Abstract
In renal cystic diseases, sustained enlargement of fluid-filled cysts is associated with severe interstitial fibrosis and progressive loss of functioning nephrons. Periostin, a matricellular protein, is highly overexpressed in cyst-lining epithelial cells of autosomal dominant polycystic disease kidneys (ADPKD) compared to normal tubule cells. Periostin accumulates in situ within the matrix subjacent to ADPKD cysts, binds to αVβ3- and αVβ5-integrins and stimulates the integrin-linked kinase to promote cell proliferation. We knocked out periostin (Postn) in pcy/pcy mice, an orthologous model of nephronophthisis type 3, to determine whether periostin loss reduces PKD progression in a slowly progressive model of renal cystic disease. At 20 weeks of age, pcy/pcy: Postn−/− mice had a 34% reduction in kidney weight/body weight, a reduction in cyst number and total cystic area, a 69% reduction in phosphorylated S6, a downstream component of the mTOR pathway, and fewer proliferating cells in the kidneys compared to pcy/pcy: Postn+/+ mice. The pcy/pcy Postn knockout mice also had less interstitial fibrosis with improved renal function at 20 weeks and significantly longer survival (51.4 compared to 38.0 weeks). Thus, periostin adversely modifies the progression of renal cystic disease by promoting cyst epithelial cell proliferation, cyst enlargement and interstitial fibrosis, all contributing to the decline in renal function and premature death.
Keywords: integrins, proliferation, ADPKD, osteoblast specific factor 2, integrin-linked kinase
INTRODUCTION
Polycystic kidney disease (PKD) is a family of hereditary disorders characterized by the formation and progressive expansion of numerous fluid-filled cysts that cause massively enlarged kidneys (1). Renal cysts are benign neoplasms that ultimately cause renal insufficiency through extensive nephron loss and replacement of normal parenchyma with fibrosis (2). Autosomal dominant PKD (ADPKD) is the most common inherited renal disorder with a frequency of 1:500 individuals and accounts for 7–9% of patients on renal replacement therapy. ADPKD is caused by mutations in PKD1 or PKD2, genes that encode polycystin-1 (PC1) and polycystin-2 (PC2), respectively (3–6). Autosomal recessive PKD (ARPKD) is less common (~1:20,000 births) and is caused by mutations in PKHD1, a gene that encodes fibrocystin. ARPKD is characterized by rapid disease progression, often leading to renal failure within the first year of life. Several signaling pathways, including those regulated by cAMP, growth factors, B-Raf/MEK/ERK, mammalian Target Of Rapamycin (mTOR), integrins, and Akt have been implicated in aberrant cell proliferation and the relentless expansion of PKD cysts (7–16).
In addition to intrinsic defects in PKD cells, factors secreted into the extracellular environment may also contribute to cyst growth and development of interstitial fibrosis. We and others have shown that mRNA levels of genes involved in tissue remodeling and extracellular matrix (ECM) production are highly elevated in cystic cells compared to normal renal cells (12, 17). In fact, periostin was one of the most highly differentially expressed genes in human ADPKD cells compared to normal tubule cells (12). Periostin is a secreted protein that binds to components of the ECM, including type I collagen and fibronectin, and has been implicated in collagen fibrillogenesis (18–20). Periostin, as well as other “matricellular” proteins, transmits signals from the ECM to the cell by binding to cell surface integrins, leading to changes in cell adhesion, migration, proliferation, survival and tissue angiogenesis (21–26). While periostin is expressed in several tissues during embryonic development (27, 28), its expression in adults is restricted to collagen-rich tissues and can be upregulated by mechanical stress (29–31). Periostin has also been found to be elevated in a variety of cancers, including breast, lung, and colon, where it promotes cell proliferation and survival (21, 22, 25, 32–36).
In ADPKD, periostin is secreted by cyst-lining cells and accumulates within the ECM adjacent to cysts (12). Periostin binds αVβ3 and αVβ5 integrins and stimulates integrin-linked kinase (ILK), leading to an acceleration of cell proliferation and in vitro cyst growth of ADPKD cells within a collagen matrix. By contrast, periostin does not stimulate the proliferation of normal renal cells, suggesting that periostin is a novel autocrine mitogen secreted by cystic cells (12). In this study, we show that upregulation of periostin expression is not limited to ADPKD, but rather is a common feature of inherited renal cystic epithelia, regardless of the underlying genetic defect. To determine if periostin contributes to PKD progression, we knocked out periostin (Postn) expression in pcy/pcy mice, a well characterized model orthologous to human nephronophthisis type three, a recessive form of PKD that typically causes renal failure in children and adolescents (37, 38). In pcy/pcy mice, cystic kidneys enlarge to several times normal size and are associated with extensive renal interstitial fibrosis by 18 weeks of age and the development of azotemia (37). Previously, we monitored PKD progression in a pcy/pcy mouse by measuring kidney volume by magnetic resonance imaging. Kidney volume increased exponentially up to 20 weeks of age, after which there was a plateau as renal parenchyma was replaced with fibrosis (39). In this study, we determined if loss of Postn expression reduced kidney weight, cystic index, cell proliferation and fibrosis in 20-week old pcy/pcy mice and extended their survival. The results indicate that periostin and its associated signaling pathways may be viable targets for therapy in PKD.
