Abstract
Objective
The purpose of these experiments was to test the hypothesis that dietary phytoestrogens would diminish experimental aortic aneurysm formation.
Materials and methods
Six-week old C57BL/6 mice were divided into groups, fed either a diet with minimal phytoestrogen content or a regular commercial rodent diet with high phytoestrogen content for two weeks. At the eight week, aortic aneurysms were induced by infusing the isolated infra-renal abdominal aorta with 0.4% elastase for five minutes. Mice were recovered and the diameter of the infused aorta was measured at post-operative days 3, 7 and 14. Abdominal aorta samples were collected for histology, cytokine array and gelatin zymography after aortic diameter measurement. Blood samples were also collected to determine serum phytoestrogens and estradiol levels. Multiple-group comparisons were done using ANOVA with post hoc Tukey tests.
Results
Compared to mice on minimal phytoestrogen diet, mice on regular rodent diet had higher levels of serum phytoestrogens (male 1138±846 ng/dL, female 310±295 ng/dL). These serum phytoestrogen levels were also much higher than their own endogenous estradiol level (109 fold higher for males, and 35.5 fold higher for females). While aortic diameters of female mice were unaffected by the phytoestrogen concentration in the diets, male mice on regular rodent diet (M+ group) developed smaller aortic aneurysms than male mice on minimal phytoestrogen diet (M− group)on post-operative day 14 (M+ 54.8±8.8% versus M− 109.3±37.6%, P<0.001). During aneurysm development (post-operative days 3 and 7), there were fewer neutrophils, macrophages and lymphocytes in the aorta from the M+ group than from the M− group. Concentrations of multiple pro-inflammatory cytokines (MMPs, IL-1β, IL-6, IL-17, IL-23, MCP-1, RANTES, INF-γ, TNF-α) from aortas of M+ group were also lower than those from the aortas of M− group. Zymography also demonstrated that M+ group had lower levels of aortic MMP-9s than M− group on post-operative day 14 (P<0.001 for pro-MMP-9, P<0.001 for active MMP-9).
Conclusions
These results suggest that dietary phytoestrogens inhibit experimental aortic aneurysm formation in male mice via a reduction of the inflammatory response in the aorta wall. The protective effect of dietary phytoestrogens on aneurysm formation warrants further investigation.
Keywords: phytoestrogen, aortic aneurysm, mouse AAA model, aneurysm phenotype, inflammatory cytokine, MMP
Introduction
Abdominal aortic aneurysms (AAAs) are a gender-related disease with a prevalence of male to female ratio 4:1. Estrogens play a protective role in AAAs development (1–3). Phytoestrogens are plant-derived chemicals that are strikingly similar to estrogens both in structure and function. Therefore, the potential benefits and risks of phytoestrogen exposure have already attracted much attention (4–9). Major sources of phytoestrogens include soybeans, alfalfa, and flaxseed. Phytoestrogens are selective estrogen receptor modulators (SERMs) and have anti-inflammatory, anti-oxidant, and anti-proliferative properties (10–14). Animal experiments have demonstrated that phytoestrogens can reduce plasma cholesterol and attenuate atherosclerosis (15–17). However, little is known regarding phytoestrogens’ effects on aortic aneurysm formation. Therefore, we hypothesized that dietary supplementation with phytoestrogens might reduce inflammation in the aortic wall and thus inhibit aneurysm formation in an experimental model.
Materials and methods
1. Experimental design
Experiment 1 (Gender-based study): Thirty-two (n=16 male,16 female) 6-week old wild type C57BL/6 mice (Jackson Lab, Bar Harbor, Maine) were divided into four groups of 8 mice based on dietary phytoestrogen exposure to determine the influence of phytoestrogen content on aortic aneurysm formation. Thus, four groups were evaluated: (1) male mice fed with minimal phytoestrogen diet (M−), (2) male mice fed regular diet (M+), (3) female mice fed minimal phytoestrogen diet (F−), and (4) female mice fed regular diet (F+). The isoflavone content, one of the major classes of phytoestrogens, ranged from non-detectable to20 mg/kg for minimal phytoestrogen diet (2016 Teklad Grobal 16% Protein Rodent Diet), while the regular diet (7012 Teklad LM485 Mouse/Rat Diet) had between 300 to 500mg/kg. The other ingredients in the both diets were similar (see www.harlan.com). Both rodent diets were commercially available and obtained on April 2011. Two weeks after mice were placed on the diets, AAAs were induced surgically (18–19).
Briefly, infra-renal abdominal aorta was isolated and infused in situ with porcine pancreatic elastase (0.4 u/ml, Sigma) for 5 minutes at a pressure of 100mmHg. Elastase solution was evacuated and the mice were allowed to recover. Mice abdominal aortic diameters (n=8/group) were measured immediately after infusion to ensure similar dilation. On post-operative day 14, the infra-renal abdominal aorta was dissected and maximal aortic diameter was measured using video microscopy with NIS-Elements D.3.10 software attached to the microscope (Nikon SMX-800, Melville, NY). Aortic dilation was determined using (maximal aortic diameter – internal control diameter)/maximal aortic diameter *100%. The internal control diameter was the diameter of un-infused infra-renal aorta just above the infused section. A dilation of 50% or more was considered to be positive for AAA formation. All measurements were performed while the animal was alive. Blood samples were collected immediately after aorta diameter measurement. Aorta samples were harvested for protein analysis (n=5/group) and histological studies (n=3/group). Serum samples were used to determine phytoestrogens and estradiol levels.
Experiment 2 (Time course study): Six-week-old wild type C57BL/6 male mice (n=24) were divided into 2 groups based on diet. As above, one group was fed with the minimal phytoestrogen 2016 diet, while the other group was maintained on the phytoestrogen-rich 7012 diet. At 8 weeks of age, all mice were underwent elastase infusion. To evaluate AAA formation at earlier time points, six mice in each group were evaluated at post-operative day 3 and 7. After aortic diameters were measured (n=6/group), samples were collected for histology (n=3/group) and protein analysis (n=3/group).
All experiments were conducted in accordance with standards approved by the Animal Care and Use Committee of University of Virginia (IACUC #3848).
2. Mass spectrometry
Mass spectrometry was performed on serum samples to identify and quantify four common phytoestrogens in serum. Four standards were used in the test (Sigma, St Louis, MO. Purity: diadzein ≥ 97%, genistein ≥ 98%, equol ≥ 99% and coumestrol ≥ 95%). The test used a LC-MS system, which consisted of a Thermo Electron TSQ Quantum Access MAX mass spectrometer system with a Protana nanospray ion source interfaced to a self-packed 8 cm × 75um id Phenomenex Jupiter 10 um C18 reversed-phase capillary column. Samples were prepared as published (20). Dry samples were re-suspended using 5% ethanol/water to a volume equal to 25% of the plasma volume processed. Samples were vortexed and spun at 14 K rpm. A 5uL aliquot of each extract was injected and four compounds eluted from the column by an acetonitrile/0.1 M acetic acid gradient at a flow rate of 0.5µL/min over 0.5 hour. Nanospray ion source was operated at −3 kV (negative ions). Data was analyzed using the peak area (MPM) for each compound in each sample and comparing it to areas obtained for standard curves generated at the start and end of the sample set. The limit of detection (LOD) and limit of quantification (LOQ) was 0.5 fmol/ml and 5 fmol/ml, respectively.
3. Radioimmunoassay
To determine circulating estradiol concentration in the serum, a 200 µl aliquot of each serum sample was used for radioimmunoassay with commercial kit from Siemens Healthcare Diagnostic INC. (Tarrytown, NY). TKE21 kit was used to determine the serum estradiol levels according to the manufacturer’s instructions at the Ligand Assay and Analysis Core Facility at the University of Virginia Center for Research in Reproduction.
