Abstract
Mutations in lamins, which are ubiquitous nuclear intermediate filaments, lead to a variety of disorders, including muscular dystrophy and dilated cardiomyopathy. Lamins provide nuclear stability, help connect the nucleus to the cytoskeleton, and can modulate chromatin organization and gene expression. Nonetheless, the diverse functions of lamins remain incompletely understood. Here, we focus on the role of lamins on nuclear mechanics and its implication in human diseases. Recent findings suggest that lamin mutations can decrease nuclear stability, increase nuclear fragility, and disturb mechanotransduction signaling, possibly explaining the muscle-specific defects in many laminopathies. At the same time, altered lamin expression has been reported in many cancers, where the resulting increased nuclear deformability could enhance the ability of cells to transit tight interstitial spaces, promoting metastasis.
Keywords: Protein assembly/structure, cell mechanics, gene regulation, cytoskeleton, laminopathy
Lamins and disease
Since the discovery in 1999 that mutations in the nuclear envelope proteins lamin A/C cause Emery-Dreifuss muscular dystrophy (EDMD) [1], lamins and lamin-associated proteins have garnered increasing interest in the scientific and medical community, resulting in the discovery of over 450 disease-associated lamin mutations to date (see http://www.umd.be/LMNA/). These ‘laminopathies’ include EDMD, dilated cardiomyopathy (DCM), Dunnigan-type familial partial lipodystrophy (FPLD), and Hutchinson-Gilford progeria syndrome (HGPS), a premature aging disorder [2]. Given that lamins A/C are expressed in nearly all cells and tissues, the high degree of tissue-specificity and the broad range of diseases caused by different mutations in the same gene are quite perplexing. Despite extensive research efforts, the molecular mechanisms underlying laminopathies remain to be fully explained.
In addition to providing structural support to the nucleus, lamins also contribute to nucleo-cytoskeletal coupling, chromatin organization, epigenetic modifications, DNA replication, transcriptional regulation and repair, and responses to oxidative stress [2]. In this review, we focus on the mechanical aspects of lamin functions, including governing nuclear deformability and fragility, physically connecting the nucleus and the cytoskeleton, and contributing to mechanotransduction signaling, i.e., the cells’ ability to respond to mechanical stimuli. These functions could be particularly relevant to muscular dystrophies and cardiomyopathies caused by mutations in lamins A/C. At the same time, striking new reports suggest that nuclear deformability, which is modulated by the expression of specific lamin isoforms, can constitute a rate-limiting factor in the ability of cells to pass through micrometer-sized constrictions in three-dimensional (3-D) environments [3,4], which has important implications in cancer progression, immune cell function, and development.
Lamins – primary components of the nuclear lamina
The lamina is a dense protein meshwork underlying the inner nuclear membrane that is composed of lamins and lamina-associated proteins. In somatic cells, the predominant lamins are lamins A and C (referred to as A-type lamins), resulting from alternative splicing of the LMNA gene, and the B-type lamins, lamin B1 and B2. While most differentiated cells express at least one A-type lamin, embryonic stem cells, the lower layer of epidermis [5], and the central nervous system [6] produce little to no lamin A. These latter findings may explain why the central nervous system is typically spared in diseases arising from LMNA mutations. In contrast, striated muscles and many mesenchymal cells have particularly high levels of A-type lamins, which may contribute to their prominent involvement in many laminopathies.
Unlike A-type lamins, B-type lamins are expressed by all cells throughout development. B-type lamins were previously considered essential for cell viability, based on knockdown studies in human cells [7] and C. elegans [8], but recent studies indicate that at least in mice, B-type lamins are dispensable in many cell types, including embryonic stem cells [9,10] and skin cells [11]. Nonetheless, lamins B1 and B2 are necessary for organogenesis [9], and mice lacking lamins B1 and/or B2 die shortly after birth with severe defects in neuronal development and migration [12]. These contrasting findings may reflect changes in experimental conditions (acute knockdown in the human and C. elegans cells versus selection of stable lamin B-deficient embryonic stem cells), or they may result from cell- or species-specific differences. Regardless, the findings consistently point to an important role of B-type lamins in organ development, particularly the brain, and should stimulate further research into the function of lamins during tissue development. A growing number of reports further suggest an involvement of lamin B1 in regulating cellular senescence [13]. B-type lamins have recently been linked to two diseases, adult-onset leukodystrophy, caused by a duplication of the LMNB1 gene [14], and acquired partial lipodystrophy (Barraquer-Simons syndrome) arising from mutations in the LMNB2 gene [15]. Nonetheless, the number of identified disease-causing mutations remains far fewer than for lamins A/C, suggesting that many lamin B mutations may result in embryonic lethality in humans.
Structural organization of nuclear lamins
Lamins, which are type V intermediate filaments, assemble into a dense network in the nuclear lamina (Fig. 1). Lamins A and C, however, are also present in the nuclear interior [16]. Due to the difficulty of imaging the chromatin- and nuclear membrane-associated lamina at high resolution in situ and the challenge to accurately reconstitute the nuclear envelope environment in vitro, the ultrastructural organization of the nuclear lamina in mammalian cells remains incompletely understood. Advances in cryo-electron tomography may eventually enable more accurate visualization of the nuclear lamina in somatic cells [17]. Ectopic expression of human lamins in Xenopus oocytes indicates that A-type lamins form a thick (up to 100 nm) network and that B-type lamins form a thin fibrous meshwork closely associated with the inner nuclear membrane, likely due to their farnesyl lipid anchor [18,19]. (See Box 1 for a comparison of common animal models used to study lamins.) Although A-type and B-type lamins can form mixed polymers during in vitro assembly [20], immunofluorescence and photobleaching studies suggest that A-type and B-type lamins form separate but overlapping networks in somatic cells [21]. Even lamins A and C segregate and assemble as homodimers in vivo, despite their ability to form heterodimers in vitro [22]. Whether the diverse lamin networks are interpenetrating or simply adjacent remains to be determined, as well as to what extent specific lamins can compensate for one another. Mice that express only prelamin A, only lamin C, or only mature lamin A lack obvious disease phenotypes [23,24], and in Drosophila ectopic expression of an A-type lamin can compensate for loss of B-type lamins in cyst stem cells [25], indicating that lamins may have (partially) redundant or overlapping roles. However, unlike lamin A, ectopic expression of lamin C only partially rescues the native stiffness of lamin A/C-deficient cells [26], suggesting a more important role for lamin A in nuclear stability.
