Abstract
Purpose
D,L-Sulforaphane (SFN) is a promising chemopreventive agent with in vivo efficacy against prostate cancer in experimental rodents. This study was undertaken to determine the role of vimentin and plasminogen activator inhibitor-1 (PAI-1) in anti-cancer effects of SFN.
Methods
Effect of SFN on levels of different proteins was determined by western blotting or immunofluorescence microscopy. RNA interference of vimentin and PAI-1 was achieved by transient transfection. Apoptosis was quantified by flow cytometry. Transwell chambers were used to determine cell migration.
Results
Exposure of PC-3 and DU145 human prostate cancer cells to SFN resulted in induction of vimentin protein, which was accompanied by down-regulation of E-cadherin protein expression. The SFN-mediated induction of vimentin was also observed in a normal human prostate epithelial cell line. RNA interference of vimentin did not have any appreciable effect on early or late apoptosis resulting from SFN exposure. On the other hand, SFN-mediated inhibition of PC-3 and DU145 cell migration was significantly augmented by knockdown of the vimentin protein. Knockdown of vimentin itself was inhibitory against cell migration. The SFN-treated cells also exhibited induction of PAI-1, which is an endogenous inhibitor of urokinase-type plasminogen activator system. Similar to vimentin, PAI-1 knockdown resulted in a modest augmentation of PC-3 cell migration inhibition by SFN. Tumors from SFN-treated Transgenic Adenocarcinoma of Mouse Prostate mice showed a 1.7-fold increase in vimentin protein level compared with control tumors.
Conclusion
The present study indicates that vimentin and PAI-1 inductions confer modest protection against SFN-mediated inhibition of prostate cancer cell migration.
Keywords: Sulforaphane, Vimentin, Prostate Cancer, Chemoprevention
Introduction
D,L-Sulforaphane (SFN) is a promising chemopreventive agent with in vivo activity in rodent cancer models [1, 2]. SFN occurs naturally as L-isomer in edible cruciferous vegetables such as broccoli [3]. Research interest in anti-cancer effects of SFN and other structurally-related small molecules (eg, phenethyl isothiocyanate) was initially sparked by data from population-based case-control studies suggesting an inverse association between dietary intake of cruciferous vegetables and the risk of different cancers including cancer of the prostate [1, 4, 5]. Chemopreventive effect of SFN was first documented against 9,10-dimethyl-1,2- benzanthracene-induced breast cancer in rats [6]. Cancer chemopreventive efficacy of SFN was subsequently extended to other chemical carcinogens [7, 8]. For example, SFN administration (both pre- and post-initiation) resulted in suppression of azoxymethane-induced colonic aberrant crypt foci in rats [7]. Previous studies from our laboratory have shown that oral administration of 6 μmol SFN (three times per week) inhibited incidence and burden of prostatic intraepithelial neoplasia and/or well-differentiated prostate cancer as well as pulmonary metastasis multiplicity in Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) mice without causing any side effects [9]. Consistent with these data, TRAMP mice fed with 240 mg of broccoli sprouts/day exhibited a significant decrease in prostate tumor growth in another study [10]. We have also shown previously that growth of PC-3 human prostate cancer cells subcutaneously implanted in male athymic mice is retarded significantly after oral treatment with SFN [11].
Cellular systems, including prostate cancer cells, have been utilized to elucidate the mechanisms underlying anti-cancer effects of SFN. Mechanisms potentially contributing to SFN-mediated inhibition of pre-initiation and post-initiation cancer development include inhibition of CYP2E1, cell cycle arrest, apoptosis induction, suppression of angiogenesis, inhibition of histone deacetylase, and epigenetic repression of human telomerase reverse transcriptase [12–19]. SFN is capable of inhibiting various oncogenic pathways, including nuclear factor-κB (NF-κB), androgen receptor, and signal transducer and activator of transcription 3 [20–23]. Interestingly, SFN treatment causes activation of Notch signaling in human prostate cancer cells, but Notch activation is largely dispensable for cellular effects of SFN [24].
Previous studies from our laboratory have indicated that benzyl isothiocyanate, which is a structural analogue of SFN, is a potent inhibitor of epithelial-mesenchymal transition (EMT) in human breast cancer cells [25]. Because EMT is associated with aggressiveness of cancers [26], the present study was undertaken to determine whether anti-cancer effect of SFN in prostate cancer cells involves inhibition of the EMT phenotype.
Materials and methods
Ethics statement
Tumor tissues from our published study [9] were used to determine the effect of SFN on expression of vimentin and PAI-1 proteins in vivo. Use of mice and their care was in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee guidelines.