RESULTS
Periostin expression in pcy/pcy kidneys
Periostin (Postn) mRNA is highly overexpressed in human ADPKD cyst epithelial cells compared to normal human kidney cells (12). Similarly, we found that Postn is overexpressed in ARPKD and several animal models of cystic disease (Table 1), suggesting that aberrant periostin expression is a general feature of PKD, regardless of the underlying genetic mutation.
Table 1.
Periostin mRNA levels in recessive models of PKD
| N | Fold Increase | |
|---|---|---|
| Human ARPKD vs. NHK cells | 3 | 19.6 |
| cpk/cpk mouse kidney vs. WT tissue (3 weeks) | 3 | 9.3 |
| jck/jck mouse kidney vs. WT tissue (20–25 weeks) | 3 | > 50 |
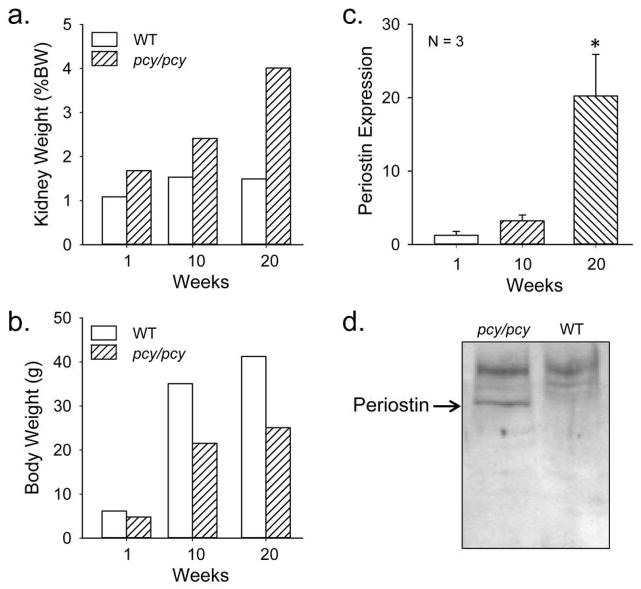
To determine if periostin was increased in cystic kidneys of pcy/pcy mice, we compared Postn expression to WT mice at 1, 10 and 20 weeks. Kidney volume of pcy/pcy mice was elevated at 1 week and continued to increase to 20 weeks (Fig. 1a), as described previously (39). By contrast, body weight of pcy/pcy mice was reduced compared to WT mice (Fig. 1b). At 20 weeks, periostin mRNA (Fig. 1c) and protein (Fig. 1d, Supplemental Figure S1) were elevated in pcy/pcy kidneys compared to age-matched WT kidneys, confirming that periostin is overexpressed in this model of slowly progressive renal cystic disease.
Figure 1. Kidney and body weight, and periostin expression in pcy/pcy and wildtype mice.
Male pcy/pcy mice and wildtype (WT) mice (2–3 mice per group) were sacrificed at 1, 10 and 20 weeks for determination of (a) total kidney weight, represented as a percentage of body weight (%BW) and (b) body weight. (c) Renal periostin (Postn) mRNA expression between pcy/pcy and WT mice was determined by quantitative real-time RT-PCR. Periostin expression was normalized to GAPDH and the expression in pcy/pcy kidneys was represented as fold-change compared to WT kidneys, calculated from Δ Ct (N = 3 per group). Data represent mean ± SE. Statistical analysis was determined by ANOVA and SNK post test. *P < 0.05. (d) Immunoblot for periostin protein (90 kDa) in kidneys of 20-week old pcy/pcy and WT mice. Expression of periostin protein was confirmed in two additional pairs of pcy/pcy and WT mice (Supplemental Figure S1).
Effects of periostin on body and kidney mass and renal cystic disease
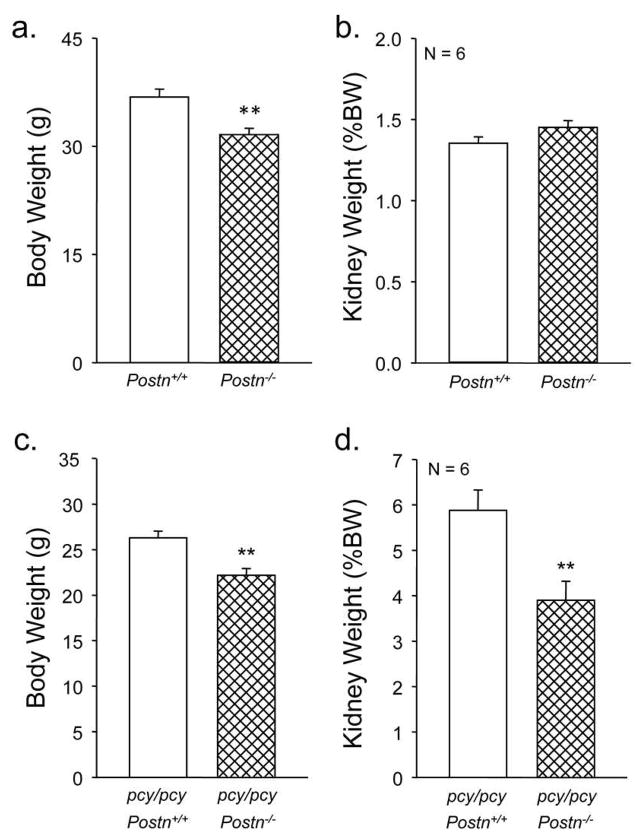
Periostin knockout mice have been reported previously (40). Consistent with this report, sex-matched Postn−/− mice were similar in general appearance to WT (Postn+/+) littermates (not shown). Also as reported, 20-week old Postn−/− mice exhibited a moderate reduction in body weight compared to WT littermates (36.8 ± 1.1 g for WT vs. 31.6 ± 0.9 g for the Postn−/−, P < 0.005) (Fig. 2a). However, there was no difference in total kidney weight (both kidneys) between Postn−/− and WT mice when corrected for body weight (%BW) (Fig. 2b).