4. Histology and immunohistochemical stainings
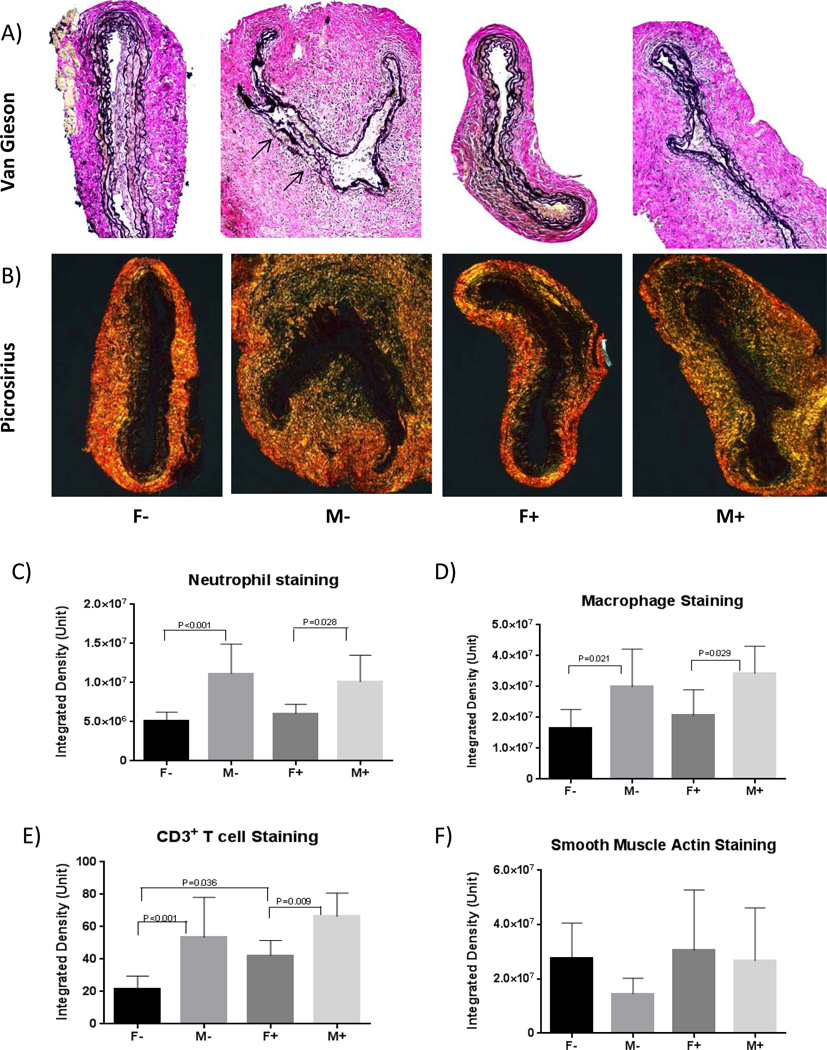
Aortic specimens were fixed in 4% buffered formaldehyde for 24 hours, transferred to 70% ethanol, and subsequently embedded in paraffin. Aortic cross-sections were stained with Verhoeff-Van Gieson and picrosirius red staining for elastin and collagen, respectively. Immunohistochemical stainings for neutrophils, migratory macrophages, CD3+ T cells and smooth muscle actin (αSMA) were performed as published (21–23). For grading, integrated optical density value of positive staining area of each section was randomly selected and measured. Four randomly selected 20× fields per aneurysm section were assessed for the density of staining by blinded observers.
5. Angiogenesis and cytokines arrays
Protein from aortic samples were isolated and pooled protein (30 µg) from each group was used in proteome profiler mouse cytokine array and proteome profiler mouse angiogenesis array according to manufacturer’s instruction (R & D Systems, cat# ARY 006, ARY 015). All samples were run in duplicate and mean value was used to represent each cytokine level.
6. Zymography
Using aortic protein isolated from the samples, gelatin zymography was performed to determine the amounts of MMP-2 and MMP-9. Precast zymography gels (Invitrogen) were loaded with 3µg of tissue protein from each aortic sample diluted into 2× Tris-glycine SDS sample buffer and electrophoretically separated under non-reducing conditions. The gels were renatured for 30 min in renaturing buffer (Invitrogen) and incubated in developing buffer (Invitrogen) for 24 hours at 37 °C rocker. Then, the gels were stained in Simply Blue Safe Stain (Invitrogen). Pro and active forms of MMP-9 and MMP-2 appeared as clear band against the blue background. Quantification was determined according the optical density using Bio-Rad Image Lab Software version 4.0.
7. Statistical analysis
The overall difference among groups, together with gender and diet factor were determined using two-way ANOVA. Post hoc Tukey’s test were used for multiple comparisons between groups. (PRISM 5, GraphPad Software, La Jolla, CA). Data are presented as mean ± standard deviation. P value less than 0.05 was considered statistically significant.
Results
1. Reduced AAA diameter in male mice on phytoestrogen-rich diet
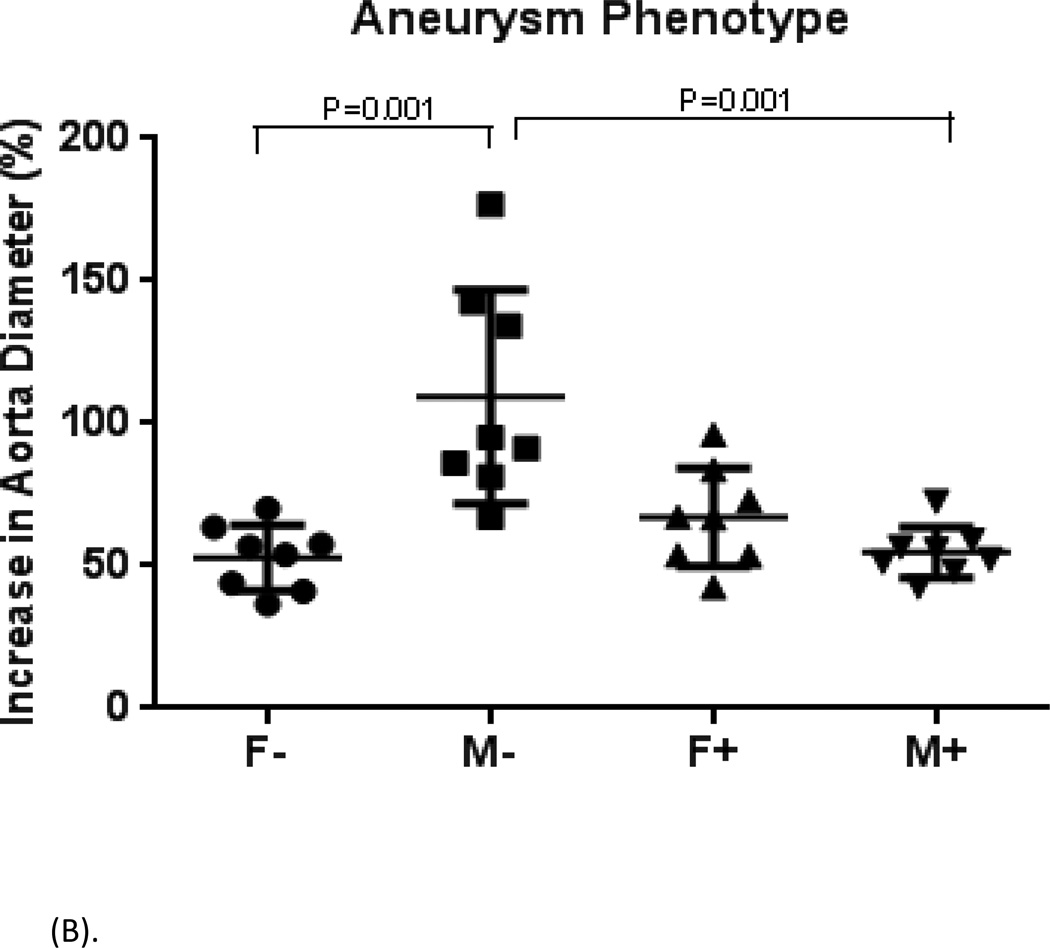
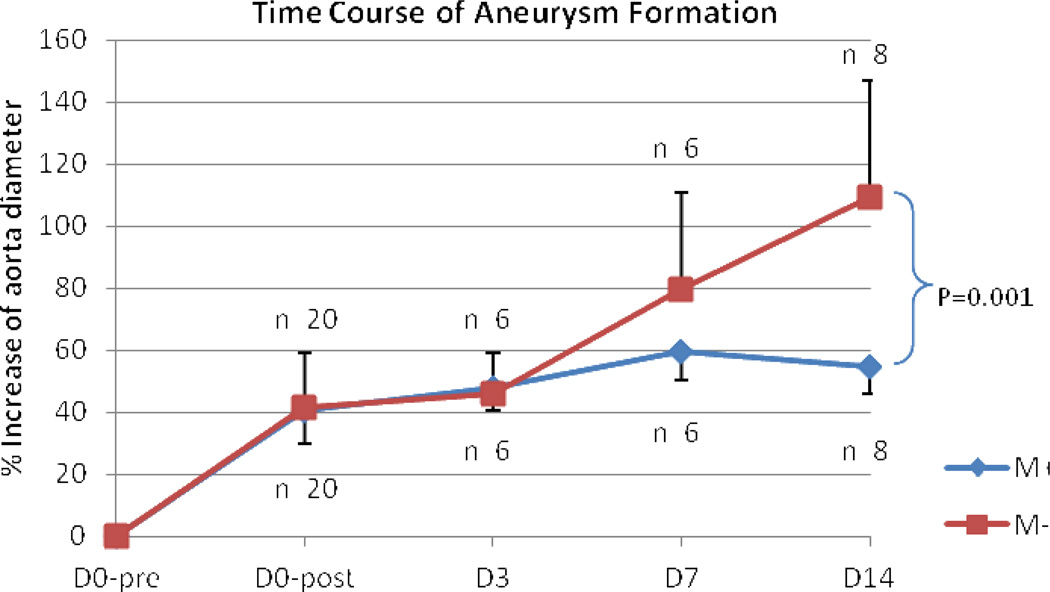