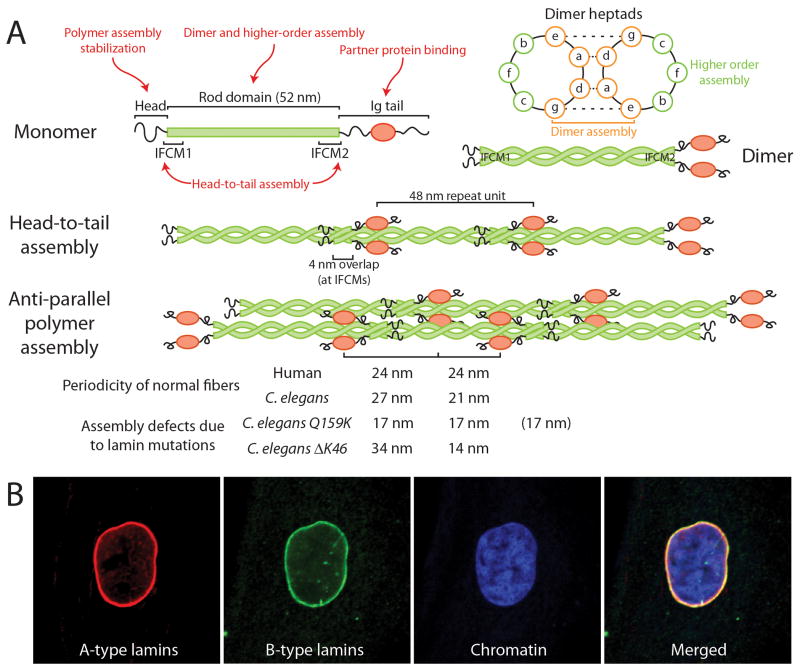
Figure 1.
Lamin assembly and defects caused by lamin mutations. (A) Experiments with mutated and truncated lamins suggest that different parts of the lamin proteins play distinct roles in lamin assembly (red text). Two parallel lamin monomers (top left) form dimers (top right) through coiled-coil interaction of the heptad repeats in their central rod domains. These dimers assemble head-to-tail by overlapping their rod domains at the two IF consensus motifs (IFCM). The head-to-tail assemblies form non-polar protofilaments composed of two anti-parallel polymers, which then laterally assemble to form mature filaments (and paracrystals) with specific repeat units, visible by electron microscopy and identified as the site of the globular Ig-fold motif. Defects in lamin assembly can be identified in vitro as changes in the spacing of the repeat unit, as demonstrated for two mutants in C. elegans (bottom), or by failure to form lamin filaments or paracrystals. Note that in the case of the Q159K mutation, it is likely that the spacing is 17/17/17, approximately corresponding to the 48 nm repeat unit, indicating a combination of three overlapping polymers instead of two. Inset, top right: a cross-section view of the heptad repeats of the coiled coil illustrates how the inner amino acids play a role in the dimer assembly, whereas amino acids that point outwards can affect higher order assembly. (B) Immunofluorescence images of human skin fibroblasts stained for lamins A/C (red), B-type lamins (green), and DNA (blue).
Box 1. Common animal models for the study of lamins.
Xenopus oocytes
In the large amphibian oocytes, which express only a B-type lamin (lamin LIII), the lamina lacks the tight association with chromatin seen in somatic cells, allowing easy access to the nuclear lamina and isolation for structural observation by electron microscopy. Its native lamina is organized into orthogonal filaments 10.5 nm wide and spaced 52 nm apart [107]. Lamins from other species ectopically expressed in amphibian oocytes assemble into a nuclear lamina enabling ultrastructural analysis. Xenopus oocytes currently present the only model in which the in vivo lamina structure can be studied.
C. elegans
These worms express a single lamin, Ce-lamin, that is functionally similar to both A- and B-type lamins [108]. Ce-lamin can be assembled into 10 nm filaments in vitro, resembling the organization of the Xenopus lamina, although it has been suggested that the 10 nm filaments obtained in vitro may represent a transitory phase that remains stable only under certain conditions [108]. The effects of specific lamin mutations in various tissues and their functional consequences can be assessed in vivo [58].
Drosophila
Flies expresses two lamin proteins, A-type Lamin C and B-type Lamin Dm0 [109]. Human lamins expressed in Drosophila localize correctly, and many protein interactions are conserved. Many human laminopathies can be modeled by introducing corresponding mutations into Drosophila Lamin C. Effects on nuclear structure and organization, as well as functional consequences such as locomotion and lethality, can be assessed in larval and adult animals [109].
Mus musculus
Mice provide important mammalian models to study laminopathies, including Lmna mutations causing DCM (N195K, H222P), EDMD (M371K), HGPS (G608G, L530P) and Charcot-Marie-Tooth disease (R298C), as well as various models lacking specific lamin isoforms. The frequently used lamin A/C-null model (Lmna−/−), which develops skeletal and cardiac defects, was recently found to express a short (54 kDa) fragment of A-type lamin by skipping exons 8–11 [110]. In a newer Lmna-null mouse model [111], homozygote mice develop more severe phenotypes, whereas heterozygotes remain healthy, implying residual function and mild toxicity of the LmnaΔ8-11 fragment. However, ectopic expression of wildtype lamin A in the original Lmna+/− cells completely rescues nuclear stiffness in these cells [43], indicating that the LmnaΔ8-11 fragment has no dominant-negative effect on nuclear mechanics. While mouse models enable the study of the effect of human lamin mutations in mammals, many models require homozygous expression of the mutant lamin, unlike the often heterozygous expression in human laminopathy patients.