Reagents
Stock solution of SFN was prepared in dimethyl sulfoxide (DMSO), stored at −20°C, and diluted with fresh complete media immediately before use. Same volume of DMSO (final concentration 0.1%) was added to the controls. Cell culture reagents including fetal bovine serum, antibiotics mixture, phosphate-buffered saline (PBS), and trypsin were purchased from Invitrogen-Life Technologies (Grand Island, NY). Antibodies against vimentin and actin were purchased from Sigma-Aldrich (St Louis, MO). An antibody against E-cadherin was from BD Biosciences (San Diego, CA), whereas antibodies against snail, slug, Zeb1, and plasminogen activator inhibitor-1 (PAI-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Vimentin and PAI-1 targeted small-interfering RNA (siRNA), and anti-vimentin antibody used for immunofluorescence microscopy were from Santa Cruz Biotechnology. A kit for flow cytometric analysis of apoptosis was from BD Biosciences. Transwell Permeable chambers used for cell migration assay were purchased from Corning (Corning, NY).
Cell lines
PC-3 and DU145 cell lines were obtained from the American Type Culture Collection (Manassas, VA) and cultured according to the supplier’s instructions. A normal human prostate stromal cell line (PrSC) and a normal human prostate epithelial cell line (PrEC) were purchased from Lonza (Walkersville, MD) and cultured according to provider’s recommendations.
Western blotting
After treatment, cells were collected and lysed as described by us previously [27]. The western blotting experiments for each protein were performed using independently prepared lysates from 3 (PC-3) or 2 (DU145, PrEC, PrSC) different experiments. Actin normalization was done for each individual experiment. Immunoreactive bands were visualized using enhanced chemiluminescence method [27]. Actin bands are the same for some western blots due to multiplexing (stripping and reprobing). Densitometric quantitation was done using UN-SCAN-IT software version 5.1 (Silk Scientific Corporation, Orem, UT).
Immunofluorescence microscopy for E-cadherin and vimentin
Cells (0.5 × 104 cells/ml) were cultured on coverslips in 12-well plates, allowed to attach by overnight incubation, and then exposed to DMSO (control), or SFN (10 or 20 μM) for 8 or 24 h. The cells were fixed with 2% paraformaldehyde for 1 h, permeabilized with PBS containing 0.05% Triton X-100 for 10 min, and blocked with 0.5% bovine serum albumin in PBS for 1 h at room temperature. Cells were incubated overnight at 4°C or at room temperature for 1 hour with anti-E-cadherin or anti-vimentin antibody, washed with PBS, and incubated with Alexa Fluor 568-conjugated secondary antibody for 1 h at room temperature. Nuclei were stained with 4′,6 diamidino-2-phenylindole (200 μg/ml; 10 min at room temperature). Cells were washed twice with PBS, mounted, and examined under a Leica DC300F fluorescence microscope. Images were captured at 100 × magnification.
RNA interference
PC-3 or DU145 cells (1 × 105 cells/well) were seeded in 6-well plates 1 day before transfection. The cells were washed thrice with serum/growth factor-free OptiMEM (Invitrogen-Life Technologies) and then transfected with 200 nM vimentin-targeted siRNA or control siRNA using OligofectAMINE. For RNA interference of PAI-1, cells were transfected using 100 nM siRNA. Forty-eight hours after transfection, cells were treated with DMSO or SFN for an additional 24 h. Cells were collected and processed for immunoblotting, apoptosis assay or migration assay.
Detection of apoptosis
Cells were treated with DMSO or SFN for 24 h, harvested by trypsinization, and washed with PBS. Cells (1 × 105) were then suspended in 100 μl of binding buffer, and stained with 4 μl Annexin V and 2 μl propidium iodide (PI) solution for 15 min at room temperature in dark. Samples were then diluted with 200 μl of binding buffer. Stained cells were analyzed using a CyAn ADP Analyzer (Beckman Coulter, Fullerton, CA).
Cell migration assay
Cell migration was determined as described previously [24]. The migrated cells on the bottom face of the membrane were fixed with methanol followed by staining with hematoxylin and eosin. Four randomly selected fields were examined under a microscope at 10 × magnification.
Statistical analysis
Each numeric value is presented as mean ± SD. Statistical significance of difference was determined by one-way ANOVA followed by Bonferroni’s multiple comparison test.