In minimal phytoestrogen diet groups, aortic dilation was significant greater in male mice compared to females (M− 109.3 ± 37.6% versus F− 52.9 ± 11.5%, P<0.001); In phytoestrogen-rich diet groups, this difference was negated. The aortic dilation of male mice was similar to that of female mice (M+ 54.8 ± 8.8% versus F+ 67.1 ± 17.4%, P=0.68). Comparing the two male groups on different diets, mice on phytoestrogen-rich diet had significant smaller aortic dilation than male mice on minimal phytoestrogen diet (M+ 54.8 ± 8.8% versus M− 109.3 ± 37.6%, P<0.001). M+ group also had decreased incidence of AAA (75%) compared with M− group (100%). There were no statistical differences between two female groups either in aortic dilation or in AAA incidence (Figure 1A, 1B). Aortic dilation increased over time following elastase perfusion. Aortic dilations were similar in male mice fed minimal phytoestrogen diet or phytoestrogen rich diet at days 3 and 7. Between days 7 and 14, dilation progressed in male mice fed minimal phytoestrogen diet, while further dilation was halted in male mice fed phytoestrogen rich diet (Figure 2).
Figure 1.
(A). Comparison of infra-renal aorta diameter. On post-operative day 14, the dilation of male mice on minimal phytoestrogen diet (M−) was more obvious than the other groups (M+, F−, F+).
(B). Comparison of infra-renal abdominal aortic dilation on post-operative day 14. N=8 mice/group. Male mice on minimal phytoestrogen diet (M−) had larger dilation than those of other groups.
Figure 2.
Comparison of infre-renal abdominal aorta dilation of male mice on different diets. Male mice on phytoestrogen-rich diet (M+) failed to dilated during the 2nd week compared with male mice on minimal phytoestrogen diet (M−). D0-pre means day 0 before infusion. D0-post means day 0 after infusion. D 3,7 and 14 mean day 3, day 7 and day 14 after infusion, respectively.
2. Significant higher level of serum phytoestrogens in mice on phytoestrogen-rich diet
At post-operative day 14, phytoestrogen levels were non-detectable in both groups of mice on minimal phytoestrogen diet (M− and F−), while the other two groups on phytoestrogen-rich diet had much higher levels of total phytoestrogen in their sera (M+ 1138±846 ng/dL, F+ 310±295 ng/dL). The predominant phytoestrogen was equol (M+ 87.3%, F+ 65.5%).The total serum phytoestrogen levels of M+ and F+ groups were significantly higher than their own endogenous estradiol levels, respectively (M+ 109 fold, F+ 35.5 fold). (Table 1)
Table 1.
Serum phytoestrogens and endogenous estrogen
| Group | Gender | Diet | Coumestrol | Diadzein | Genistein | Equol | Total phytoestrogen | Estradiol |
|---|---|---|---|---|---|---|---|---|
| F− | Female | 7012 Teklad | ND | ND | ND | ND | ND | 10.11±1.16 |
| M− | Male | 7012 Teklad | ND | ND | ND | ND | ND | 11.74±0.77 |
| F+ | Female | 2016 Teklad | ND | 63±99 | 44±45 | 203±152 | 310±295 | 8.74±1.44 |
| M+ | Male | 2016 Teklad | ND | 85±66 | 60±40 | 993±794 | 1138±846 | 10.40±1.04 |
All units were ng/dL; ND= non-detectable; Eight mice per group. In mice on phytoestrogen-rich diet (2016 Teklad), the total serum phytoestrogen levels were much higher than their endogenous estradiol levels (109 fold in male and 35.5 fold in female).