Lamin binding partners
For A-type lamins, at least 54 binding-partners have been identified to date; for the lesser studied lamins B1 and B2, a combined 25 partners have been reported, some of which are also common to A-type lamins [27]. As a note of caution, some of the reported interactions may be indirect or might not occur under physiological conditions, and expression of many binding partners is likely cell-type specific [27,28]. Many nuclear envelope proteins and potential lamin-binding partners are still incompletely characterized, and new partners continue to be discovered. Recent examples include Samp1 [29], MLIP [30] and SLAP75 [31]. Broadly speaking, lamin binding partners can be categorized into structural proteins (such as actin, nesprin, SUN proteins and titin) and signaling molecules and transcription factors (e.g., pRb, ERK1/2, c-Fos, and SREBP), although several proteins may possess both structural and regulatory functions. Importantly, interaction between lamins and lamin-binding partners can take place at the nuclear envelope and/or the nuclear interior, which may provide an additional mechanism to regulate interactions. For a comprehensive overview of lamin-binding partners and their diverse functions, see a recent review [27].
Broken nuclei lead to broken hearts (and muscle) – the effect of lamin mutations
To date, it remains unclear how mutations in lamins, which are nearly ubiquitously expressed, can lead to often highly tissue-specific disorders [2]. The ‘structural hypothesis’ suggests that lamin mutations increase nuclear fragility, resulting in cell death and progressive failure in tissues such as muscle that are exposed to repetitive mechanical stress. The ‘gene regulation hypothesis’ proposes that lamin mutations interfere with tissue-specific genes: lamin mutations may inhibit binding to tissue-specific factors [27] or lead to abnormal gene activation/silencing during differentiation [32]. A third hypothesis proposes that lamin mutations impair stem cell function: mutations may cause abnormal differentiation or depletion of the stem cell niche through defects in proliferation, survival, or differentiation efficiency [25]. These hypotheses are not mutually exclusive, and it is likely that laminopathies arise from a combination of defects in lamin function [2]. For example, abnormal differentiation could be related to dysregulated signaling pathways and disturbed chromatin organization, but also mechanical defects such as impaired nucleo-cytoskeletal coupling [33]. Recent results provide support for each of these hypotheses, as detailed below.
Lamin mutations can reduce nuclear stability
Nuclear deformability is largely determined by nuclear lamina composition [34–36] and chromatin organization [37,38], which is sensitive to changes in lamin expression [39]. Consequently, expression levels of lamins A (and C) can help predict nuclear deformability, with increasing levels corresponding to stiffer and more viscous nuclei [26,40]. Lamin A/C-deficient nuclei are not only more deformable but also more fragile: lamin A/C-deficient cells show spontaneous transient nuclear rupture [41] and are more susceptible to nuclear breakage and cell death when exposed to mechanical stress [36]. B-type lamins undoubtedly play an important role in nuclear structure and contribute to nuclear shape and stability, particularly in cells lacking A-type lamins. However, in cells expressing both types of lamins, levels of A-type lamins correlate more strongly with nuclear stiffness than B-type lamins [26,40], suggesting that lamins A and C play a more dominant role in determining nuclear stiffness. This functional difference may be due to different structural properties of the diverse lamin networks, as experiments ectopically expressing human lamins in Xenopus oocytes found that A-type lamins assembled into substantially thicker networks than B-type lamins [42]. In addition to lamin content, several other factors may influence nuclear deformability by affecting the organization of the lamin network, including the phosphorylation status of lamins and the structural contribution of lamin-binding proteins.
While earlier studies were directed at cells lacking specific lamins, techniques to assess nuclear mechanics and fragility (e.g., micropipette aspiration, microindentation, and substrate strain application) are now finding widespread application to investigate the effects of specific disease-causing lamin mutations. A recent study of a broad panel of lamin A mutants expressed in lamin A/C-deficient cells found that whereas reintroduction of wildtype lamin A completely restored nuclear stiffness to levels of wildtype cells, many mutations linked to EDMD and DCM failed to restore nuclear stiffness; on the other hand, FPLD mutations were functionally indistinguishable from wildtype lamin A [43]. In vivo, muscle tissue from EDMD patients [44], and mouse [45,46] and Drosophila models of EDMD and DCM [43,47] contain severely elongated nuclei, indicative of reduced nuclear stiffness. Importantly, lamin mutations responsible for striated muscle disease also increase nuclear envelope fragility. Cultured laminopathy patient fibroblasts have higher rates of nuclear rupture in vitro [41], and skeletal muscle and cardiac tissue of mice and human patients carrying EDMD and DCM mutations, respectively, show anecdotal evidence of nuclear rupture in vivo, including mitochondria inside the nucleus [48] and discontinuities in the nuclear envelope visible by electron microscopy [49]. Taken together, these results point to a disease mechanism by which lamin mutations cause muscle-specific disease by compromising nuclear envelope stability and integrity, resulting in nuclear rupture and consequently cell death in tissues subjected to mechanical stress.
Contrary to the effect of EDMD- and DCM-causing mutations, lamin A mutations responsible for Hutchinson-Gilford progeria syndrome (HGPS) cause increased stiffness in HGPS patient cells [50,51] and when expressed in Xenopus oocytes [19]. Interestingly, fibroblasts from HGPS patients nonetheless have an increased susceptibility to mechanically induced cell death [50]. Increased sensitivity to mechanical stress is particularly relevant to vascular smooth muscle cells in HGPS, as patients and mouse models exhibit a progressive loss of vascular smooth muscle cells in large arteries, which are subjected to repetitive vessel strains.
Lamin mutations cause defects in lamin assembly
The mechanical defects described above are likely caused by aberrations in the nuclear lamina assembly. Similar to other intermediate filament (IF) family members, lamins are composed of a mostly α-helical central rod domain flanked by a short N-terminal head and a long tail domain, which contains a globular Ig-fold [52]. In contrast to cytoplasmic IFs, many questions remain regarding the higher order assembly of nuclear lamins. Most in vitro studies have focused on lamins from C. elegans (Ce-lamin), which form ≈10nm thick filaments in vitro, resembling those observed in the lamina of Xenopus oocytes (see Box 1). Mammalian lamins can be induced to assemble into various structures in vitro, ranging from short head-to-tail polymers and filaments to paracrystals, depending on the experimental conditions [43,52]. The physiological relevance of these varying lamin forms remains unclear.