Results
SFN treatment caused vimentin induction in prostate cancer cells
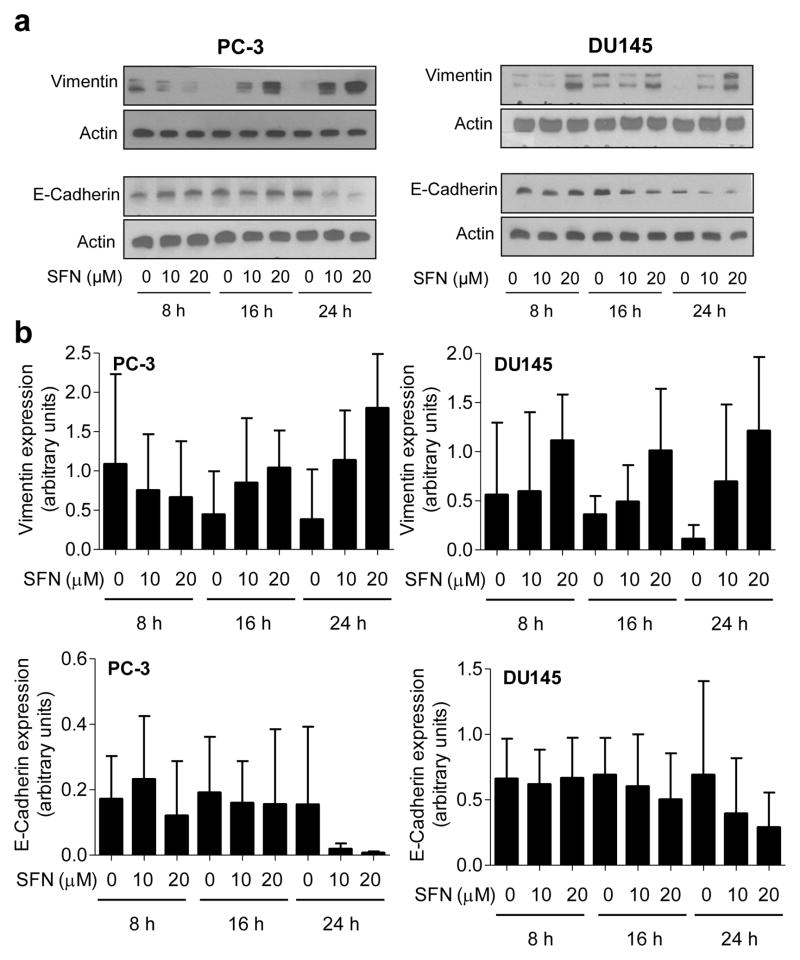
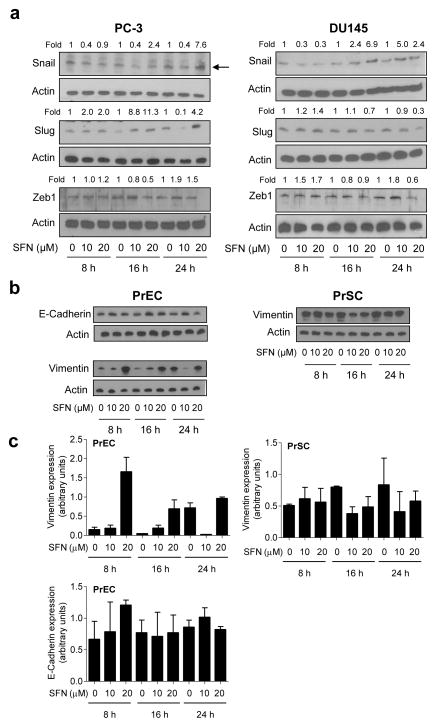
Initial objective of this study was to determine if SFN treatment inhibits EMT in human prostate cancer cells. Induction of vimentin concomitant with suppression of E-cadherin expression is a biochemical hallmark of EMT [26]. Effect of SFN treatment on levels of EMT-related proteins was determined by western blotting, and the results are shown in Figure 1a. Exposure of PC-3 and DU145 human prostate cancer cells to 10 and 20 μM SFN resulted in induction of vimentin protein (Fig. 1b). Immunoblotting for vimentin revealed a doublet in both PC-3 and DU145 cells possibly due to post-translational modification of the protein. The SFN-mediated induction of vimentin was accompanied by suppression of E-cadherin protein level especially at the 24 h time point in PC-3 cells and 16 and 24 h time points in DU145 cells (Fig. 1a,b). Moreover, an increase in protein levels of E-cadherin transcriptional repressors snail, slug, and Zeb1 was also observed in SFN-treated PC-3 and DU145 cells (Fig. 2a).
Fig. 1.
SFN treatment causes induction of vimentin protein expression in prostate cancer cells. a Western blotting for vimentin and E-cadherin proteins using lysates from PC-3 and DU145 cells after treatment with DMSO (control) or the indicated concentrations of SFN for specified time periods. b Densitometric quantitation of vimentin and E-cadherin protein expression changes (arbitrary units) from western blots shown in panel a. Results shown are mean ± SD (n=2–3). Quantitation was normalized against actin protein band intensity for each individual experiment.
Fig. 2.
