, Hardy J, Pocock J. Microglia, Alzheimer’s Disease, and Complement. International Journal of Alzheimer’s Disease. 2012;2012(3):1–10. doi: 10.1155/2012/983640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature Publishing Group; 2009. pp. 1–7. [DOI] [PubMed] [Google Scholar]
- 34.O’bryan MK, Baker HW, Saunders JR, Kirszbaum L, Walker ID, Hudson P, et al. Human seminal clusterin (SP-40,40) Isolation and characterization Journal of Clinical Investigation. 1990;85(5):1477–1486. doi: 10.1172/JCI114594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski T, et al. The cerebrospinal-fluid soluble form of Alzheimer’s amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. The Biochemical journal. 1993;293 (Pt 1):27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi-Miura NH, Ihara Y, Fukuchi K, Takeda M, Nakano Y, Tobe T, et al. SP-40,40 is a constituent of Alzheimer’s amyloid. Acta neuropathologica. 1992;83(3):260–4. doi: 10.1007/BF00296787. [DOI] [PubMed] [Google Scholar]
- 37.McGeer PL, Kawamata T, Walker DG. Distribution of clusterin in Alzheimer brain tissue. Brain Res. 1992;579(2):337–41. doi: 10.1016/0006-8993(92)90071-g. [DOI] [PubMed] [Google Scholar]
- 38.Kida E, Choi-Miura NH, Wisniewski KE. Deposition of apolipoproteins E and J in senile plaques is topographically determined in both Alzheimer’s disease and Down’s syndrome brain. Brain Res. 1995;685(1–2):211–6. doi: 10.1016/0006-8993(95)00482-6. [DOI] [PubMed] [Google Scholar]
- 39.Xie Z, Harris-White ME, Wals PA, Frautschy SA, Finch CE, Morgan TE. Apolipoprotein J (clusterin) activates rodent microglia in vivo and in vitro. J Neurochem. 2005;93(4):1038–1046. doi: 10.1111/j.1471-4159.2005.03065.x. [DOI] [PubMed] [Google Scholar]
- 40.Cole GM, Ard MD. Influence of lipoproteins on microglial degradation of Alzheimer’s amyloid beta-protein. Microsc Res Tech. 2000;50(4):316–24. doi: 10.1002/1097-0029(20000815)50:4<316::AID-JEMT11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Crehan H, Hardy J, Pocock J. Blockage of CR1 prevents activation of rodent microglia. Neurobiology of Disease. 2013;54(C):139–149. doi: 10.1016/j.nbd.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Rogers J, Li R, Mastroeni D, Grover A, Leonard B, Ahern G, et al. Peripheral clearance of amyloid beta peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27(12):1733–9. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Forabosco P, Ramasamy A, Trabzuni D, Walker R, Smith C, Bras J, et al. Insights into TREM2 biology by network analysis of human brain gene expression data. Neurobiol Aging. 2013;34(12):2699–714. doi: 10.1016/j.neurobiolaging.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cribbs D, Berchtold N, Perreau V, Coleman P, Rogers J, Tenner A, et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–15. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55(11):1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- 47.Hua K, Schindler MK, Mcquail JA, Forbes ME, Riddle DR. Regionally Distinct Responses of Microglia and Glial Progenitor Cells to Whole Brain Irradiation in Adult and Aging Rats. PLoS ONE. 2012;7(12):e52728. doi: 10.1371/journal.pone.0052728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long J, Kalehua A, Muth N, Calhoun M, Jucker M, Hengemihle J, et al. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19(5):497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 49.Vaughan DW, Peters A. Neuroglial cells in the cerebral cortex of rats from young adulthood to old age: an electron microscope study. J Neurocytol. 1974;3(4):405–29. doi: 10.1007/BF01098730. [DOI] [PubMed] [Google Scholar]
- 50.Tremblay M-È, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60(4):541–558. doi: 10.1002/glia.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–76. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 53.Marques F, Sousa JC, Sousa N, Palha JA. Blood--brain-barriers in aging and in Alzheimer’s disease. Mol Neurodegener. 2013;8(1):38. doi: 10.1186/1750-1326-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Streit WJ, Xue Q-S. The Brain’s Aging Immune System. Aging Dis. 2010;1(3):254–261. [PMC free article] [PubMed] [Google Scholar]
- 55.Conde J, Streit W. Effect of aging on the microglial response to peripheral nerve injury. Neurobiol Aging. 2006;27(10):1451. doi: 10.1016/j.neurobiolaging.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence That Aging And Amyloid Promote Microglial Cell Senescence. Rejuvenation Research. 2007;10(1):61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- 57.Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2013:n/a–n/a. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker DJ, Wijshake T, Tchkonia T, Lebrasseur NK, Childs BG, Sluis Bvd, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011:1–6. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sieweke MH, Allen JE. Beyond Stem Cells: Self-Renewal of Differentiated Macrophages. Science. 2013;342(6161):1242974–1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 61.Kamphuis W, Orre M, Kooijman L, Dahmen M, Hol EM. Differential cell proliferation in the cortex of the appsweps1de9 alzheimer’s disease mouse model. Glia. 2012;60(4):615–629. doi: 10.1002/glia.22295. [DOI] [PubMed] [Google Scholar]