Due to the challenge of crystallizing filamentous proteins, crystallographic analysis has been restricted to smaller lamin fragments, including the coil 2B of lamin A/C [53] and lamin B1 [54], along with the lamin A/C globular tail [55]. Combined with in vitro assembly experiments using lamin mutations and partial deletions, these studies indicate that the supramolecular assembly of lamins largely depends on interactions within the lamin rod domains and the N-terminal head domain [52]. These studies have recently been expanded to investigate the effect of specific mutations on lamin assembly. Mutations causing DCM, EDMD and HGPS in the first IF consensus motif and in coil 2 of human [43] and C. elegans lamin [56–59] disturb lamin assembly, leading to shortened and irregular filament and paracrystal assembly in vitro and increased mobility and solubility of mutant lamins in vivo (Fig. 1). The EDMD and DCM-causing mutations in the lamin rod domain impair head-to-tail polymer assembly or lateral filament association rather than lamin dimerization [43], although some mutations could also disturb dimer formation [60,61]. Supporting the notion that EDMD and DCM mutations primarily affect higher order assembly of lamins A/C, the crystal structure of coil2B was recently solved for two DCM mutations, and neither altered the secondary structure of the wildtype protein [62]. Intriguingly, studies with a fragment of the lamin A/C coil 2B domain, comprising a small portion of the tail (residues 328–398), suggest that in addition to the typical left-handed heptad, lamins can form right-handed quindecad coiled-coils [63], so that during head-to-tail polymer formation the IF consensus motifs may “unzip” from the heptad and latch onto the adjacent lamin dimer. Head-to-tail polymer assembly might therefore require that the IF consensus motifs can be easily disassembled, and mutations that increase heptad stability may impede higher order assembly [60].
While the rod and head domains are essential for lamin assembly, other regions are also important for proper protein function. The lamin A/C tail domain, including the globular Ig-fold, harbors most of the interaction sites for lamin-binding partners [27]. Specific mutations can affect the Ig-fold structure in distinct ways. Mutations causing striated muscle disease are typically located in the interior of the Ig-fold beta sandwich, which can destabilize the Ig-fold [55]. In contrast, mutations responsible for FPLD are clustered in a small region on the surface of the Ig-fold. The FPLD mutations, as shown for the R482W mutant, do not affect the crystal structure of the Ig-fold [64], but may instead impair interaction with specific binding partners such as SREBP1 [55]. Lastly, the HGPS mutation that results in a 50 amino acid deletion in the lamin A tail leads to stronger intramolecular binding, impeding molecular interactions that would require accessibility of the Ig fold [65].
Lamin mutations can disrupt nucleo-cytoskeletal coupling
While many lamin mutations that cause DCM and EDMD impair nuclear stability, other mutations have little or no effect on nuclear stiffness [43], suggesting that alternative mechanisms must contribute to the disease. One potential mechanism that has gained increasing prominence is the role of lamins in nucleo-cytoskeletal coupling through interactions with Linker of Nucleoskeleton to Cytoskeleton (LINC) complex components. The LINC complex is composed of SUN protein trimers at the inner nuclear membrane that connect across the luminal space to nesprins proteins on the outer nuclear membrane, which can in turn interact with various cytoskeletal components [66,67]. Intact LINC complex function was recently identified to be crucial for a multitude of cellular functions, including force transmission between the nucleus and the cytoskeleton [68], nuclear positioning in secretory epithelial cells [69], retrograde nuclear movement in migrating fibroblasts via transmembrane actin-associated nuclear (TAN) lines [70], positioning of synaptic nuclei in muscle fibers [71] and the retina [72], and cytoskeletal organization [36]. Many molecular details underlying LINC complex function, including its dynamic regulation, remain incompletely understood, and recent studies have implicated additional nuclear envelope proteins in nucleo-cytoskeletal coupling, including Samp1 [29,73] and emerin [74]. Lamins A/C can bind to SUN1 [75], SUN2 [76], Samp1 [73], emerin and various nesprin isoforms [77], highlighting their importance in nucleo-cytoskeletal coupling, presumably by anchoring LINC complex components to the nuclear lamina and interior.
Loss of lamins A/C and mutations associated with striated muscle disease can interfere with coupling to SUN proteins [77,78], emerin [59,77], Klaroid (a Drosophila nesprin analog) [79], Nesprin-1 [78], Nesprin-2 [80], nuclear pore components [79], and DNA [81]. Conversely, other mutations increase binding to SUN [77] and emerin [82]. Consequently, loss of lamins A/C and lamin mutations responsible for EDMD and DCM can disrupt nucleo-cytoskeletal coupling and related functions. Cells carrying EDMD and DCM mutations have defective intracellular force transmission between the cytoskeleton and nucleus [43], and expression of EDMD and DCM mutations, but not FPLD mutation, prevents retrograde nuclear movement by failing to anchor TAN lines to the nucleus [83]. Lamins also contribute to proper cytoskeletal organization, as lamin A/C–deficient cells have reduced cytoskeletal stiffness and disturbed cytoskeletal networks [36], similar to defects observed in cells after LINC complex disruption. Evidence of impaired nucleo-cytoskeletal coupling can also be found in tissues from EDMD and DCM patients and mouse and Drosophila models, which display discontinuous neuromuscular junctions, reduced numbers of synaptic nuclei, abnormal clustering of nuclei, and sarcomere disorganization around the nucleus [44,78,84]. These aberrations could provide further explanation for the muscle-specific phenotype of certain laminopathies.
The role of B-type lamins in nucleo-cytoskeletal coupling is slowly emerging. Lamin B1-deficient cells have spontaneously rotating nuclei, indicating loss of nuclear anchoring, and lamin B1 and B2 are essential for neuronal migration during brain development, a process that involves nuclear movement along microtubules [15,85]. However, the underlying molecular defects remain unclear.