SFN treatment causes induction of vimentin protein in a normal prostate epithelial cell line (PrEC) but not in a normal prostate stromal cell line (PrSC). a Western blotting for snail, slug, and Zeb1 proteins using lysates from PC-3 and DU145 cells after treatment with DMSO (control) or the indicated concentrations of SFN for specified time periods. b Western blotting for vimentin and E-cadherin using lysates from PrEC and PrSC cells (E-cadherin protein was not detectable in the PrSC cell line) after treatment with DMSO (control) or the indicated concentrations of SFN for specified time periods. Blots were stripped and reprobed with anti-actin antibody. Numbers on top of bands are fold changes relative to corresponding DMSO-treated control. Each experiment was repeated at least twice. c Densitometric quantitation of vimentin and E-cadherin protein expression changes (arbitrary units) from western blots shown in panel b. Results shown are mean ± SD (n=2). Quantitation was normalized against actin protein band intensity for each individual experiment.
Next we raised the question of whether SFN-mediated induction of vimentin was unique to cancer cells. We addressed this question using a normal human prostate epithelial cell line (PrEC) as well as a normal human prostate stromal cell line (PrSC). As can be seen in Figure 2b and c, basal expression of E-cadherin was detectable in PrEC cells, whereas vimentin protein was expressed in both cells. E-cadherin protein expression was not detectable in the PrSC cell line. Similar to prostate cancer cells, however, SFN treatment resulted in induction of vimentin protein level in PrEC cells (Fig. 2b,c). On the other hand, SFN-mediated induction of vimentin was either modest or not observed in the PrSC cell line (Fig. 2b,c). These results indicated that normal and cancerous epithelial cells were sensitive to SFN-mediated induction of vimentin.
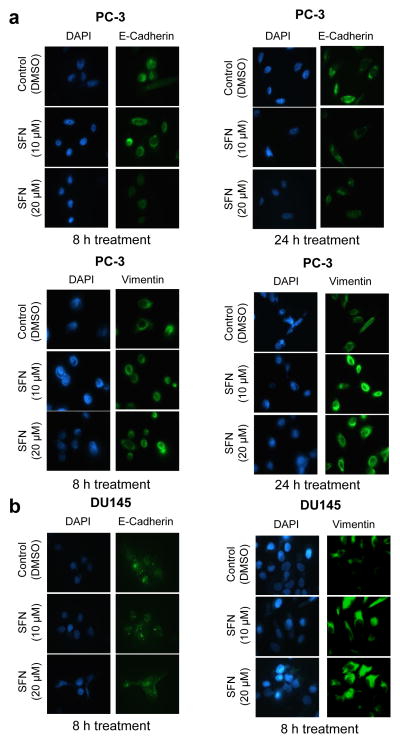
Immunofluorescence microscopy was performed using PC-3 and DU145 cells to confirm SFN-mediated changes in E-cadherin and vimentin proteins. In agreement with the results of immunoblotting, SFN-treated PC-3 (Fig. 3a) and DU145 cells (Fig. 3b) exhibited down-regulation of E-cadherin protein but induction of vimentin. Collectively, these results indicated induction of vimentin after treatment with SFN in prostate epithelial cells.
Fig. 3.
SFN treatment causes induction of vimentin protein in PC-3 and DU145 cells. Immunofluorescence microscopy for the effect of SFN treatment on E-cadherin and vimentin protein levels in a PC-3 and b DU145 cells. Each experiment was repeated at least twice.
Effect of vimentin knockdown on apoptosis induction by SFN
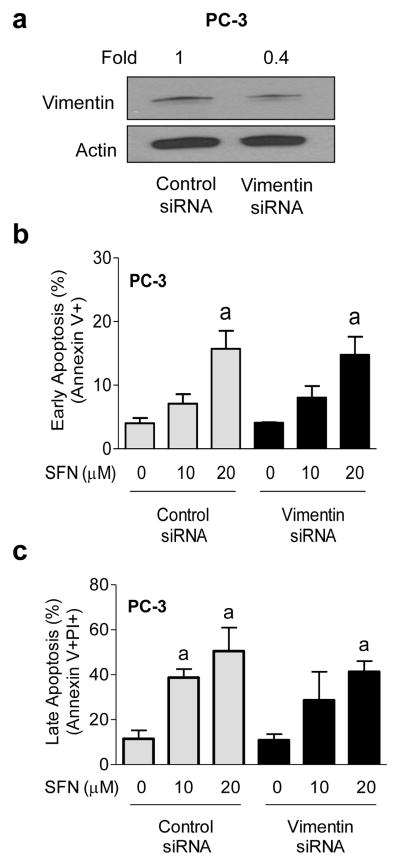
We next performed RNA interference studies to determine the functional significance of vimentin induction in anti-cancer effects of SFN. Initially, we performed a dose-response study using 100, 200, and 300 nM vimentin-targeted siRNA to optimize the conditions for knockdown. Knockdown was partial but comparable with 200 and 300 nM siRNA (results not shown). The cells were transfected with 200 nM siRNA in follow-up experiments. Protein level of vimentin was decreased by about 60% upon transient transfection of PC-3 cells with a vimentin-targeted siRNA in comparison with cells transfected with a control (non-specific) siRNA (Fig. 4a). In control siRNA transfected PC-3 cells, SFN treatment resulted in a dose-dependent increase in early (Fig. 4b) as well as late apoptosis (Fig. 4c). The SFN-induced apoptosis was maintained even after RNA interference of vimentin. The possibility that lack of an effect of vimentin RNA interference on SFN-induced apoptosis is due to incomplete knockdown can’t be excluded. Because vimentin induction did not have any meaningful impact on early or late apoptosis resulting from SFN exposure in PC-3 cells, these experiments were not performed in the DU145 cell line.