3. Decreased inflammatory cell infiltration in the early stages of AAA formation in male mice on phytoestrogen-rich diet
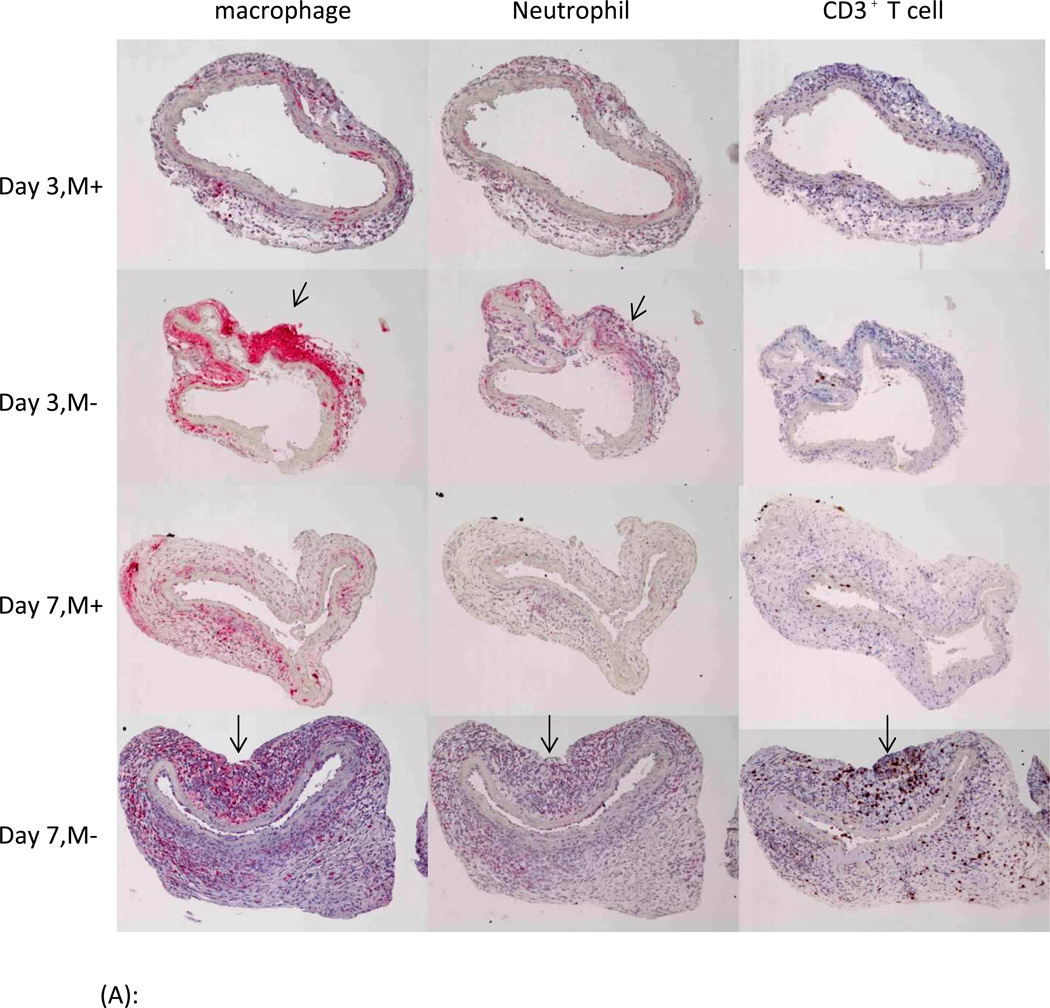
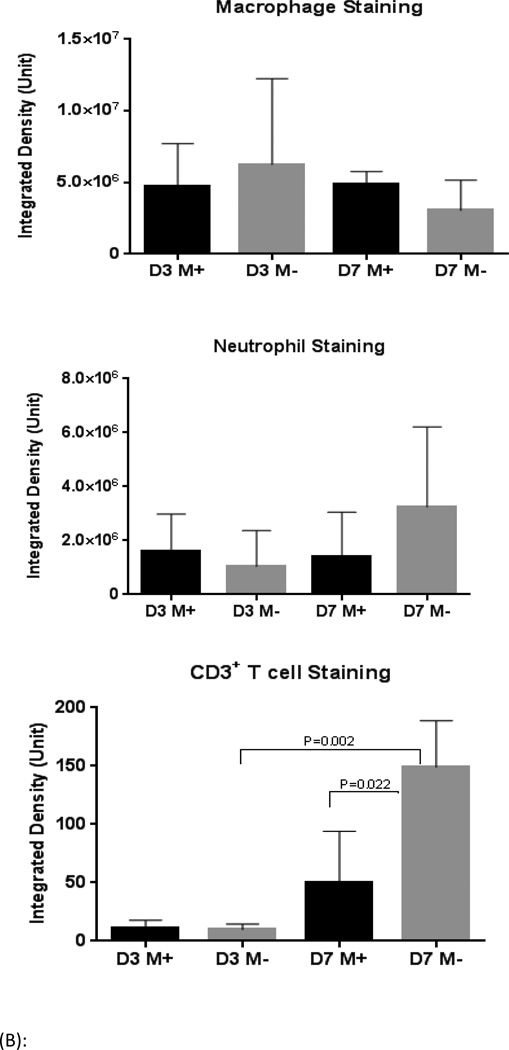
Compared with M− group, male aortas from mice fed phytoestrogen rich diet (M+) had fewer lymphocytes on post-operative day 7 (P=0.02). The phytoestrogen rich diet group also had fewer neutrophils on post-operative day 7 and fewer macrophages on post-operative day 3 (Figure 3A, 3B). By post-operative day 14, aorta samples of M− group demonstrated obvious elastin fragmentation and disorganization, increased collagen deposition in the media and adventitia of aorta; while the elastin and collagen contents in M+ group were relatively intact, similar to the female groups (Figure 4 A, B). Male groups still contained more inflammatory cells in the aorta compare with the female groups on post-operative day 14. However, no diet-related differences in inflammatory cell staining were found at this time point (Figure 4 C, D, E). There were no differences in smooth muscle actin-α staining among groups.
Figure 3.
(A): Representative immunohistochemitry samples of post-operative day 3 & 7 demonstrating macrophage, neutrophil and lymphocyte infiltration in aorta. Macrophage and neutrophil were red, CD3+ T cell was brown in their respective staining. On postoperative day 3 and 7, there were more inflammatory cells in M− group than in M+ group (marked by arrows).
(B): Quantification of immunohistochemistry evaluatiing neutrophil, macrophage and T cell in aorta samples of post-operative day 3 & 7. N=3 mice/group. M− group had more T cells in aorta than M+ group. D3 and D7 mean post-operative day 3 and 7, respectively.
Figure 4.
Representative samples of post-operative day 14, demonstrating elastin fragmentation (Van Gieson) and collagen disruption (Picrosirius red) in M− group (A–B). Quantification of immunohistochemistry evaluating neutrophils, macrophages, T-cells, and smooth muscle cells (C–F). N=3 mice/group.