Figure 2. Effect of periostin expression on kidney weight in pcy/pcy mice.
At 20 weeks, body weights of male pcy/pcy: Postn+/+ and pcy/pcy: Postn−/− mice were measured and kidneys were collected to determine total kidney weight, represented as %BW. (a) Postn−/− (CD-1) mice had a lower body weight than periostin-expressing littermates consistent with mild dwarfism in mice lacking periostin expression (40). **P < 0.001, compared to Postn+/+ mice. (b) By contrast, kidney weight (%BW) was not different between Postn−/− and Postn+/+ mice. (c) As expected, pcy/pcy: Postn−/− mice also had a lower body weight compared to pcy/pcy: Postn+/+ mice. (d) There was a 34% reduction in kidney weight (%BW) of pcy/pcy: Postn−/− mice compared to pcy/pcy:Postn+/+ littermates. Data are means ± SE. Statistical analysis was determined by an unpaired t-test. **P < 0.001, compared to pcy/pcy: Postn+/+.
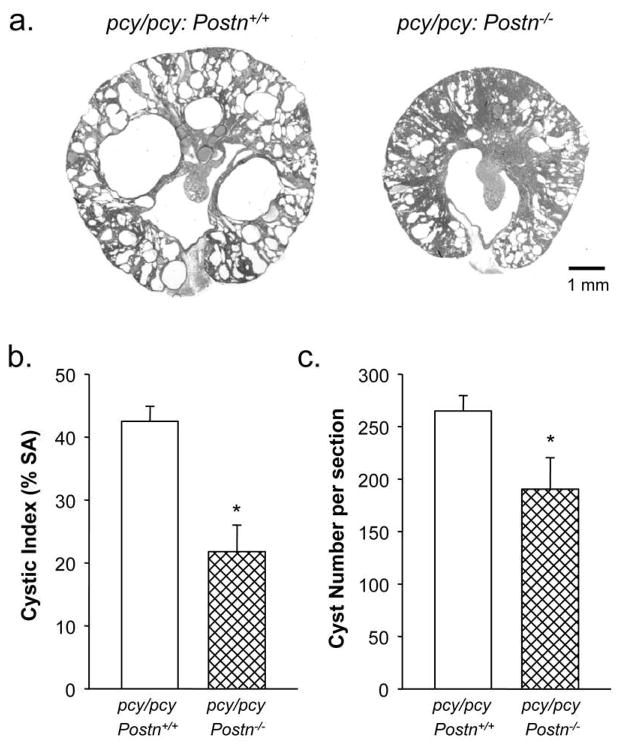
To examine the effects of periostin on cystic disease, Postn−/− mice were bred with pcy/pcy mice to generate pcy/pcy:Postn−/− mice. As expected, loss of periostin expression, confirmed by immunoblot analysis (Supplemental Figure S1), caused a reduction in body weight of pcy/pcy: Postn−/− mice from 26.3 ± 0.7 to 22.2 ± 0.7 g (P < 0.01) at 20 weeks of age, similar to the effect in WT mice (Fig. 2a, c). However, in contrast to the lack of an effect of Postn knockout on kidney mass in normal mice, pcy/pcy:Postn−/− mice showed a dramatic decrease in KW/BW (5.9 ± 0.5 vs. 3.9 ± 0.4 %, P < 0.001) compared to pcy/pcy:Postn+/+ mice (Fig. 2d). To understand the ameliorating effects of periostin loss on kidney enlargement, kidneys from pcy/pcy:Postn−/− were sectioned for microscopic examination. Compared to pcy/pcy:Postn+/+ mice, kidneys from pcy/pcy:Postn−/− mice showed a significant reduction in cystic area (Fig. 3a). Measurements of cyst surface area in three non-overlapping representative kidney sections demonstrated decreased cystic area in the Postn knockout mice from 42.5 ± 2.4 to 21.8 ± 4.2%; P < 0.005 (Fig. 3b). There was also a 28% reduction in the number of cysts per section (Fig. 3c). Thus, loss of periostin expression results in a significant reduction in pathologic enlargement of pcy/pcy kidneys by reducing cyst number and total cystic area.
Figure 3. Effect of periostin expression on renal cyst development in pcy/pcy mice.
(a) Representative kidney sections of male pcy/pcy: Postn+/+ and pcy/pcy: Postn−/− mice. Scale bar is 1 mm. (b) Cross-sectional surface area of cysts is represented as % of total area minus the area of the renal pelvis. Data are mean ± SE from three representative sections per kidney. pcy/pcy: Postn−/− kidneys had a marked reduction in cystic area compared to pcy/pcy: Postn+/+ kidneys. (c) The number of cysts (≥ 50 μm) per section was also significantly reduced in the pcy/pcy: Postn−/− kidneys (190 ± 30 vs. 265 ± 15). Data are means ± SE. Statistical analysis was determined by an unpaired t-test. *P < 0.05, compared to the pcy/pcy: Postn+/+ kidneys.