- 62.Bondolfi L, Calhoun M, Ermini F, Kuhn HG, Wiederhold K-H, Walker L, et al. Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. J Neurosci. 2002;22(2):515–22. doi: 10.1523/JNEUROSCI.22-02-00515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luccarini I, Grossi C, Traini C, Fiorentini A, Dami TE, Casamenti F. Aβ plaque-associated glial reaction as a determinant of apoptotic neuronal death and cortical gliogenesis: A study in APP mutant mice. Neuroscience Letters. 2012;506(1):94–99. doi: 10.1016/j.neulet.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 64.Serrano-Pozo A, Gómez-Isla T, Growdon JH, Frosch MP, Hyman BT. A Phenotypic Change But Not Proliferation Underlies Glial Responses in Alzheimer Disease. Am J Pathol. 2013;182(6):2332–2344. doi: 10.1016/j.ajpath.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jørgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Experimental Neurology. 1993;120(1):70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- 66.Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, et al. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48(2):196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 67.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. Journal of neuroimmunology. 1989;24(3):173–82. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 68.Styren SD, Civin WH, Rogers J. Molecular, cellular, and pathologic characterization of HLA-DR immunoreactivity in normal elderly and Alzheimer’s disease brain. Experimental Neurology. 1990;110(1):93–104. doi: 10.1016/0014-4886(90)90054-v. [DOI] [PubMed] [Google Scholar]
- 69.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neuroscience Letters. 1987;79(1–2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 70.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77(1):1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 71.Sierra A, Gottfried-Blackmore AC, Mcewen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 72.Koenigsknecht-Talboo J, Meyer-Luehmann M, Parsadanian M, Garcia-Alloza M, Finn MB, Hyman BT, et al. Rapid Microglial Response Around Amyloid Pathology after Systemic Anti-A Antibody Administration in PDAPP Mice. Journal of Neuroscience. 2008;28(52):14156–14164. doi: 10.1523/JNEUROSCI.4147-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, et al. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging. 2013:1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Tremblay M-È, Lowery RL, Majewska AK. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. Journal of Neuroscience. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 77.Schafer Dorothy P, Lehrman Emily K, Kautzman Amanda G, Koyama R, Mardinly Alan R, Yamasaki R, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, De Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451(7179):720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis JB, McMurray HF, Schubert D. The amyloid beta-protein of Alzheimer’s disease is chemotactic for mononuclear phagocytes. Biochem Biophys Res Commun. 1992;189(2):1096–100. doi: 10.1016/0006-291x(92)92317-q. [DOI] [PubMed] [Google Scholar]
- 80.Maeda K, Nakai M, Maeda S, Kawamata T, Yamaguchi T, Tanaka C. Possible different mechanism between amyloid-beta (25-35)-and substance P-induced chemotaxis of murine microglia. Gerontology. 1997;43 (Suppl 1):11–5. doi: 10.1159/000213881. [DOI] [PubMed] [Google Scholar]
- 81.Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer’s amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43(2):190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 82.McLarnon JG. Microglial chemotactic signaling factors in Alzheimer’s disease. Am J Neurodegener Dis. 2012;1(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 83.Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer’s disease. Neurochem Int. 2001;39(5–6):333–40. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 84.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 85.Naert G, Rivest S. CC Chemokine Receptor 2 Deficiency Aggravates Cognitive Impairments and Amyloid Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. Journal of Neuroscience. 2011;31(16):6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryu JK, Cho T, Choi HB, Wang YT, Mclarnon JG. Microglial VEGF Receptor Response Is an Integral Chemotactic Component in Alzheimer’s Disease Pathology. Journal of Neuroscience. 2009;29(1):3–13. doi: 10.1523/JNEUROSCI.2888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, et al. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197(12):1657–66. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yates SL, Burgess LH, Kocsis-Angle J, Antal JM, Dority MD, Embury PB, et al. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem. 2000;74(3):1017–25. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 89.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Experimental Neurology. 2001;171(1):29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 90.Gordon S, Martinez FO. Alternative Activation of Macrophages: Mechanism and Functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 91.Sheffield L, Berman N. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19(1):47. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 92.Wong A, Patel N, Patel N, Wei M, Morgan T, de Beer M, et al. Macrosialin increases during normal brain aging are attenuated by caloric restriction. Neuroscience Letters. 