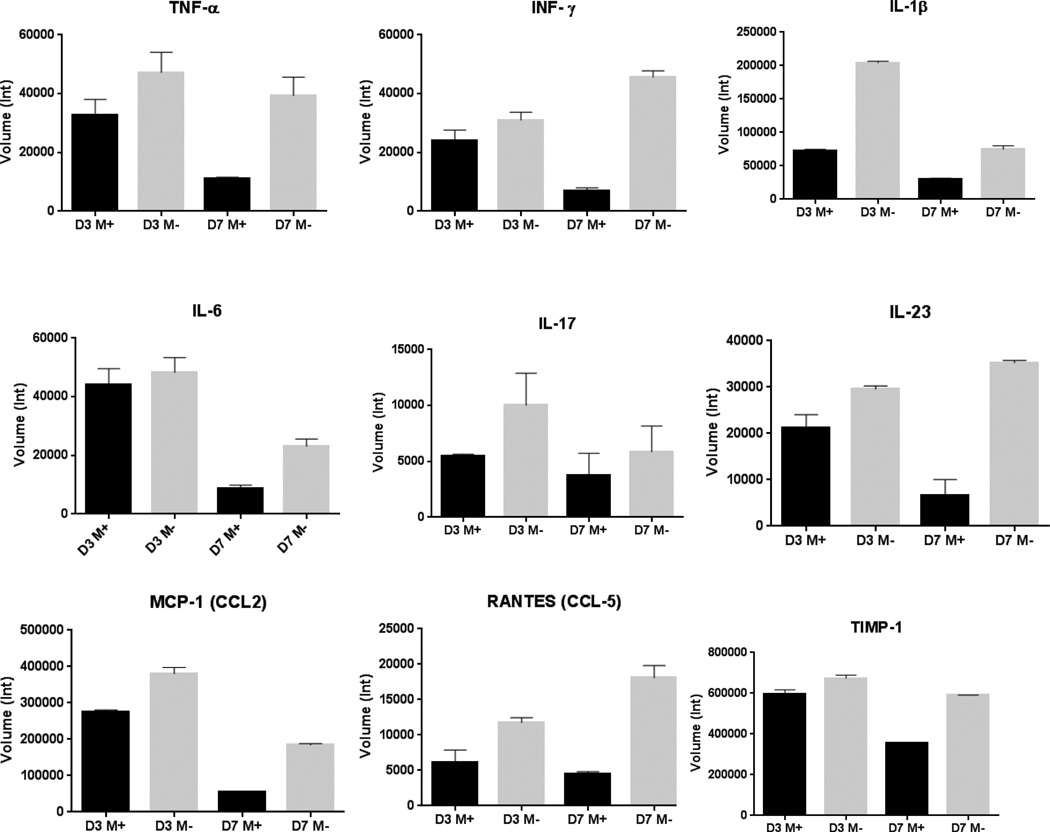
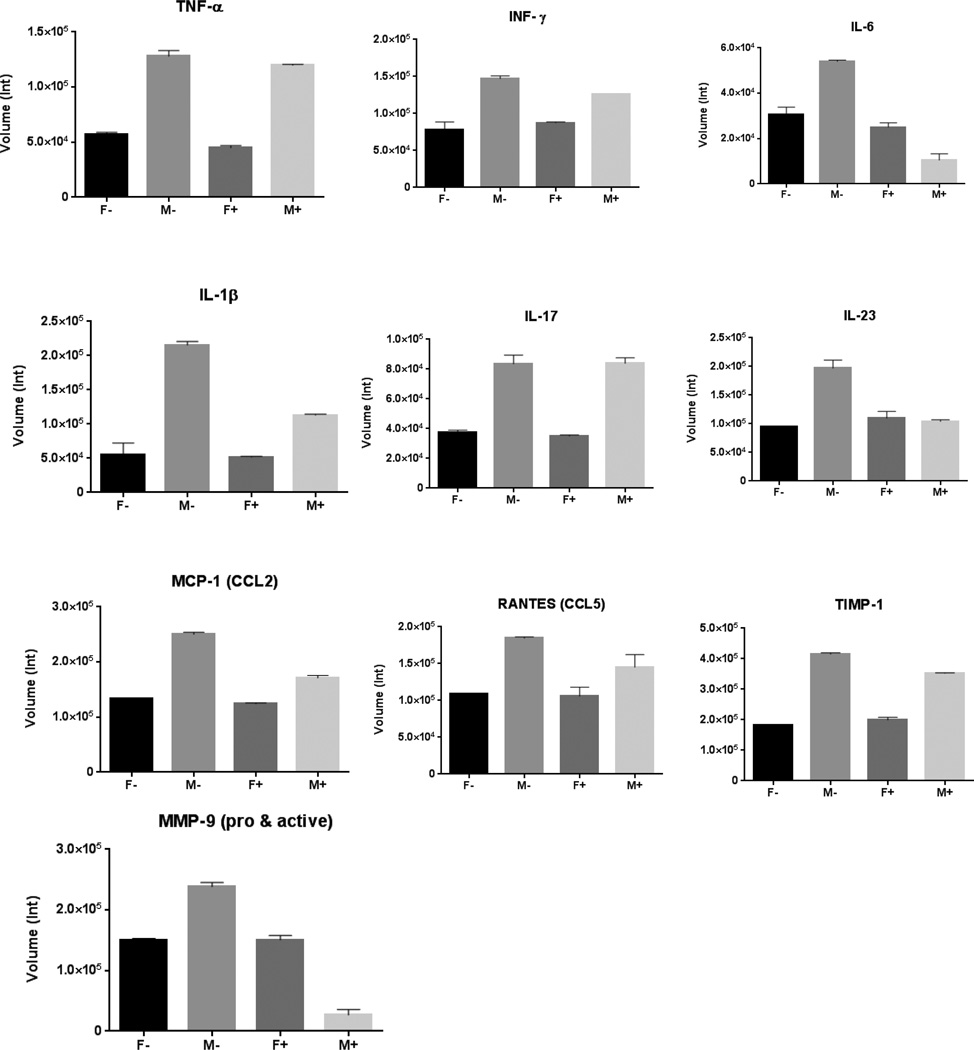
4. Lower inflammatory cytokine levels in the aorta of male mice on phytoestrogen-rich diet
Several dozen cytokines of interest and MMPs were analyzed in the study. According to the arrays result, the following matrix metalloproteinases and cytokines were found more closely related with aneurysm formation in this animal model: Matrix metalloproteinases-9 (MMP-9), tissue inhibitors of metalloproteinases-1 (TIMP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-17 (IL-17), interleukin-23 (IL-23), monocyte chemoattractant protein-1 (MCP-1), regulated on activation, normal T cell expressed and secreted (RANTES), tumor necrosis factor-α (TNF-α) and interferon-γ (INF-γ) were all altered. The array analysis indicated that most of the above MMPs and inflammatory cytokines were up-regulated in M− group at post-operative day 3, 7 or 14, while M+ group had relatively lower levels of the above proteins in their aortas (Table 2, Figure 5 and Figure 6).
Table 2.
Up-regulated cytokine levels in M− group at different time points
| Cytokine | Day 3 | Day 7 | Day 14 |
|---|---|---|---|
| TNF-a | 0.45 | 2.58 | 0.07 |
| INF-r | 0.29 | 5.63 | 0.17 |
| IL-1β | 1.81 | 1.48 | 0.91 |
| IL-6 | 0.09 | 1.70 | 4.15 |
| IL-17 | 0.82 | 0.56 | 0.00 |
| IL-23 | 0.40 | 4.36 | 0.89 |
| MCP-1 | 0.39 | 2.36 | 0.46 |
| RANTES | 0.93 | 2.99 | 0.27 |
| TIMP-1 | 0.13 | 0.66 | 0.18 |
| MMP9 (pro & active) | 7.97 | ||
Note: All numbers are the fold of increase compared with M+ group.
M− : male mice on minimal phytoestrogeb diet.
M+: male mice on phytoestrogen-rich diet.
Figure 5.
Cytokine array of mouse aorta samples of post-operative day 3 & 7. Three pooled samples per group. Multiple inflammatory cytokines were up-regulated in M− group compared with M+ group on the same experiment end point. D3,D7 mean post-operative day 3 and 7,respectively. M+ means male mice on phytoestrogen-rich diet; M− means male mice on minimal phytoestrogen diet.
Figure 6.
Cytokine array of post-operative day 14 mouse aorta samples. Five pooled samples/group. IL-6, IL-1β, IL-23, MCP-1 and MMP-9 were up-regulated in male mice on minimal phytoestrogen diet (M−) compared with M+ group. There were also gender differences in the levels of multiple inflammatory cytokines.