Fig. 4.
Effect of vimentin knockdown on SFN-induced apoptosis. a Western blotting for vimentin protein using lysates from PC-3 cells transiently transfected with a control siRNA or a vimentin-targeted siRNA. Flow cytometric analysis of b early (Annexin V+) or c late apoptosis (Annexin V+ and propidium iodide+) in PC-3 cells transiently transfected with a control siRNA or a vimentin-targeted siRNA and treated for 24 h with DMSO or SFN. Results shown are mean ± SD (n=3). aSignificantly different (P < 0.05) compared with corresponding DMSO-treated control by one-way ANOVA followed by Bonferroni’s multiple comparison test. Difference was not significant between control siRNA and vimentin siRNA transfected cells at any dose of SFN. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
Vimentin knockdown modestly augmented SFN-mediated inhibition of cancer cell migration
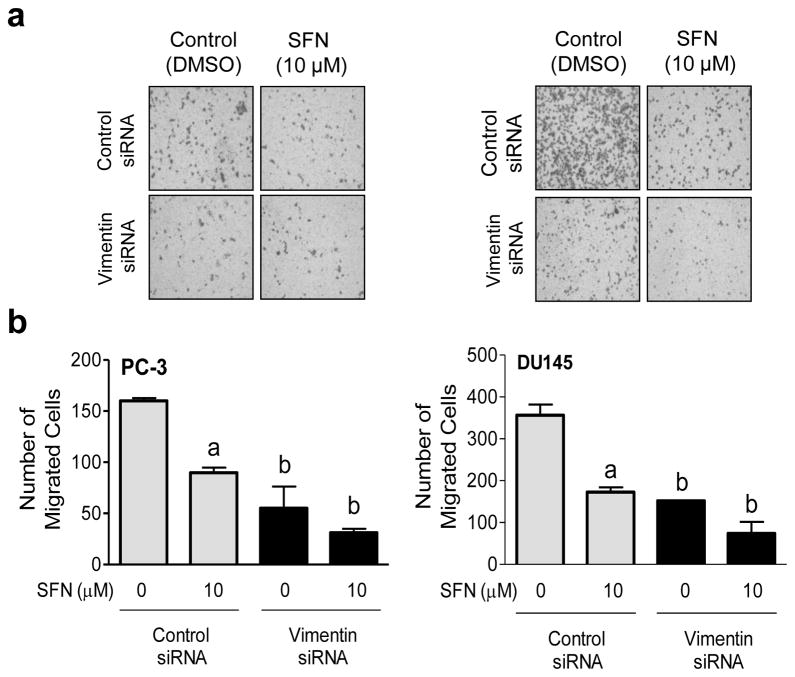
Because vimentin protein is implicated in cancer cell motility [28], we questioned whether SFN-mediated inhibition of prostate cancer cell migration was affected by vimentin protein. Figure 5a shows microscopic images for PC-3 and DU145 cell migration after knockdown of vimentin protein and/or treatment with SFN. The PC-3 and DU145 cell migration was decreased significantly after 24 h treatment with 10 μM SFN (Fig. 5b). Vimentin protein knockdown itself was inhibitory against PC-3 and DU145 cell migration. Furthermore, the SFN-mediated inhibition of prostate cancer cell migration was modestly but significantly augmented by RNA interference of vimentin in both cell lines (Fig. 5b). These results indicated that vimentin induction by SFN conferred modest protection against in vitro inhibition of prostate cancer cell migration by this agent.
Fig. 5.