Effect of periostin on cell proliferation in pcy/pcy kidneys
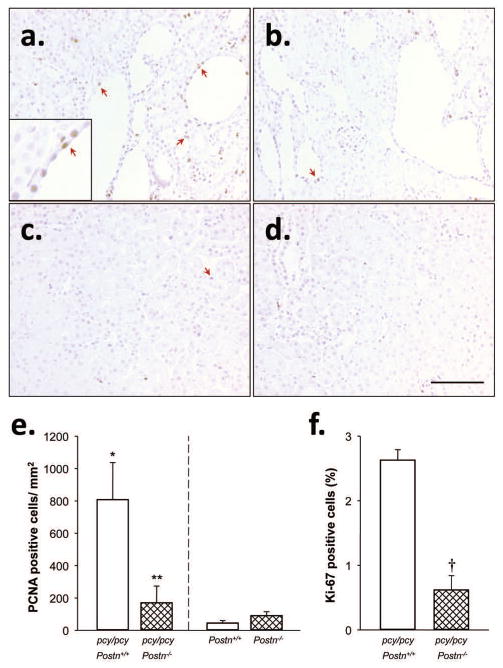
Previously, periostin was shown to stimulate ADPKD cell proliferation through binding αV-integrins and activation of ILK (12), suggesting that periostin provides a proliferative signal that contributes to renal cyst expansion. To test this, we measured the number of proliferating cells in 20 week-old kidneys of pcy/pcy:Postn−/− mice compared to pcy/pcy:Postn+/+ mice by immunohistochemistry using antibodies to proliferating cell nuclear antigen (PCNA) and Ki-67, nuclear markers for cell proliferation. There were visibly fewer PCNA-positive cells (brown precipitate, arrows) in pcy/pcy: Postn−/− compared to pcy/pcy: Postn+/+ kidney sections (Fig. 4a, b), as confirmed by manual counting positive cells in these sections (Fig. 4e). In contrast, Postn knockout in mice without renal cystic disease did not affect the number of PCNA-positive cells in the kidneys (Fig. 4c–e).
Figure 4. Effect of periostin on cell proliferation in pcy/pcy mice.
Representative kidney sections from (a) pcy/pcy: Postn+/+, (b) pcy/pcy: Postn−/−, (c) Postn+/+ (CD-1), and (d) Postn−/− (CD-1) mice were stained with an antibody to proliferating cell nuclear antigen (PCNA), a proliferation marker. All images were taken at the same magnification. Bar scale is 100 μm. The inset in the lower left corner of Fig. 4a shows a higher magnification image of brown-stained nuclei of PCNA-positive cells. Sections were counter stained with hematoxylin. (e) The number of PCNA-positive cells per section area (excluding cyst lumens) from three random microscope images was determined by an observer blinded to the identity of the slides. There were significantly fewer PCNA-positive cells in pcy/pcy mice lacking periostin, compared to pcy/pcy:Postn+/+ kidneys. (f) To further examine the effect of Postn deletion on cell proliferation, three tissue sections/kidney (N = 3 kidneys) were stained with an antibody to Ki-67, another proliferation marker. Ki-67 was visualized using a secondary antibody conjugated to GFP and the number of Ki-67 positive cells was quantified by counting GFP-positive cells using a Nikon immunofluorescence microscope controlled by Metamorph software. Total cell numbers were determined by counting nuclei stained with DAPI and data are presented as % of Ki-67 positive cells. There were fewer Ki-67 positive cells in pcy/pcy: Postn−/− kidney sections compared to the pcy/pcy: Postn+/+ kidney sections, confirming the PCNA results. Data are means ± SE. Statistical analysis was determined by unpaired t-test. *P < 0.05, compared to Postn+/+ (CD-1); **P < 0.05 and †P < 0.001, compared to pcy/pcy: Postn+/+.
To confirm the PCNA results, we measured the number of Ki-67 positive cells by immunofluorescence, normalizing for total cell number using the nuclear stain DAPI. As with PCNA staining, kidney sections from pcy/pcy: Postn−/− mice (N = 3) had significantly fewer Ki-67 positive cells than those from pcy/pcy: Postn+/+ mice (2.6 ± 0.2 vs. 0.6 ± 0.2 per field, P < 0.001, Fig. 4f). Taken together, these data support the hypothesis that periostin expression is an important contributor to the proliferation of cystic epithelial cells and promotes cyst expansion and kidney enlargement in pcy/pcy mice.