2005;390(2):76–80. doi: 10.1016/j.neulet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 93.Schindler M, Forbes M, Robbins M, Riddle D. Aging-dependent changes in the radiation response of the adult rat brain. International journal of radiation oncology, biology, physics. 2008;70(3):826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hart AD, Wyttenbach A, Perry VH, Teeling JL. Age related changes in microglial phenotype vary between CNS regions: Grey versus white matter differences. Brain Behavior and Immunity. 2012;26(5):754–765. doi: 10.1016/j.bbi.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harry GJ. Microglia during development and aging. Pharmacology and Therapeutics. 2013;139(3):313–326. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65(3):199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- 97.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9(4):339–49. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 98.Strohmeyer R, Ramirez M, Cole GJ, Mueller K, Rogers J. Association of factor H of the alternative pathway of complement with agrin and complement receptor 3 in the Alzheimer’s disease brain. Journal of neuroimmunology. 2002;131(1–2):135–46. doi: 10.1016/s0165-5728(02)00272-2. [DOI] [PubMed] [Google Scholar]
- 99.Arends YM, Duyckaerts C, Rozemuller JM, Eikelenboom P, Hauw JJ. Microglia, amyloid and dementia in alzheimer disease. A correlative study Neurobiol Aging. 2000;21(1):39–47. doi: 10.1016/s0197-4580(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 100.Di Patre PL, Read SL, Cummings JL, Tomiyasu U, Vartavarian LM, Secor DL, et al. Progression of clinical deterioration and pathological changes in patients with Alzheimer disease evaluated at biopsy and autopsy. Arch Neurol. 1999;56(10):1254–61. doi: 10.1001/archneur.56.10.1254. [DOI] [PubMed] [Google Scholar]
- 101.Landreth GE, Reed-Geaghan EG. Current Topics in Microbiology and Immunology. 2009;336:137–153. doi: 10.1007/978-3-642-00549-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cameron B, Landreth GE. Inflammation, microglia, and alzheimer’s disease. Neurobiology of Disease. 2010;37(3):503–509. doi: 10.1016/j.nbd.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Von Bernhardi R, Tichauer JE, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112(5):1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- 104.Nakanishi H, Wu Z. Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behavioural brain research. 2009;201(1):1–7. doi: 10.1016/j.bbr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, et al. Inflammatory Response in the Hippocampus of PS1M146L/APP751SL Mouse Model of Alzheimer’s Disease: Age-Dependent Switch in the Microglial Phenotype from Alternative to Classic. Journal of Neuroscience. 2008;28(45):11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging. 2013;34(5):1397–1411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-β expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behavior and Immunity. 2012;26(5):766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behavior and Immunity. 2009;23(3):309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee DC, Ruiz CR, Lebson L, Selenica M-LB, Rizer J, JBH, et al. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol Aging. 2013;34(6):1610–1620. doi: 10.1016/j.neurobiolaging.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lively S, Schlichter LC. Age-Related Comparisons of Evolution of the Inflammatory Response After Intracerebral Hemorrhage in Rats. Transl Stroke Res. 2012;3(S1):132–146. doi: 10.1007/s12975-012-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91(9):1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of neuroimmunology. 1999;93(1–2):139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- 113.Njie EG, Boelen E, Stassen FR, Steinbusch HWM, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33(1):195, e1–12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lai AY, Dibal CD, Armitage GA, Winship IR, Todd KG. Distinct activation profiles in microglia of different ages: A systematic study in isolated embryonic to aged microglial cultures. Neuroscience. 2013:1–11. doi: 10.1016/j.neuroscience.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 115.Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Experimental Neurology. 2003;182(1):135–41. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 116.Yu WH, Go L, Guinn BA, Fraser PE, Westaway D, McLaurin J. Phenotypic and functional changes in glial cells as a function of age. Neurobiol Aging. 2002;23(1):105–15. doi: 10.1016/s0197-4580(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 117.Colton C, Mott R, Sharpe H, Xu Q, Van Nostrand W, Vitek M. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3(1):27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meda L, Baron P, Prat E, Scarpini E, Scarlato G, Cassatella MA, et al. Proinflammatory profile of cytokine production by human monocytes and murine microglia stimulated with beta-amyloid [25–35] Journal of neuroimmunology. 1999;93(1–2):45–52. doi: 10.1016/s0165-5728(98)00188-x. [DOI] [PubMed] [Google Scholar]
- 120.Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bianca VD, Dusi S, Bianchini E, Dal Prà I, Rossi F. beta-amyloid activates the O-2 forming NADPH oxidase in microglia, monocytes, and neutrophils. A possible inflammatory mechanism of neuronal damage in Alzheimer’s disease. J Biol Chem. 1999;274(22):15493–9. doi: 10.1074/jbc.274.22.15493. [DOI] [PubMed] [Google Scholar]