Vimentin knockdown modestly augments SFN-mediated inhibition of prostate cancer cell migration. a Microscopic images depicting PC-3 and DU145 cell migration after transfection with a control siRNA or a vimentin-targeted siRNA and 24 h treatment with DMSO or 10 μM SFN. b Quantitation of cell migration. Results shown are mean ± SD (n=3). Significantly different (P < 0.05) compared with acorresponding DMSO-treated control and bbetween control siRNA transfected cells and vimentin siRNA transfected cells at each dose (0 or 10 μM SFN) by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
SFN treatment caused induction of PAI-1 in human prostate cancer cells
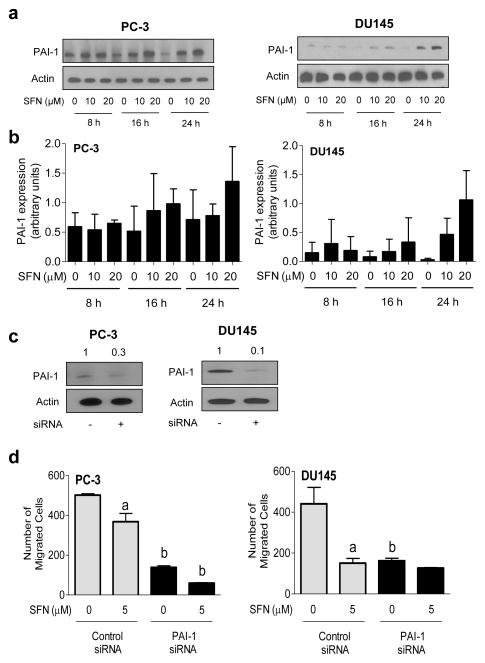
PAI-1 is an endogenous inhibitor of urokinase-type plasminogen activator system, which constitutes an integral component of extracellular matrix proteolysis and cell-extracellular matrix interaction as well as cell signaling involving receptor tyrosine kinases [29, 30]. Effect of SFN treatment on protein level of urokinase-type plasminogen activator or its receptor was not consistent (results not shown). On the other hand, SFN treatment resulted in an increase in PAI-1 protein levels in both PC-3 and DU145 cells (Fig. 6a,b). The SFN-mediated induction of PAI-1 was, however, variable between PC-3 and DU145 cells. For example, induction of PAI-1 protein level resulting from SFN exposure was observed at 16, and 24 h time points in PC-3 cells (Fig. 6a,b). On the other hand, SFN-mediated induction of PAI-1 protein was robust only at the 24 h time point in DU145 cells. The reasons for the cell line-specific differences in induction of PAI-1 by SFN treatment are not yet clear. We next proceeded with RNA interference studies to determine functional significance of SFN-mediated induction of PAI-1 protein. The level of PAI-1 protein was decreased between 70–90% upon its RNA interference (Fig. 6c). Similar to vimentin, knockdown of PAI-1 protein significantly inhibited PC-3 and DU145 cell migration (Fig. 6d). In addition, the SFN-mediated inhibition of cell migration was modestly but significantly augmented by RNA interference of PAI-1 at least in the PC-3 cell line. These results indicated that PAI-1 induction also conferred modest protection against SFN-mediated inhibition of PC-3 cell migration.
Fig. 6.
SFN treatment causes induction of PAI-1 protein in prostate cancer cells. a Western blotting for PAI-1 protein using lysates from PC-3 and DU145 cells after treatment with DMSO (control) or the indicated concentrations of SFN for specified time periods. b Densitometric quantitation of PAI-1 protein expression changes (arbitrary units) from western blots shown in panel a. Results shown are mean ± SD (n=2–3). Quantitation was normalized against actin protein band intensity for each individual experiment. c Western blotting for PAI-1 protein using lysates from PC-3 and DU145 cells transiently transfected with a control siRNA or a PAI-1-targeted siRNA. Blots were stripped and reprobed with anti-actin antibody. d Quantitation of cell migration in PC-3 and DU145 cells transfected with control siRNA or PAI-1-targeted siRNA after 24 h treatment with DMSO or 5 μM SFN. Results shown are mean ± SD (n=3). Significantly different (P < 0.05) compared with acorresponding DMSO-treated control and bbetween control siRNA transfected cells and PAI-1 siRNA transfected cells at each dose (0 or 5 μM SFN) by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated at least twice.
SFN administration caused induction of vimentin protein in TRAMP tumor
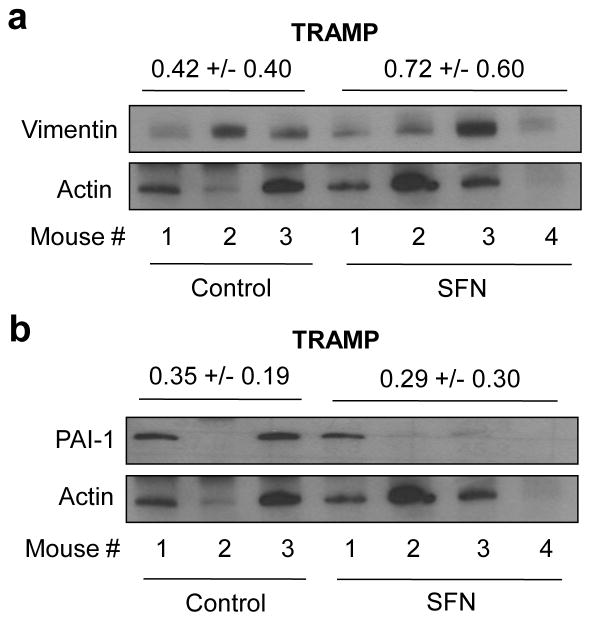
We have shown previously that SFN administration inhibits prostate cancer development in TRAMP mice [9]. We used tumor tissues from this study to determine the in vivo effect of SFN treatment on vimentin and PAI-1 protein expression. Mean intensity for vimentin protein was about 70% higher in the tumors from SFN-treated TRAMP mice compared with control mice (Fig. 7a). On the other hand, SFN-mediated induction of PAI-1 in vivo was not observed at least in the TRAMP tumors (Fig. 7b).