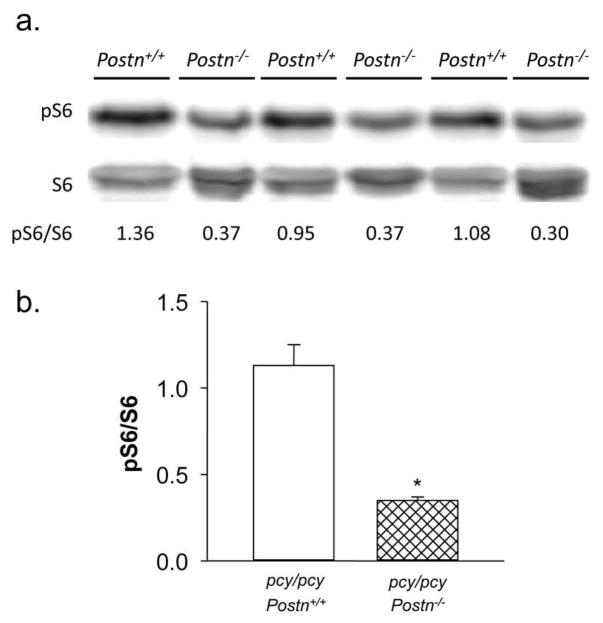
To examine the mechanism by which periostin contributes to cell proliferation, we examined effects of Postn knockout on the mTOR pathway. This signaling pathway has been implicated in cyst epithelial cell proliferation, and there is increased phosphorylation of downstream targets of mTOR pathway, S6K and S6, in cystic kidneys of human ADPKD, ARPKD and PKD animals, including the pcy/pcy mice (8, 41–43). The mTOR pathway is also known to be a downstream target of ILK (44), which we have shown to be activated by periostin (12). To determine whether Postn knockout affects mTOR signaling in pcy/pcy mice, kidney lysates were prepared and amounts of phosphorylated S6 (pS6) were determined by immunoblot analysis (Fig. 5). The level of pS6, normalized to total S6 (pS6/S6), was significantly decreased (0.35 ± 0.02 vs. 1.13 ± 0.12, N = 3, P < 0.05), despite an increase in total S6 in pcy/pcy: Postn−/− kidneys. There was also a 26% decrease in band intensity for pS6K (P < 0.05, data not shown) in pcy/pcy: Postn−/− kidneys. These data suggest that diminished activity of the mTOR pathway contributes to the reduction in cell proliferation and cystic area in kidneys of pcy/pcy:Postn−/− mice.
Figure 5. Effect of periostin expression on mTOR signaling in pcy/pcy mice.
(a) Immunoblot analysis was used to compare the effect of Postn deletion on phosphorylation levels of the ribosomal protein S6 (pS6), a downstream target of the mTOR signaling pathway, in kidneys of pcy/pcy mice. Levels of pS6 were normalized to total S6. The number below the bands indicates the pS6/S6 for each sample. (b) Summary of the effect of Postn knockout in pcy/pcy mice on renal pS6/S6 levels. Data are means ± SE. Statistical analysis was determined by unpaired t-test. *P < 0.05, compared to pcy/pcy: Postn+/+ kidney, N = 3.
Influence of periostin on renal interstitial fibrosis in pcy/pcy mice
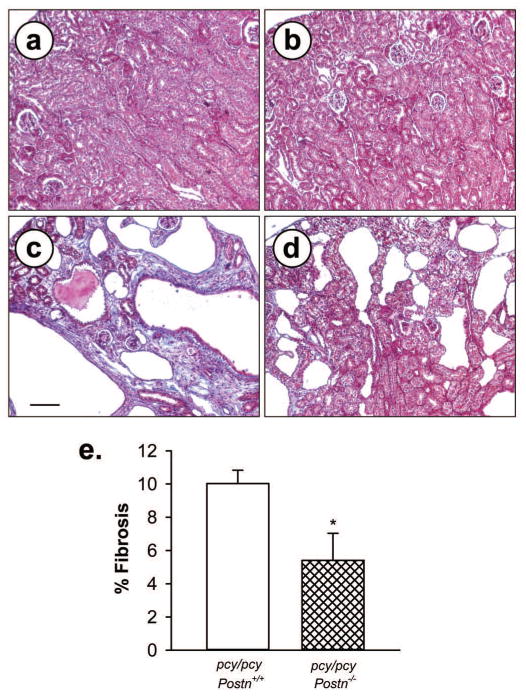
Periostin directly interacts with components of the ECM, including collagen and fibronectin, and promotes collagen cross-linking and fibrogenesis (18–20). In addition to modulating biomechanical properties of tissues, periostin enhances pro-fibrotic TGF-β signaling and can induce fibrosis (45, 46). To assess the role of periostin in renal interstitial fibrosis in pcy/pcy mice, kidney sections were stained with Masson trichrome. There was intense blue staining of the collagen deposition adjacent to cysts of 20-week old pcy/pcy kidneys and this staining was significantly reduced in pcy/pcy:Postn−/− kidneys (Fig. 6). Under a polarizing light, picrosirius red, which specifically stains collagen types 1 and III, visualizes pathogenic fibrils within the interstitium. We found that % area of collagen fibrils within the renal interstitium was reduced in pcy/pcy mice lacking periostin compared to pcy/pcy:Postn+/+ mice; however, this difference did not reach statistical significance (P = 0.06) (Fig. 7). Nevertheless, in conjunction with the results from analysis of trichrome staining (Fig. 6), these results suggest that periostin directly or indirectly contributes to renal fibrosis in this model of PKD.
Figure 6. Effect of periostin expression on interstitial collagen deposition in pcy/pcy mice.
Representative kidney sections from (a) Postn+/+, (b) Postn−/−, (c) pcy/pcy: Postn+/+ and (d) pcy/pcy: Postn−/− mice were stained with Masson trichrome to visualize collagen deposition. There was intense staining of collagen (blue color) within the interstitial areas adjacent cysts in pcy/pcy mice expressing Postn. By contrast, collagen staining was markedly reduced in cystic areas of pcy/pcy: Postn−/− mice. All images are the same magnification. Bar scale is 100 μm. (e) Fibrosis was determined by visual assessment of the trichrome-stained slides and represented as % fibrotic area to total area of the whole tissue section. Sections (n = 6 per group) were de-identified and fibrosis was determined by visual measurement of % fibrotic area (62). Data are means ± SE. Statistical analysis was determined by unpaired t-test. *P < 0.05, compared to pcy/pcy: Postn+/+ kidney.