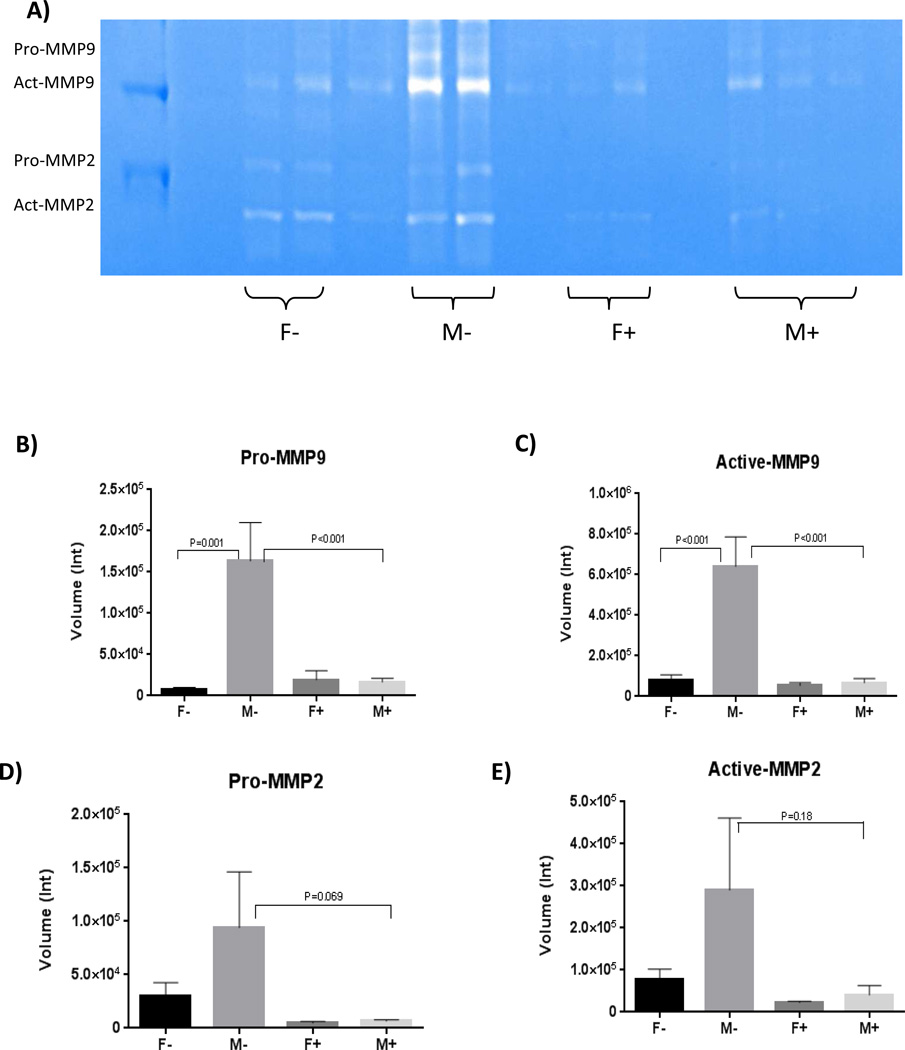
5. Less MMP-9s in the aorta of male mice on phytoestrogen-rich diet
MMPs are known to be critical in blood vessel matrix degradation and AAA formation. Therefore, gelatin zymography was used to further evaluate MMPs activities. On post-operative day 14, the male group fed phytoestrogen rich diet (M+) had much lower MMP-9s levels in their aortas than male group fed minimal phytoestrogen diet (M−) (P<0.001 for pro-MMP-9, P<0.001 for active MMP-9). Within the two groups of mice fed minimal phytoestrogen diet (F− versus M−), the female group also had less MMP-9s compared with the male group (P=0.001 for pro-MMP-9, P<0.001 for active-MMP-9) (Figure 7). MMP-2s had similar trends at post-operative day 14 as MMP-9s, but the differences were not significant. The present study found no differences in MMPs levels among all groups on post-operative day 3 and 7 (data not shown).
Figure 7.
Gelatin zymography of post-operative day 14 samples (A) demonstrating significant higher level of both pro and active form of MMP-9s in male mice on minimal phytoestrogen diet (B–C). There were similar trends for pro and active form of MMP-2s.
Discussion
In the present study, we tested four phytoestrogens that are most commonly found in a commercial rodent diet. Our study showed that mice on the regular commercial rodent diet had much higher levels of serum phytoestrogens than mice on the minimal phytoestrogen diet. The phytoestrogens in our study were mainly in the form of equol (87.3 % in male, 65.5% in female). Equol is the metabolite of daidzein with a longer half life and greater biological activity. We also tested endogenous serum estradiol levels in order to compare it with phytoestrogen levels in the blood. Our results showed that the total phytoestrogen levels in mice on the regular commercial rodent diet were alarmingly higher than their own endogenous estradiol levels (109 fold for male, 35.5 fold for female). Phytoestrogens come mainly from soybean in the diet. Phytoestrogen concentration in soybean varies from harvest to harvest, so does the phytoestrogen content in each batch of rodent diet. Unfortunately, not all investigators doing experimental AAA studies are aware of the influence of phytoestrogens on AAA formation. Our study demonstrated that it is critical to use minimal phytoestrogen diet in experimental AAA investigations.
Gender-related differences in aneurysm formation have been well documented in both human and animal studies. Male gender is a well-known risk factor for AAA development (24–26). Experiments with rodent AAA models have demonstrated that male animals develop a positive AAA phenotype compared with female animals (19, 27–28). In the present study, gender-related differences in AAA phenotype between the two groups of mice on minimal phytoestrogen diet (M− versus F− group) were clearly demonstrated (109.3 ± 37.6% versus 52.9 ± 11.5%, P<0.001). However, gender-related differences were obviated with phytoestrogen rich diets, as both male and female mice had similar aortic dilation. Both male and female mice on phytoestrogen rich diet had decreased aortic dilation in comparison with male mice on the minimal phytoestrogen diet. Male mice on phytoestrogen-rich diet (M+ group) had a much lower aortic dilation than male mice on minimal phytoestrogen diet (M− group) (54.8 ± 8.8% versus 109.3 ± 37.6%, P<0.001), while the aortic dilation of both female groups were low and similar. These results suggested that the phytoestrogen-rich diet blunted the aortic dilation that is normally seen in the male mice in the gender-related AAA study. Since female mice are protected against AAA formation, it is suspected that phytoestrogens would not have a further impact on AAA protection.
Statistic analysis showed that the interaction of gender and diet factor accounted for 31.33% of the total aortic dilation variance (F=19.56, P=0.0001). This interaction indicates that the male and female mice had different responses to the same diet factor. The gender factor in this experiment accounted for approximately 12.97% of the total variance in aortic dilation (F=8.094, P=0.0082). The effect is considered very significant. The diet factor in the experiment accounted for about 10.83% of the total variance (F= 6.760, P=0.0147) and the effect is considered significant.
When evaluating aneurysm formation over time, our results showed that phytoestrogen-rich diet did not make a significant difference in aortic dilation during the early stages of AAA (post-operative day 3 and 7). Male mice on minimal phytoestrogen diet gained much of their aorta dilation during the second week (P=0.012), which is one of the characteristics of the elastase-induced model (18). On the other hand, the aortic diameter of male mice on phytoestrogen-rich diet remained stable over the second half of the experimental model (P= 0.999), indicating the importance of diet. Diet factor in the time course experiment accounted for approximately 4.409% of all the variance (F=22.21, P<0.0001). However, the above results do not mean that dietary phytoestrogens affect only the later stage of aneurysm formation. Aorta dilation in the early stages of the model reflect mainly the mechanical pressure and elastase digestion on the blood vessel; while the later stages of the AAA model include aortic wall degradation caused by inflammation, which mimics the human aneurysm formation. The logical implication of this observation was that dietary phytoestrogens might reduce aneurysm formation through anti-inflammation properties.