Fig. 7.
SFN administration causes induction of vimentin in TRAMP tumors. Western blotting for a vimentin and b PAI-1 using tumor supernatants from control (n=3) and SFN treated mice (n=4). The membrane was first probed with anti-vimentin antibody, and then stripped and reprobed with anti-PAI-1 antibody. The membrane was subsequently stripped and reprobed with anti-actin antibody to correct for differences in protein loading. Densitometric quantitation data for vimentin and PAI-1 protein expression are shown above corresponding protein bands.
Discussion
The present study reveals that SFN is a potent inducer of vimentin in human prostate cancer cells. Vimentin is a major constituent of the intermediate filament system and responsible for maintenance of cellular integrity [28]. The SFN-mediated induction of vimentin is not confined to prostate cancer cells as this effect is also observed in a normal human prostate epithelial cell line (PrEC). Moreover, the SFN-mediated prevention of prostate cancer in TRAMP mice is associated with induction of vimentin (present study). Several transcription factors are implicated in regulation of vimentin, including nuclear NF-κB, AP-1, PEA3, and Sp1, and β-catenin [31–34]. Even though further work is needed to determine the mechanism underlying SFN-mediated induction of vimentin, this effect is probably not mediated by NF-κB as this transcription factor is inhibited by SFN treatment in prostate cancer cells [20].
Vimentin is overexpressed in various epithelial cancers including prostate cancer [28]. In prostate cancer, vimentin is mainly detectable in poorly-differentiated cancers and bone metastases [35, 36]. On the other hand, vimentin expression is largely undetectable in well- or moderately-differentiated tumors [35, 36]. Vimentin expression is associated with motility in prostate cancer cell lines [35]. Consistent with literature data [35, 36], knockdown of vimentin itself is inhibitory against cell migration in both PC-3 and DU145 cells. We also provide evidence to indicate that vimentin induction by SFN modestly impedes its inhibitory effect on cell migration. The precise mechanism by which vimentin protein knockdown augments SFN-mediated inhibition of prostate cancer cell migration is elusive but may involve Scribble. This protein is involved in cell migration, and protected from proteasomal degradation upon interaction with vimentin [37]. It has been suggested that vimentin upregulation during EMT leads to stabilization of Scribble thereby promoting cell migration [37].
Another interesting observation of the present study is that SFN treatment causes induction of PAI-1, which is an endogenous inhibitor of the urokinase-type plasminogen activator system [29, 30]. Similar to vimentin, PAI-1 knockdown itself inhibits PC-3 and DU145 cell migration. The SFN-mediated inhibition of cell migration is modestly augmented by knockdown of PAI-1 protein at least in PC-3 cells. The role of PAI-1 in prostate cancer is somewhat fuzzy based on literature review. For example, overexpression of PAI-1 was shown to be associated with adverse pathologic characteristics as well as higher overall risk of aggressive disease recurrence in men with localized prostate cancer treated with radical prostatectomy [38]. On the other hand, overexpression of PAI-1 was shown to inhibit PC-3 cell growth in nude mice through endothelial apoptosis in the newly established tumor vasculature [39]. Similarly, exogenously applied PAI-1 inhibited angiogenesis in vitro and retarded LNCaP tumor growth in severe-combined immune deficient mice [40]. Nevertheless, the present study suggests that PAI-1 induction confers a modest protection against cell migration inhibition by SFN.
Acknowledgments
This study was supported by the grant CA115498-07, awarded by the National Cancer Institute. This research project used the Flow Cytometry Facility supported in part by a grant from the National Cancer Institute at the National Institutes of Health (P30 CA047904).
Footnotes
Conflict of interest: None.