Figure 7. Effect of periostin expression on fibrillar collagen in pcy/pcy kidneys.
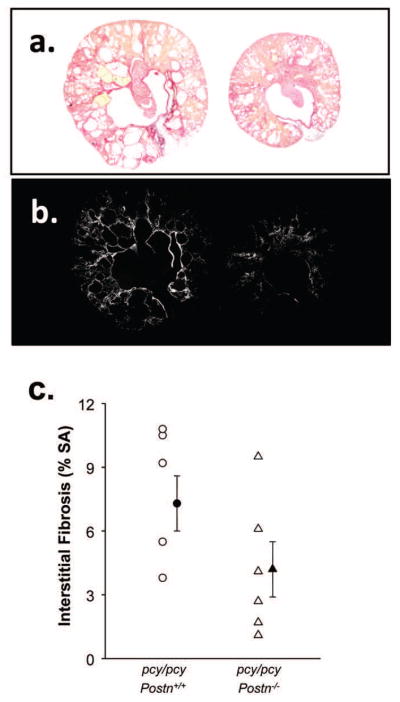
(a) Kidney sections of pcy/pcy: Postn+/+ (left) and pcy/pcy: Postn−/− (right) mice were stained with picrosirius red (59, 60) and illuminated with a polarized light to visualize type I and III collagen fibrils (b). Images were digitized with the use of a computer-video analysis system and converted to gray scale for measurement of the area of white pathogenic collagen fibers as a % of total surface area (SA) (c). There was a trend for less collagen fibrils in tissue sections of pcy/pcy: Postn−/− kidneys compared to pcy/pcy: Postn+/+ kidneys; however, the difference did not reach statistical significance (P = 0.06) by an unpaired t-test. Data are means ± SE.
Effect of periostin on renal function and survival of pcy/pcy mice
Since Postn knockout significantly reduced cystic index, fibrosis and kidney enlargement in pcy/pcy mice, we determined if the loss of Postn expression improved renal function. As expected, 20 week-old pcy/pcy: Postn+/+ mice had elevated blood urea nitrogen (BUN: 48.7 ± 7.2 mg/dl; normal range is 8–33 mg/dl) with four of the six mice with values above 40 mg/dl (Fig. 8). By contrast, pcy/pcy: Postn−/− mice had a BUN of 31.3 ± 5.7 mg/dl, P < 0.05, demonstrating that gene knockout of Postn significantly improved renal function in pcy/pcy mice.
Figure 8. Effect of periostin expression on blood urea nitrogen (BUN) in pcy/pcy mice.
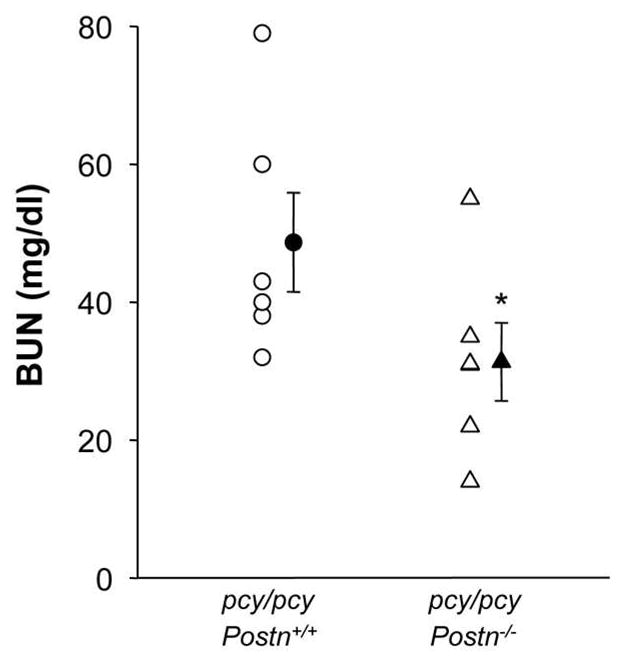
At 20 weeks, pcy/pcy: Postn+/+ mice had a BUN of 48.7 ± 7.2 mg/dl with five of the six mice with values above 33 mg/dl. The normal range for BUN in healthy adult mice is 8 to 33 mg/dl. By contrast, the mean BUN for pcy/pcy: Postn−/− mice was 31.3 ± 5.7 mg/dl (P < 0.05), consistent with improved renal function. Data are means ± SE. Statistical analysis was determined by unpaired t-test.