In the elastase induced AAA model, the inflammation in aorta wall usually peaks around 5–7 days after surgery, then elastin and collagen disruption and remodeling of the aorta occurs. Our histology demonstrated that there was more inflammatory cell infiltration in the aortas of male mice on minimal phytoestrogen diet on post-operative day 7 compared to male mice fed a diet rich in phytoestrogens. On post-operative day 14, the histology showed increased elastin fragmentation and collagen deposition in the animals on minimal phytoestrogen diet. In contrast, male mice on the phytoestrogen-rich diet experienced less inflammation during the early stage of this model, indicating phytoestrogen’s anti-inflammatory effects. Also in this model, aortic dilation usually peaks around post-operative day 14, then, the aorta begins to heal. In the present study, there were still more inflammatory cells left in male samples than in female samples on post-operative day 14. Endogenous estrogen is believed to account for this gender-related difference. We are not sure why there were no diet–related differences at this time point, but hypothesized that the anti-inflammatory effects of phytoestrogen may be detected more easily at the peak of inflammation. By day 14, the acute injury is basically resolving, so gender-related differences at this particular point might have more influence on the recovery process, not in the formation of AAA.
To explore the mechanisms behind diet-related phenotypic differences, multiple MMPs and cytokines in aortic samples were examined. Although the function of each of these pro-inflammatory molecules in aneurysm formation have been studied extensively (27–37), little is known regarding how these inflammatory biomarkers in aorta are regulated in the presence of dietary phytoestrogens. In our study, each of these inflammatory cytokines in male mice on minimal phytoestrogen diet (M−) was up-regulated at different time points. These findings demonstrate that phytoestrogens impact AAA formation process through not one, but multiple cytokine pathways.
Of all the MMPs and cytokines evaluated in the present study, the most interesting ones were MMP-9/TIMP-1 pair. First, the results demonstrated that the rodent diets in the study have a significant impact on MMPs levels in the aorta. For pro-MMP9, diet factors accounted for approximately 22.55% of the total variance (F=9.90, P=0.0071), which was considered very significant. The diet factor for active-MMP9 was about 30.35% of the total variance (F=19.89. P=0.0005, extremely significant). The diet factor of pro-MMP2 and active-MMP2 was 24.6% (F=5.66, P=0.0321, significant) and 17.73% (F=3.75, P=0.0734, not significant), respectively. Second, on post-operative day 14, male mice on minimal phytoestrogen diet (M−) had significantly higher levels of MMP-9 activity, but the increase of TIMP-1 was not in proportion to the increase in MMP-9. This change in MMP/TIMP-1 ratio was consistent with the aortic remodeling time frame and the positive aneurysm phenotype of M− group. Considering MMPs’ critical role in matrix degradation, the high level of MMP-9 in the second week may play an important role in the aorta dilation in M− group.
The anti-inflammatory mechanisms of phytoestrogens are complicated. It could be estrogen receptor(ER)-dependent or ER-independent, or both. Phytoestrogens have the ability to bind both ER-α and ER-β, but with a preference for ER-β. These exogenous phytoestrogens may compete with endogenous estrogens on the same ERs. Although phytoestrogens’ affinities for ERs are weak, they can be present in large amounts in the blood. Depending on their concentrations, they can elicit estrogenic or anti-estrogenic effects (38–40). The ER-independent anti-inflammatory effect probably relies on phytoestrogen’s antioxidant, anti-proliferative and anti-angiogenic properties. Although the present study demonstrated that phytoestrogens reduced the inflammation in the aorta of male mice, the results did not indicate if this anti-inflammatory effect was ER dependent or ER-independent. Experiments with ER knock-out animals would be helpful to detail the mechanisms. Since MMPs play such an important role in AAA formation, using MMP-9 and TIMP-1 knockout mice in future experiments could also help us understand the relationship between phytoestrogen and MMPs. Other AAA animal models (e.g. angiotensin II model) would be useful to investigate the different mechanisms at play in a model with advanced inflammation.
In summary, the present study showed that dietary phytoestrogens inhibit male experimental aneurysm formation by reducing the inflammatory response in the aorta of male mice. Further studies should reveal more details of the mechanisms involved. Since phytoestrogen’s effect may be tissue specific, their selectivity may provide beneficial effect for AAA prevention without the side effects of traditional hormone therapy. Further studies should examine the potential of dietary phytoestrogens for AAA risk reduction in humans.
Acknowledgments
The authors would like to thank Erin D. Jeffery of W.M Keck Biomedical Mass Spectrometry Lab of University of Virginia for her skillful technical assistance with serum phytoestrogen tests. We also thank the university of Virginia Center for Research in Reproduction Ligand and Analysis Core, supported by the Eunice Kennedy Shriver NICHD/NIH (SCCPIR) Grant U54-HD28934, for conducting the serum sex hormone assays.
Source of Funding
This work was supported by NIH RO1 HL081629 (G.R.U).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Woodrum DT, Ford JW, Cho BS, Hannawa KK, Stanley JC, Henke PK, Upchurch GR., Jr Differential effect of 17-beta-estradiol on smooth muscle cell and aortic explant MMP2. J Surg Res. 2009 Jul;155(1):48–53. doi: 10.1016/j.jss.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin-McNulty B, Tham DM, da Cunha V, Ho JJ, Wilson DW, Rutledge JC, Deng GG, Vergona R, Sullivan ME, Wang Y-X. 17β-Estradiol attenuates development of angiotensin II–induced aortic abdominal aneurysm in apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1627–1632. doi: 10.1161/01.ATV.0000085842.20866.6A. [DOI] [PubMed] [Google Scholar]
- 3.Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS, Henke PK, Stanley JC, Eagleton MJ, Upchurch GR., Jr Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41(1):108–114. doi: 10.1016/j.jvs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Cassidy A. Potential risks and benefits of ohytoestrogen-rich diets. Int J Nutr Res. 2003 Mar;73(2):120–126. doi: 10.1024/0300-9831.73.2.120. [DOI] [PubMed] [Google Scholar]
- 5.van der Schouw YT, de Kleijn MJ, Peeters PH, Grobbee DE. Phytoestrogens and cardiovascular disease risk. Nutr Metab Cardiovasc Dis. 2000 Jun;10(3):154–167. [PubMed] [Google Scholar]
- 6.Hall WL, Vafeiadou K, Hallund J, Bugel S, Koebnick C, Reimann M, Ferrari M, Branca F, Talbot D, Dadd T, Nilsson M, Dahlman-Wright K, Gustafsson JA, Minihane AM, Williams CM. Soy-isoflavone-enchied foods and inflammartory biomarkers of cardiovascular disease risk in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr. 2005 Dec;82(6):1260–1268. doi: 10.1093/ajcn/82.6.1260. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JJ, Anthony M, Messina M, Garne SC. Effects of phyto-oestrogens on tissues. Nutr Res Rev. 1999 Jun;12(1):75–116. doi: 10.1079/095442299108728875. [DOI] [PubMed] [Google Scholar]
- 8.McCarty MF. Isoflavones made simple – genistein’s agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006;66(6):1094–1114. doi: 10.1016/j.mehy.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Jackman KA, Woodman OL, Sobey CG. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr Med Chem. 2007;14(26):2824–2828. doi: 10.2174/092986707782360178. [DOI] [PubMed] [Google Scholar]