- 122.Klegeris A, Walker DG, McGeer PL. Activation of macrophages by Alzheimer beta amyloid peptide. Biochem Biophys Res Commun. 1994;199(2):984–91. doi: 10.1006/bbrc.1994.1326. [DOI] [PubMed] [Google Scholar]
- 123.Varnum MM, Ikezu T. The Classification of Microglial Activation Phenotypes on Neurodegeneration and Regeneration in Alzheimer’s Disease Brain. Arch Immunol Ther Exp. 2012;60(4):251–266. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25(3):294–7. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 126.Qin L, Liu Y, Hong J-S, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61(6):855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behavior and Immunity. 2010;24(7):1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, et al. Fractalkine and CX3CR1 regulate hippocampal neurogenesis in adult and aged rats. NBA. 2011;32(11):2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cox FF, Carney D, Miller A-M, Lynch MA. CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behavior and Immunity. 2012;26(5):789–796. doi: 10.1016/j.bbi.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 130.Frank M, Barrientos R, Biedenkapp J, Rudy J, Watkins L, Maier S. mRNA up- regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 131.Al-Ali S, Al-Hussain S. An ultrastructural study of the phagocytic activity of astrocytes in adult rat brain. Journal of anatomy. 1996;188(Pt 2):257. [PMC free article] [PubMed] [Google Scholar]
- 132.Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature Cell Biology. 2011;13(8):1–9. doi: 10.1038/ncb2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, et al. TLR2 Is a Primary Receptor for Alzheimer’s Amyloid β Peptide To Trigger Neuroinflammatory Activation. The Journal of Immunology. 2012;188(3):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 135.Michaud J-P, Halle M, Lampron A, Theriault P, Prefontaine P, Filali M, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proceedings of the National Academy of Sciences. 2013;110(5):1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, et al. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128(Pt 8):1778–89. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- 137.Du Yan S, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 138.Hickman SE, Allison EK, El Khoury J. Microglial Dysfunction and Defective β-Amyloid Clearance Pathways in Aging Alzheimer’s Disease Mice. Journal of Neuroscience. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El Khoury J, Hickman S, Thomas C, Cao L, Silverstein S, Loike J. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996 doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 140.D’Andrea MR, Cole GM, Ard MD. The microglial phagocytic role with specific plaque types in the Alzheimer disease brain. Neurobiol Aging. 2004;25(5):675–83. doi: 10.1016/j.neurobiolaging.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 141.Solito E, Sastre M. Microglia Function in Alzheimer’s Disease. Front Pharmacol. 2012;3:1–10. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, et al. Functional Impairment of Microglia Coincides with Beta-Amyloid Deposition in Mice with Alzheimer-Like Pathology. PLoS ONE. 2013;8(4):e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-β fibrils. J Alzheimers Dis. 2011;25(2):279–93. doi: 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor RC, Dillin A. Aging as an Event of Proteostasis Collapse. Cold Spring Harbor Perspectives in Biology. 2011;3(5):a004440–a004440. doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mandrekar S, Jiang Q, Lee CYD, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia Mediate the Clearance of Soluble A through Fluid Phase Macropinocytosis. Journal of Neuroscience. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miners JS, Barua N, Kehoe PG, Gill S, Love S. Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol. 2011;70(11):944–59. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- 147.Shimizu E, Kawahara K, Kajizono M, Sawada M, Nakayama H. IL-4-induced selective clearance of oligomeric beta-amyloid peptide(1-42) by rat primary type 2 microglia. J Immunol. 2008;181(9):6503–13. doi: 10.4049/jimmunol.181.9.6503. [DOI] [PubMed] [Google Scholar]
- 148.Qiu WQ. Insulin-degrading Enzyme Regulates Extracellular Levels of Amyloid beta -Protein by Degradation. Journal of Biological Chemistry. 1998;273(49):32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 149.Prokop S, Miller KR, Heppner FL. Microglia actions in Alzheimer’s disease. Acta neuropathologica. 2013 doi: 10.1007/s00401-013-1182-x. [DOI] [PubMed] [Google Scholar]
- 150.Grathwohl SA, Kälin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, et al. Formation and maintenance of Alzheimer’s disease β-amyloid plaques in the absence of microglia. Nat Neurosci. 2009:1–3. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7(9):940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94(24):13287–92. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lucin KM, O’Brien CE, Bieri G, Czirr E, Mosher KI, Abbey RJ, et al. Microglial Beclin 1 Regulates Retromer Trafficking and Phagocytosis and Is Impaired in Alzheimer’s Disease. Neuron. 2013;79(5):873–886. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]