Lamins, mechanotransduction, and gene regulation
The cell nucleus has long been proposed to act as a cellular mechanosensor [86]. Thus, altered nucleo-cytoskeletal coupling and nuclear deformability resulting from lamin mutations may also affect the ability of cells to translate mechanical stimuli into biochemical signals, as evidenced by impaired activation of mechanosensitive genes in lamin A/C- and emerin-deficient cells in vitro [36,87] and in vivo [88]. However, it remains unclear whether the diminished mechanotransduction signaling results from impaired nuclear mechanosensing or altered downstream signaling. On the one hand, disruption of nucleo-cytoskeletal coupling with dominant negative nesprin and SUN protein constructs, which abolish nuclear deformation when cells are subjected to mechanical strain, has no effect on the mechanical activation of the genes impaired in lamin A/C-deficient cells [68], arguing against a role of the nucleus as a mechanosensor. On the other hand, externally applied forces can alter intranuclear protein mobility [89,90] and depletion of lamins A/C prevents force-induced dissociation of protein complexes inside Cajal bodies [89]. A recent report further found that exposing isolated nuclei to shear stress causes partial unfolding of the lamin A/C Ig-fold, exposing a buried cysteine, which could trigger further signaling events [40]. Furthermore, the ratio of A-type lamins to B-type lamins in cells and tissues strongly correlates with the overall stiffness of the surrounding tissue, with most of the effect being attributed to higher levels of A-type lamins in response to increased tissue stiffness [40]; in vitro, increasing substrate stiffness results in higher levels of lamins A/C and suppresses their phosphorylation [40], which could further increase nuclear stiffness. These findings support the notion that applied forces can directly induce structural changes and signaling inside the nucleus, with the nucleus and the nuclear lamina in particular playing a central role in cellular mechanosensing and adaptation of cells to their mechanical environment. Interestingly, loss of lamin A/C or mutations leading to emerin mislocalization result in impaired intracellular localization of the mechanosensitive transcriptional co-factor myocardin-related transcription factor A (MRTF-A), which plays important roles in cardiac development and function [91]. This effect was caused by dysregulation of the actin-polymerizing function of emerin at the nuclear envelope, affecting nuclear (and cytoskeletal) actin dynamics that are critical in regulating MRTF-A intracellular localization and impairing activation of MRTF-A/SRF downstream genes [91].
Lamins may also modulate gene expression independently of mechanical stimulation, as lamins A/C associate with a number of transcriptional regulators, including c-Fos, ERK1/2, SREBP1, and pRb [27]. Mutations and (functional) loss of lamins can affect intranuclear localization and stability of these transcription factors, as well as the affinity of lamins for specific binding partners. In addition, lamins can modulate gene expression by controlling gene positioning through lamina-associated domains (LADs), which may controls silencing and activation of tissue-specific genes [92]. A detailed discussion of the various gene regulatory roles of lamins can be found elsewhere [2,27].
Given these facts, it is not surprising that lamin mutations can disturb numerous important signaling pathways: striated muscle disease mutations result in abnormal activation of mitogen-activated protein kinases (MAPK) ERK [45], JNK [93] and p38α [94], as well as target of rapamycin (mTOR) [95,96]; HGPS mutations cause abnormalities in the Wnt pathway [97] and SIRT1 activity [98]. The identification of abnormal signaling pathways provides attractive targets for the development of therapies for laminopathies: treatment with MAPK inhibitors [45,93,94] or rapamycin [95,96] have already been shown to improve phenotypes in EDMD and DCM mouse models. For patients with HGPS, clinical trials with farnesyltransferase inhibitors (FTIs), statins, and bisphosphonates, aimed at modulating the abnormal processing of mutant lamin A, are currently underway [99]. An initial study with farnesyltransferase inhibitors resulted in modestly improved cardiovascular symptoms, but did not rescue the stunted growth of the patients [100]. While the various therapeutic approaches may not always address the root cause of the disease, they nonetheless present powerful means to alleviate many of the most pressing symptoms, immediately benefiting laminopathy patients.
Lamins, cell migration and cancer
An increasing number of reports have recently implicated lamins in human cancers, as cancers of the ovary, colon, gut, blood, prostate, lung and breast often have altered expression of lamins (reviewed in [101]). In the case of colorectal cancer, both increased [102] and decreased levels of lamin A/C [103] have been shown to correlate with increased aggressiveness, with decreased levels associated with tumor recurrence in advanced stage patients [103]. Given the diverse functions of lamins, changes in lamin expression may affect cancer progression through a variety of mechanisms, including altered proliferation, signaling, and migration [101,104]. One possible model to explain how low levels of lamins A/C can contribute to cancer progression is based on the emerging importance of nuclear deformability during cell motility in three dimensional (3-D) environments. It is now becoming clear that nuclear deformability can constitute a rate-limiting factor in the passage of cells through narrow constrictions, as convincingly demonstrated for cancer cells and immune cells migrating through dense collagen matrices [3], breast cancer cells migrating through microfabricated channels [105], and neutrophils passing through microfluidic constrictions [4]. In the latter case, cells with decreased levels of lamin A were able to pass more easily through constrictions smaller than the size of the nucleus [4]. Conversely, expression of an HGPS lamin variant, progerin, which stiffens the nucleus, impairs cell migration through confining 3-D environments [106], further supporting the idea that nuclear deformability is an important factor in 3-D migration. These findings suggest that downregulation of A-type lamins, whether during physiological processes such as granulopoiesis or in a subpopulation of cancer cells, results in increased nuclear deformability and can thereby facilitate transit of cells through narrow constrictions, for example, in the interstitial space or during intra- and extravasation and passage through narrow capillaries. However, given that lamin levels are not uniformly altered across different cancer types and lamin expression even varies within individual tumors, it remains unclear whether lamins primarily modulate cancer metastasis by changing the mechanical properties of cells or through effects of lamins on cell proliferation, signaling and differentiation.
Concluding remarks
Inside the body, cells are continuously exposed to physical forces and the mechanical constraints of their microenvironment. It is now emerging that lamins play a crucial role in the ability of cells to adapt to their mechanical environment by providing structural support to the nucleus and modulating mechanotransduction signaling. However, it remains unclear to what extent lamins and other nuclear proteins can act as direct nuclear mechanosensors or whether they primarily serve as signaling and structural hubs at the nuclear/cytoskeletal interface. Similarly, the role of lamins in stem cell function and differentiation, e.g., by responding to mechanical cues or tethering genes to transcriptionally repressive locations, remains to be addressed in more detail, as embryonic stem cells lacking all lamins still undergo normal differentiation in vitro and in vivo. In addition, more work will be required to address the specific effects of lamin mutations associated with different diseases. Patient-derived induced pluripotent stem cells (iPSCs) that can be differentiated into various cell types provide an important tool to study tissue-specific defects of lamin mutations and are finding increasing use in investigating laminopathies. Understanding whether muscle-specific laminopathies primarily result from structural defects or disturbed signaling is particularly relevant to the development of therapeutic approaches; correcting altered signaling is currently more amenable to treatment with small molecules than structural defects, which may require gene therapy or silencing of the mutant gene(s). While much of our current understanding of the diverse functions of lamins has come from studying the effects of disease-causing lamin mutation, it is now becoming apparent that lamins may also play an important role in cancer progression, whether by providing physical limits to the ability of the nucleus deform through microscopic constrictions in interstitial spaces or by modulating cytoskeletal dynamics and other cell functions.