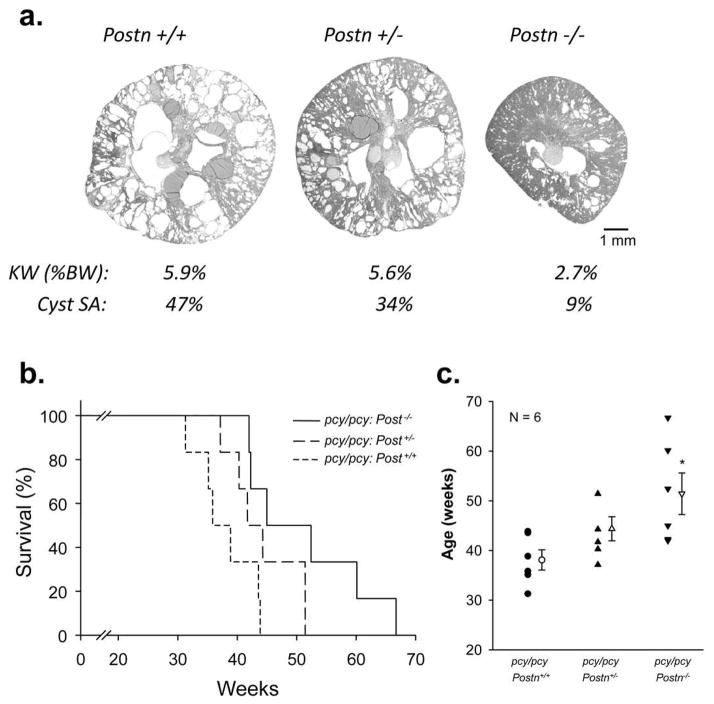
To further examine the effect of Postn expression on disease severity, we compared the kidney morphology of sex-matched pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− litermates at 20 weeks. Loss of one allele (Postn+/−) caused a small reduction in kidney weight and decreased cystic area from 47% to 34%; whereas, complete loss of Postn expression reduced kidney weight (%BW) from 5.9 to 2.7% and decreased cystic area to 9%. Previously, periostin expression in Postn+/− newborn mice was found to be ~30 to 50% of the normal level (40). These data suggest that there was a gene dosage effect of periostin on PKD progression in pcy/pcy mice.
Next, we compared the lifespan of pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− mice that were fed a standard diet ad libitum and monitored daily until natural death. Periostin expressing pcy/pcy mice lived to 38.1 ± 2.0 weeks, slightly longer than the 30–36 week time span previously reported (37). Interestingly, mice heterozygous at the periostin locus (pcy/pcy: Postn+/−) survived to 44.4 ± 2.4 weeks (Fig. 9b, c). Complete knockout of periostin (pcy/pcy: Postn−/−) resulted in a significant increase in survival to 51.4 ± 4.2 weeks with all the mice in this group living longer than the mean age of death for the pcy/pcy:Postn+/+ mice (38.1 ± 2.0 weeks). Taken together, these studies demonstrate that renal expression of periostin in pcy/pcy mice accelerates disease progression by stimulating cyst epithelial cell proliferation and interstitial fibrosis, thereby contributing to a decline in renal function and death.
Figure 9. Effect of periostin expression on the lifespan of pcy/pcy mice.
The lifespan of pcy/pcy mice is 30–36 weeks of age and death is typically caused by renal failure (37). To determine whether periostin expression affects the survival of pcy/pcy mice, littermates of pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− mice were given standard diet ad libitum until natural death. (a) Representative images of kidney sections, kidney weight (KW) as %BW, and % cystic area from pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− mice at 20 weeks of age. (b) Kaplan-Meier curve of pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− mice. (c) Summary of the age of death. pcy/pcy: Postn+/+ mice died at 38.1 ± 2.0 weeks. By contrast, pcy/pcy mice with the loss of one Postn allele (pcy/pcy: Postn+/−) survived to 44.4 ± 2.4 weeks and pcy/pcy mice with a complete loss of periostin expression survived to 51.4 ± 4.2 weeks (*P < 0.05, compared to pcy/pcy: Postn+/+). Data are means ± SE. Statistical analysis was determined by ANOVA and SNK post test.
DISCUSSION
Periostin is a member of the matricellular proteins that also includes thrombospondins, osteopontin, βig-H3, SPARC and connective tissue growth factors. These small molecules are secreted into the ECM, where they modulate matrix accumulation and collagen fibrillogenesis and bind cell surface integrins to regulate a variety of cell functions, including cell adhesion, migration, proliferation and survival (18, 21, 22, 24, 25, 32). In this study, we found that periostin contributes importantly to renal cyst enlargement and disease progression in pcy/pcy mice. Global knockout of Postn in pcy/pcy mice 1) caused a significant reduction in the KW/BW ratio, 2) decreased renal mTOR activity, 3) reduced the number of proliferating cells, cyst number and cystic area, 4) decreased renal interstitial fibrosis, 5) preserved renal function, and 6) significantly extended the lifespan of the mice.
Our data suggest that periostin is an important contributing factor for the progression of PKD by promoting the proliferation of cystic cells in vivo. We have previously shown that periostin activates ILK, which promotes human ADPKD cell proliferation (12). Here, we show that Postn loss in the pcy/pcy mouse model diminished the activity of mTOR, a signaling pathway implicated in the aberrant proliferation of renal epithelial cells and formation of cysts in human ADPKD, ARPKD and animal models of PKD, including the pcy/pcy mouse (8, 41–43). Moreover, mTOR is known to be activated downstream of ILK, via AKT-dependent phosphorylation of tuberin (TSC2) (44). These results suggest that a periostin-ILK-AKT-mTOR signaling axis may be an important mechanism underlying periostin-stimulated cyst cell proliferation.