- 10.Ondricek AJ, Kashyap AK, Thamake SI, Vishwanatha JK. A comparative study of phytoestrogen action in mitigating apoptosis induced by oxidative stress. In Vivo. 2012 Sep-Oct;26(5):765–775. [PubMed] [Google Scholar]
- 11.Tiulea C, Peev C, Brezovan D, Dehelean C, Motoc A. A comparison regarding antiproliferative action between soy total extract and genistein. Rom J Morphol Embryol. 2011;52(3 Suppl):1065–1069. [PubMed] [Google Scholar]
- 12.Fotsis T, Pepper MS, Montesano R, Aktas E, Breit S, Schweigerer L, Rasku S, Wahala K, Adlercreutz H. Baillieres Phytoestrogens and inhibition of angiogenesis. Clin Endocrinol Metab. 1998 Dec;12(4):649–666. doi: 10.1016/s0950-351x(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Zhu J, Mi M, Chen W, Pan Q, Wei M. Anti-angiogenic genistein inhibits VEGF-induced endothelial cell activation by decreasing PTK activity and MAPK activation. Med Oncol. 2012 Mar;29(1):349–357. doi: 10.1007/s12032-010-9770-2. [DOI] [PubMed] [Google Scholar]
- 14.Xu JX, Zhang Y, Zhang XZ, Ma YY. Anti-angiogenic effects of genistein on synovium in a rat model of type II collagen-induced arthritis. Zhong Xi Yi Jie He Xue Bao. 2011 Feb;9(2):186–193. doi: 10.3736/jcim20110212. [DOI] [PubMed] [Google Scholar]
- 15.Kirk EA, Sutherland P, Wang SA, Chait A, LeBoeuf RC. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J Nutr. 1998 Jun;128(6):954–959. doi: 10.1093/jn/128.6.954. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi J, Piskula MK, Izumi T, Tobe K, Saito M, Kataoka S, Obata A, Kikuchi M. Isoflavone aglycone-rich extract without soy protein attenuates atherosclerosis development in cholesterol-fed rabbits. J Nutr. 2000 Aug;130(8):1887–1893. doi: 10.1093/jn/130.8.1887. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clinic Endoc & Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 19.DiMusto PD, Lu G, Ghosh A, Roelofs KJ, Su G, Zhao Y, Lau CL, Sadiq O, McEvoy B, Laser A, Diaz JA, Wakefield TW, Henke PK, Eliason JL, Upchurch GR., Jr Increased PAI-1 in females compared with males is protective for abdominal aortic aneurysm formation in a rodent model. Am J Physiol Heart Circ Physiol. 2012 Apr 1;302(7):H1378–H1386. doi: 10.1152/ajpheart.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morandi S, Locati D, Ferrario F, Chiesa G, Arnoldi A. A simple method for the characterization and quantification of soy isoflavone metabolites in the serum of MMTV-Neu mice using high-performance liquid chromatography/electrospray ionization mass spectrometry with multiple reaction monitoring. Rapid Commun Mass Spectrom. 2005;19:153–161. doi: 10.1002/rcm.1760. [DOI] [PubMed] [Google Scholar]
- 21.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, Linden J. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Ann Thorac Surg. 2009;88(4):1071–1078. doi: 10.1016/j.athoracsur.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, LaPar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, Linden J, Lau CL. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29(12):1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Xiao A, diPierro CG, Carpenter JE, Abdel-Fattah R, Redpath GT, Lopes MB, Hussaini IM. An extensive invasive intracranial human glioblastoma xenograft model: role of high level matrix metalloproteinase 9. Am J Pathol. 2010;176(6):3032–3049. doi: 10.2353/ajpath.2010.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederle FA, Johnson GR, Wilson SE. Abdominal aortic aneurysm in women. J Vasc Surg. 2001;34(1):122–126. doi: 10.1067/mva.2001.115275. [DOI] [PubMed] [Google Scholar]
- 25.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25(3):561–568. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson H, Sonesson B, Bergqvist D. Incidence and prevalence of abdominal aortic aneurysms, estimated by necropsy studies and population screening by ultrasound. Ann N Y Acad Sci. 1996;800:1–24. doi: 10.1111/j.1749-6632.1996.tb33294.x. [DOI] [PubMed] [Google Scholar]
- 27.DiMusto PD, Lu G, Ghosh A, Roelofs KJ, Sadiq O, McEvoy B, Su G, Laser A, Bhamidipati CM, Ailawadi G, Henke PK, Eliason JL, Upchurch GR., Jr Increased JNK in males compared with females in a rodent model of abdominal aortic aneurysm. J Surg Res. 2012 Aug;176(2):687–959. doi: 10.1016/j.jss.2011.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, Lu G, Henke PK, Stanley JC, Weiss SJ, Thompson RW, Upchurch GR., Jr Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004 Nov;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 29.Sharma AK, Lu G, Jester A, Johnston WF, Zhao Y, Hajzus VA, Saadatzadeh MR, Su G, Bhamidipati CM, Mehta GS, Kron IL, Laubach VE, Murphy MP, Ailawadi G, Upchurch GR., Jr Experimental abdominal aortic aneurysm formation is mediated by IL-17 and attenuated by mesenchymal stem cell treatment. Circulation. 2012 Sep 11;126(11) Suppl 1:S38–S45. doi: 10.1161/CIRCULATIONAHA.111.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR, Jr, Ailawadi G. Genetic and pharmacologic disruption of interleukin-1β signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ailawadi G, Knipp BS, Lu G, Roelofs KJ, Ford JW, Hannawa KK, Bishop K, Thanaporn P, Henke PK, Stanley JC, Upchurch GR., Jr A nonintrinsic regional basis for increased infrarenal aortic MMP-9 expression and activity. J Vasc Surg. 2003 May;37(5):1059–1066. doi: 10.1067/mva.2003.163. [DOI] [PubMed] [Google Scholar]
- 32.Moehle CW, Bhamidipati CM, Alexander MR, Mehta GS, Irvine JN, Salmon M, Upchurch GR, Jr, Kron IL, Owens GK, Ailawadi G. Bone marrow-derived MCP1 required for experimental aortic aneurysm formation and smooth muscle phenotypic modulation. J Thorac Cardiovasc Surg. 2011 Dec;142(6):1567–1574. doi: 10.1016/j.jtcvs.2011.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrlichman LK, Ford JW, Roelofs KJ, Tedeschi-Filho W, Futchko JS, Ramacciotti E, Eliason JL, Henke PK, Upchurch GR., Jr Gender-dependent differential phosphorylation in the ERK signaling pathway is associated with increased MMP2 activity in rat aortic smooth muscle cells. J Surg Res. 2010 May 1;160(1):18–24. doi: 10.1016/j.jss.2009.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR., Jr Female gender attenuates cytokine and chemokine expression and leukocyte recruitment in experimental rodent abdominal aortic aneurysms. Ann N Y Acad Sci. 2006 Nov;1085:367–379. doi: 10.1196/annals.1383.027. [DOI] [PubMed] [Google Scholar]
- 35.Sinha I, Hannawa KK, Eliason JL, Ailawadi G, Deogracias MP, Bethi S, Ford JW, Roelofs KJ, Grigoryants V, Henke PK, Stanley JC, Upchurch GR., Jr Early MT-1 MMP expression following elastase exposure is associated with increased cleaved MMP-2 activity in experimental rodent aortic aneurysms. Surgery. 2004 Aug;136(2):176–182. doi: 10.1016/j.surg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Colonnello JS, Hance KA, Shames ML, Wyble CW, Ziporin SJ, Leidenfrost JE, Ennis TL, Upchurch GR, Jr, Thompson RW. Transient exposure to elastase induces mouse aortic wall smooth muscle cell production of MCP-1 and RANTES during development of experimental aortic aneurysm. J Vasc Surg. 2003 Jul;38(1):138–146. doi: 10.1016/s0741-5214(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 37.Eagleton MJ, Peterson DA, Sullivan VV, Roelofs KJ, Ford JA, Stanley JC, Upchurch GR., Jr Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res. 2002 May 1;104(1):15–21. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- 38.Johnston I, Williamson G. Phytochemical Functional Foods. CRC Press, Inc; 2003. [Google Scholar]
- 39.Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytotherapy Research. 2003;17:845–869. doi: 10.1002/ptr.1364. [DOI] [PubMed] [Google Scholar]
- 40.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]