Lamins provide an ideal research avenue to study the interplay between (nuclear) structure and cellular function, given their central role in modulating nuclear and cytoskeletal structure, gene expression, and a plethora of other cellular functions. Enormous progress has been made in recent years in the understanding of lamins, with insights gained from these studies providing hope and promise to the many patients affected by devastating laminopathies.
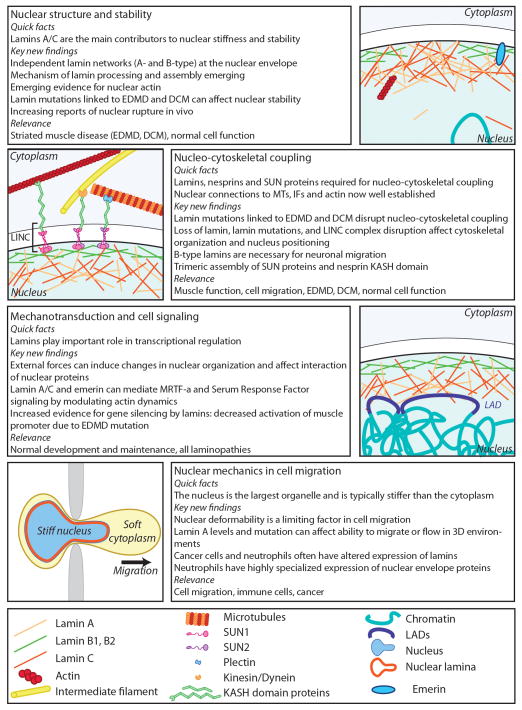
Figure 2.
Overview of the diverse functions of lamins in nuclear and cellular mechanics and mechanotransduction.
Table 1.
Consequences of lamin mutations on nuclear structure and mechanics
| Evidence of altered nuclear stability | Experimental system | References |
|---|---|---|
| Nuclear rupture |
In vitro: in patient fibroblasts carrying mutations that lead to HGPS, FPLD, RD and HCM In vivo: in the heart tissue of human and mouse carrying DCM mutations |
[41] [48,49] |
| Stiffer nuclei | In vitro: in HGPS patient cells and in Xenopus oocytes overexpressing lamin A with a mutation that causes HGPS | [19,50] |
| Softer nuclei |
In vitro: in fibroblasts carrying mutations that lead to DCM and EDMD In situ: In Drosophila larval muscle expressing dominant-negative lamin mutations |
[43] |
| Elongated nuclei in vivo | In the heart tissue of human, mice, and flies carrying DCM mutations | [43–47] |
| Defects in lamin assembly | In vitro assembly of lamin filaments and paracrystals from recombinant lamin | [43,56–59] |
Highlights.
Lamins modulate nuclear mechanics, chromatin organization and gene regulation
Lamin mutations cause a broad spectrum of diseases with often tissue-specific defects
Lamin mutations can disrupt nuclear stability and nucleo-cytoskeletal connections
Lamins play an important role in mechanosensing and mechanotransduction signaling
Many cancers have altered lamin expression, which may facilitate metastatic spreading
Acknowledgments
We apologize to all authors whose work could not be cited due to space constraints. This work was supported by National Institutes of Health awards [R01 NS059348 and R01 HL082792], a National Science Foundation CAREER award [CBET-1254846], the Department of Defense Breast Cancer Idea Award [BC102152], an award from the Progeria Research Foundation [PRF2011-0035], and a Pilot Project Award by the Cornell Center on the Microenvironment & Metastasis through Award Number U54CA143876 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patricia M. Davidson, Email: patricia.davidson@cornell.edu, Weill Institute for Cell and Molecular Biology, Cornell University, Weill Hall, Room 234, 526 Campus Rd, Ithaca, NY 14853
Jan Lammerding, Department of Biomedical Engineering/Weill Institute for Cell and Molecular Biology, Cornell University, Weill Hall, Room 235, 526 Campus Rd, Ithaca, NY 14853
References
- 1.Bonne G, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–8. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber K, Kennedy B. When Lamins Go Bad: Nuclear Structure and Disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf K, et al. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowat AC, et al. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem. 2013;288:8610–8. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanif M, et al. Differential expression of A-type and B-type lamins during hair cycling. PLoS One. 2009;4:e4114. doi: 10.1371/journal.pone.0004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung HJ, et al. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc Natl Acad Sci U S A. 2012;109:E423–31. doi: 10.1073/pnas.1111780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harborth J, et al. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–65. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, et al. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, et al. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science. 2011;334:1706–10. doi: 10.1126/science.1211222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y, et al. Proliferation and differentiation of mouse embryonic stem cells lacking all lamins. Cell Res. 2013 doi: 10.1038/cr.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SH, et al. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet. 2011;20:3537–44. doi: 10.1093/hmg/ddr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffinier C, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreesen O, et al. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol. 2013;200:605–617. doi: 10.1083/jcb.201206121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmer Ta, Misteli T. The lamin protein family. Genome Biol. 2011;12:222. doi: 10.1186/gb-2011-12-5-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffinier C, et al. LINCing lamin B2 to neuronal migration: growing evidence for cell-specific roles of B-type lamins. Nucleus. 2010;1:407–411. doi: 10.4161/nucl.1.5.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam S, Goldman R. Insights into the Differences between the A-and B-Type Nuclear Lamins. Adv Biol Regul. 2012;52:108–113. doi: 10.1016/j.advenzreg.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridman K, et al. Advances in tomography: probing the molecular architecture of cells. Nat Rev Mol Cell Biol. 2012;13:736–42. doi: 10.1038/nrm3453. [DOI] [PubMed] [Google Scholar]
- 18.Jung HJ, et al. Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc Natl Acad Sci U S A. 2013;110:E1923–32. doi: 10.1073/pnas.1303916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann A, et al. Amphibian oocyte nuclei expressing lamin A with the progeria mutation E145K exhibit an increased elastic modulus. Nucleus. 2011;2:310–9. doi: 10.4161/nucl.2.4.16119. [DOI] [PubMed] [Google Scholar]
- 20.Kapinos LE, et al. Characterization of the head-to-tail overlap complexes formed by human lamin A, B1 and B2 “half-minilamin” dimers. J Mol Biol. 2010;396:719–31. doi: 10.1016/j.jmb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Shimi T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–21. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolb T, et al. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus. 2011;2:425–33. doi: 10.4161/nucl.2.5.17765. [DOI] [PubMed] [Google Scholar]
- 23.Davies BS, et al. Investigating the purpose of prelamin A processing. Nucleus. 2011;2:4–9. doi: 10.4161/nucl.2.1.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fong L, Ng J. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J Clin Invest. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H, et al. The Nuclear Lamina Regulates Germline Stem Cell Niche Organization via Modulation of EGFR Signaling. Cell Stem Cell. 2013;13:73–86. doi: 10.1016/j.stem.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lammerding J, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem. 2006;281:25768–80. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 27.Simon DN, Wilson KL. Partners and post-translational modifications of nuclear lamins. Chromosoma. 2013;122:13–31. doi: 10.1007/s00412-013-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korfali N, et al. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3:552–64. doi: 10.4161/nucl.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudise S, et al. Samp1 is functionally associated with the LINC complex and Atype lamina networks. J Cell Sci. 2011;124:2077–85. doi: 10.1242/jcs.078923. [DOI] [PubMed] [Google Scholar]