References
- 1.Singh SV, Singh K. Cancer chemoprevention with dietary isothiocyanates mature for clinical translational research. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 4.Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, Paffenbarger RS., Jr Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 5.Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst. 1999;91:605–613. doi: 10.1093/jnci/91.7.605. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 8.Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-Acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J Mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 9.Singh SV, Warin R, Xiao D, Powolny AA, Stan SD, Arlotti JA, Zeng Y, Hahm ER, Marynowski SW, Bommareddy A, Desai D, Amin S, Parise RA, Beumer JH, Chambers WH. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keum YS, Khor TO, Lin W, Shen G, Kwon KH, Barve A, Li W, Kong AN. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: Implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 11.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 12.Barcelo S, Gardiner JM, Gescher A, Chipman JK. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis. 1996;17:277–282. doi: 10.1093/carcin/17.2.277. [DOI] [PubMed] [Google Scholar]
- 13.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 14.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 15.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 16.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable-derived cancer chemopreventive agent (2005) Cancer Res. 65:2035–2043. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 17.Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 18.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 19.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappa B and NF-kappa B-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Singh SV. D,L-sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol Cancer Ther. 2009;8:1946–1954. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H, Kim EH, Eom YW, Kim WH, Kwon TK, Lee SJ, Choi KS. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 2006;66:1740–1750. doi: 10.1158/0008-5472.CAN-05-1568. [DOI] [PubMed] [Google Scholar]
- 23.Hahm ER, Singh SV. Sulforaphane inhibits constitutive and interleukin-6-induced activation of signal transducer and activator of transcription 3 in prostate cancer cells. Cancer Prev Res. 2010;3:484–494. doi: 10.1158/1940-6207.CAPR-09-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahm ER, Chandra-Kuntal K, Desai D, Amin S, Singh SV. Notch activation is dispensable for D,L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS One. 2012;7:e44957. doi: 10.1371/journal.pone.0044957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sehrawat A, Singh SV. Benzyl isothiocyanate inhibits epithelial-mesenchymal transition in cultured and xenografted human breast cancer cells. Cancer Prev Res. 2011;4:1107–1117. doi: 10.1158/1940-6207.CAPR-10-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- 27.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 28.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–3046. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwaan HC, McMahon B. The role of plasminogen-plasmin system in cancer. Cancer Treat Res. 2009;148:43–66. doi: 10.1007/978-0-387-79962-9_4. [DOI] [PubMed] [Google Scholar]
- 30.Hildenbrad R, Allgayer H, Marx A, Stroebel P. Modulators of the urokinase-type plasminogen activation system for cancer. Expert Opin Investig Drugs. 2010;19:641–652. doi: 10.1517/13543781003767400. [DOI] [PubMed] [Google Scholar]
- 31.Lilienbaum A, Paulin D. Activation of the human vimentin gene by the Tax human T-cell leukemia virus. I. Mechanisms of regulation by the NF-kappa B transcription factor. J Biol Chem. 1993;268:2180–2188. [PubMed] [Google Scholar]
- 32.Rittling SR, Coutinho L, Amram T, Kolbe M. AP-1/jun binding sites mediate serum inducibility of the human vimentin promoter. Nucleic Acids Res. 1989;17:1619–1633. doi: 10.1093/nar/17.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JH, Vercamer C, Li Z, Paulin D, Vandenbunder B, Stehelin D. PEA3 transactivates vimentin promoter in mammary epithelial and tumor cells. Oncogene. 1996;13:1667–1675. [PubMed] [Google Scholar]
- 34.Zhang X, Diab IH, Zehner ZE. ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucleic Acids Res. 2003;31:2900–2914. doi: 10.1093/nar/gkg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Yan Q, Long X, Chen X, Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008;26:571–577. doi: 10.1002/cbf.1478. [DOI] [PubMed] [Google Scholar]
- 36.Lang SH, Hyde C, Reid IN, Hitchcock IS, Hart CA, Bryden AA, Villette JM, Stower MJ, Maitland NJ. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52:253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 37.Phua DC, Humbert PO, Hunziker W. Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol Biol Cell. 2009;20:2841–2855. doi: 10.1091/mbc.E08-02-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A, Lotan Y, Ashfaq R, Roehrborn CG, Raj GV, Aragaki CC, Montorsi F, Shariat SF. Predictive value of the differential expression of the urokinase plasminogen activation axis in radical prostatectomy patients. Eur Urol. 2009;55:1124–1133. doi: 10.1016/j.eururo.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Chen SC, Henry DO, Reczek PR, Wong MK. Plasminogen activator inhibitor-1 inhibits prostate tumor growth through endothelial apoptosis. Mol Cancer Ther. 2008;7:1227–1236. doi: 10.1158/1535-7163.MCT-08-0051. [DOI] [PubMed] [Google Scholar]
- 40.Swiercz R, Keck RW, Skrzypczak-Jankun E, Selman SH, Jankun J. Recombinant PAI-1 inhibits angiogenesis and reduces size of LNCaP prostate cancer xenografts in SCID mice. Oncol Rep. 2001;8:463–470. doi: 10.3892/or.8.3.463. [DOI] [PubMed] [Google Scholar]