- 30.Ahmady E, et al. Identification of a novel muscle A-type lamin-interacting protein (MLIP) J Biol Chem. 2011;286:19702–13. doi: 10.1074/jbc.M110.165548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux KJ, et al. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196:801–10. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peric-Hupkes D, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–13. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi ML, Lammerding J. Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem Soc Trans. 2011;39:1729–34. doi: 10.1042/BST20110686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahl KN, et al. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci. 2004;117:4779–86. doi: 10.1242/jcs.01357. [DOI] [PubMed] [Google Scholar]
- 35.Broers JLV, et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–80. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 36.Lammerding J, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pajerowski JD, et al. Physical plasticity of the nucleus in stem cell differentiation. Proc Natl Acad Sci. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowat AC, et al. Towards an integrated understanding of the structure and mechanics of the cell nucleus. Bioessays. 2008;30:226–36. doi: 10.1002/bies.20720. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan T, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swift J, et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science (80–) 2013;341:1240104–1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Vos WH, et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet. 2011;20:4175–86. doi: 10.1093/hmg/ddr344. [DOI] [PubMed] [Google Scholar]
- 42.Schäpe J, et al. Influence of lamin A on the mechanical properties of amphibian oocyte nuclei measured by atomic force microscopy. Biophys J. 2009;96:4319–25. doi: 10.1016/j.bpj.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwerger M, et al. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roncarati R, et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muchir A, et al. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18:241–7. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mounkes LC, et al. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum Mol Genet. 2005;14:2167–80. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- 47.Dialynas G, et al. The role of Drosophila Lamin C in muscle function and gene expression. Development. 2010;137:3067–77. doi: 10.1242/dev.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta P, et al. Genetic and ultrastructural studies in dilated cardiomyopathy patients: a large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res Cardiol. 2010;105:365–77. doi: 10.1007/s00395-010-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattin ME, et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum Mol Genet. 2013;22:3152–3164. doi: 10.1093/hmg/ddt172. [DOI] [PubMed] [Google Scholar]
- 50.Verstraeten VLRM, et al. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–93. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahl KN, et al. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–6. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann H, et al. Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest. 2009;119:1772–1783. doi: 10.1172/JCI38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strelkov SV, et al. Crystal structure of the human lamin A coil 2B dimer: implications for the head-to-tail association of nuclear lamins. J Mol Biol. 2004;343:1067–80. doi: 10.1016/j.jmb.2004.08.093. [DOI] [PubMed] [Google Scholar]
- 54.Ruan J, et al. Crystal structures of the coil 2B fragment and the globular tail domain of human lamin B1. FEBS Lett. 2012;586:314–8. doi: 10.1016/j.febslet.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Dhe-Paganon S, et al. Structure of the globular tail of nuclear lamin. J Biol Chem. 2002;277:17381–4. doi: 10.1074/jbc.C200038200. [DOI] [PubMed] [Google Scholar]
- 56.Wiesel N, et al. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc Natl Acad Sci U S A. 2008;105:180–5. doi: 10.1073/pnas.0708974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben-Harush K, et al. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386:1392–402. doi: 10.1016/j.jmb.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 58.Bank EM, et al. Structural and physiological phenotypes of disease-linked lamin mutations in C. elegans. J Struct Biol. 2012;177:106–12. doi: 10.1016/j.jsb.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Bank EM, et al. A laminopathic mutation disrupting lamin filament assembly causes disease-like phenotypes in Caenorhabditis elegans. Mol Biol Cell. 2011;22:2716–28. doi: 10.1091/mbc.E11-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gangemi F, Degano M. Disease-associated mutations in the coil 2B domain of human lamin A/C affect structural properties that mediate dimerization and intermediate filament formation. J Struct Biol. 2013;181:17–28. doi: 10.1016/j.jsb.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 61.Bhattacharjee P, et al. Structural alterations of lamin a protein in dilated cardiomyopathy. Biochemistry. 2013;52:4229–41. doi: 10.1021/bi400337t. [DOI] [PubMed] [Google Scholar]
- 62.Bollati M, et al. Structures of the lamin A/C R335W and E347K mutants: implications for dilated cardiolaminopathies. Biochem Biophys Res Commun. 2012;418:217–21. doi: 10.1016/j.bbrc.2011.12.136. [DOI] [PubMed] [Google Scholar]
- 63.Kapinos LE, et al. Simultaneous formation of right- and left-handed anti-parallel coiled-coil interfaces by a coil2 fragment of human lamin A. J Mol Biol. 2011;408:135–46. doi: 10.1016/j.jmb.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 64.Magracheva E, et al. Structure of the lamin A/C R482W mutant responsible for dominant familial partial lipodystrophy (FPLD) Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:665–70. doi: 10.1107/S1744309109020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin Z, et al. Structure and stability of the lamin A tail domain and HGPS mutant. J Struct Biol. 2011;175:425–33. doi: 10.1016/j.jsb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sosa Ba, et al. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–47. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gundersen GG, Worman HJ. Nuclear Positioning. Cell. 2013;152:1376–1389. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lombardi ML, et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:1–20. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roux KJ, et al. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106:2194–9. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luxton GWG, et al. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–9. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lei K, et al. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106:10207–12. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu J, et al. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–73. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borrego-Pinto J, et al. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci. 2012;125:1099–105. doi: 10.1242/jcs.087049. [DOI] [PubMed] [Google Scholar]
- 74.Salpingidou G, et al. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ostlund C, et al. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci. 2009;122:4099–108. doi: 10.1242/jcs.057075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang Y, et al. Subcellular localization of SUN2 is regulated by Lamin A and Rab5. PLoS One. 2011;6:e20507. doi: 10.1371/journal.pone.0020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haque F, et al. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–98. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Méjat A, et al. Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J Cell Biol. 2009;184:31–44. doi: 10.1083/jcb.200811035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dialynas G, et al. LMNA variants cause cytoplasmic distribution of nuclear pore proteins in Drosophila and human muscle. Hum Mol Genet. 2012;21:1544–56. doi: 10.1093/hmg/ddr592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, et al. Mutations in LMNA Modulate the Lamin A - Nesprin-2 Interaction and Cause LINC Complex Alterations. PLoS One. 2013;8:e71850. doi: 10.1371/journal.pone.0071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bruston F, et al. Loss of a DNA binding site within the tail of prelamin A contributes to altered heterochromatin anchorage by progerin. FEBS Lett. 2010;584:2999–3004. doi: 10.1016/j.febslet.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rajendran V, et al. In silico investigation of molecular mechanism of laminopathy caused by a point mutation (R482W) in lamin A/C protein. Amino Acids. 2012;43:603–15. doi: 10.1007/s00726-011-1108-7. [DOI] [PubMed] [Google Scholar]
- 83.Folker ES, et al. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A. 2011;108:131–6. doi: 10.1073/pnas.1000824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park YE, et al. Nuclear changes in skeletal muscle extend to satellite cells in autosomal dominant Emery-Dreifuss muscular dystrophy/limb-girdle muscular dystrophy 1B. Neuromuscul Disord. 2009;19:29–36. doi: 10.1016/j.nmd.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 85.Ji JY, et al. Cell nuclei spin in the absence of lamin b1. J Biol Chem. 2007;282:20015–26. doi: 10.1074/jbc.M611094200. [DOI] [PubMed] [Google Scholar]
- 86.Wang N, et al. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 87.Lammerding J, et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–91. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cupesi M, et al. Attenuated hypertrophic response to pressure overload in a lamin A/C haploinsufficiency mouse. J Mol Cell Cardiol. 2010;48:1290–7. doi: 10.1016/j.yjmcc.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poh YC, et al. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Booth-Gauthier EA, et al. Force-induced changes in subnuclear movement and rheology. Biophys J. 2012;103:2423–31. doi: 10.1016/j.bpj.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho CY, et al. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guelen L, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 93.Muchir A. MEK1/2 Inhibitors to Treat Dilated Cardiomyopathy Caused by LMNA Mutations. In: Da Silva Xavier G, editor. Biochemistry, Genetics and Molecular Biology. 2012. [Google Scholar]
- 94.Muchir A, et al. Abnormal p38α mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet. 2012;21:4325–33. doi: 10.1093/hmg/dds265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramos FJ, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi JC, et al. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Sci Transl Med. 2012;4:144ra102. doi: 10.1126/scitranslmed.3003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meshorer E, Gruenbaum Y. Gone with the Wnt/Notch: stem cells in laminopathies, progeria, and aging. J Cell Biol. 2008;181:9–13. doi: 10.1083/jcb.200802155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu B, et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16:738–50. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 99.Young SG, et al. Targeting protein prenylation in progeria. Sci Transl Med. 2013;5:171ps3. doi: 10.1126/scitranslmed.3005229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gordon L, Kleinman M. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson Gilford progeria syndrome. Proc Natl Acad Sci. 2012;109:1–6. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Las Heras JI, et al. Cancer biology and the nuclear envelope: A convoluted relationship. Semin Cancer Biol. 2012 doi: 10.1016/j.semcancer.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Willis ND, et al. Lamin A/C is a risk biomarker in colorectal cancer. PLoS One. 2008;3:e2988. doi: 10.1371/journal.pone.0002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Belt EJT, et al. Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer. 2011;47:1837–45. doi: 10.1016/j.ejca.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 104.Kong L, et al. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis. 2012;33:751–9. doi: 10.1093/carcin/bgs022. [DOI] [PubMed] [Google Scholar]
- 105.Fu Y, et al. Nuclear deformation during breast cancer cell transmigration. Lab Chip. 2012;12:3774–8. doi: 10.1039/c2lc40477j. [DOI] [PubMed] [Google Scholar]
- 106.Booth-Gauthier Ea, et al. Hutchinson-Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates. Integr Biol (Camb) 2013;5:569–77. doi: 10.1039/c3ib20231c. [DOI] [PubMed] [Google Scholar]
- 107.Aebi U, et al. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 108.Bank EM, Gruenbaum Y. Caenorhabditis elegans as a model system for studying the nuclear lamina and laminopathic diseases. Nucleus. 2011;2:350–7. doi: 10.4161/nucl.2.5.17838. [DOI] [PubMed] [Google Scholar]
- 109.Schulze SR, et al. A comparative study of Drosophila and human A-type lamins. PLoS One. 2009;4:e7564. doi: 10.1371/journal.pone.0007564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jahn D, et al. A truncated lamin A in the Lmna −/− mouse line: implications for the understanding of laminopathies. Nucleus. 2012;3:463–74. doi: 10.4161/nucl.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kubben N, et al. Post-natal myogenic and adipogenic developmental: defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207. doi: 10.4161/nucl.2.3.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]