Periostin is one of the most highly over-expressed genes in human ADPKD cells compared to normal human kidney epithelial cells (12). In this study, we show that periostin is also highly overexpressed in human ARPKD and multiple mouse models of renal cystic disease. This finding implies that periostin overexpression may be a general feature of cystic kidney disease. The mechanism responsible for aberrant periostin expression in cystic disease remains unclear; however, its expression may reflect its role in development and repair. Periostin is highly expressed in the nephrogenic zone, an active site of cell proliferation and vascularization during renal development (47); however, it is not normally expressed in adult kidney. It is possible that persistent expression of periostin has a pro-proliferative effect on these immature developing tubule cells, contributing to cyst growth. Indeed, ADPKD cystic epithelial cells have been characterized as being arrested at a stage of incomplete differentiation (48). Periostin has also been shown to be elevated by mechanical stress (20). It is possible that fluid accumulation by active fluid secretion into the cyst lumen increases internal pressure to elicit a stress response in mural epithelial cells, leading to periostin expression.
Periostin also plays an important role in tissue repair, including repair after acute myocardial infarction and cardiac fibrosis (49–52). Since renal cyst expansion results in chronic injury (53), aberrant periostin expression may be involved in a futile repair mechanism (9) that contributes to cell proliferation, cyst expansion and ultimately renal fibrosis.
Matricellular proteins, such as periostin, are being considered as potential therapeutic targets to prevent fibrosis. Currently, the role of periostin in other chronic kidney diseases remains unclear. Periostin expression appeared de novo within the distal tubules after 5/6 nephrectomy (54) and, following glomerular injury, increased expression was observed in the glomerular tuft, vascular pole, Bowman’s capsule and in discreet areas of interstitial fibrosis (55). Periostin staining of human renal biopsies appeared to correlate negatively with renal function, supporting a role for periostin in glomerulosclerosis (55).
It should be noted that 6 different splice variants of periostin, varying in size from 751 to 836 amino acids (83 to 93 kDa) have been identified (26). These isoforms of periostin are determined by the presence or absence of exons 17–21 due to in-frame alternative splicing. It remains unclear whether the various isoforms have tissue specific expression or unique functions. Since it is possible that the splice variants facilitate diverse effects on the regulation of matrix production, fibrosis, and cell proliferation, understanding the contribution of the various isoforms may be important for designing therapies that target periostin function.
In summary, periostin expression is elevated in the kidneys of pcy/pcy mice throughout the progression of cystic disease. Global knockout of Postn reduced renal mTOR activity, percentage of proliferating cells, cyst growth, kidney enlargement, interstitial fibrosis and the decline in renal function. Genetic ablation of Postn also significantly extended the lifespan of pcy/pcy mice. We conclude that aberrant expression of periostin and possibly other matricellular proteins that are expressed during renal development and tissue repair may be important modulators of cystic disease progression.
METHODS
Experimental animals and study design
The pcy/pcy mouse phenotype is caused by mutations in Nphp3, which is orthologous to human nephronophthisis type 3 (38, 56–58). Renal cysts form as segmental dilations predominantly in collecting ducts and distal tubules in utero and extend to all nephron segments in later stages of the disease (37). pcy/pcy kidneys enlarge to several times normal size and azotemia begins to develop by 18 weeks in association with interstitial fibrosis and progressive decline in renal function, a disease course that is similar to human ADPKD. Generally, pcy/pcy mice survive to 30–36 weeks, making this a useful model for investigating the effects of genetic manipulations on disease progression.
Postn−/− mice were generated by insertion of the β-galactosidase gene into exon 1 of the periostin gene, causing a loss of periostin expression (40). This homologous recombination strategy removed the DNA encoding for the translation start site, all of first exon and the part of the first intron, replacing it with the bacterial β-galactosidase reporter gene. Newborn and one-week old Postn−/− mice are indistinguishable from wild-type littermates; whereas, adult mice have mild dwarfism and defects in periosteum, cartilage, cardiac valves and periodontal ligament, tissues that normally express periostin throughout adulthood (40).
Postn knockout in normal CD-1 mice caused a modest reduction in body weight, but had no effect of kidney mass as a percent on total body weight (Fig. 2a–b). We bred pcy/pcy mice with Postn+/− mice to generate pcy/pcy: Postn+/− mice and these mice were backcrossed into pcy/pcy mice (CD-1) for 6–10 generations. In the first group, littermates of pcy/pcy: Postn−/− and pcy/pcy: Postn+/+ mice were sacrificed at 20 weeks of age, blood was collected for measurement of BUN and then both kidneys were removed causing fatal exsanguinations. The left kidney was homogenized in lysis buffer for protein extraction, and the right kidney was immersed in 4% paraformaldehyde, embedded in paraffin, and sectioned for immunohistochemistry. In the second group of pcy/pcy mice, we determined whether periostin expression affected survival by comparing pcy/pcy: Postn+/+, pcy/pcy: Postn+/− and pcy/pcy: Postn−/− litermates. All animals were provided food and water ad libitum and monitored daily. The protocol for use of these mice was approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Periostin PCR genotyping
Genotyping for Postn was determined by PCR analysis of genomic DNA with forward (5′-AGTGTGCAGATGTTTGCTTG-3′) and reverse (5′-ACGAAATACAGTTTGGTAATCC-3′) primers to detect the wild-type allele (~300 bp) and with forward (5′-GCATCGAGCTGGGTAATAAGGGTTGGCAAT-3′) and reverse (5′-GACACCAGACCAACTGGTAATGGTAGCGAC-3′) primers to detect the LacZ in the targeted allele (~800 bp) (40).
Quantitative real-time PCR
Total RNA was isolated from human ARPKD cells and mouse kidney tissues, loaded onto the column of an RNAeasy Mini Kit and eluted with RNase free water (12). RNA quality was verified and cDNA was synthesized using Invitrogen SuperScript III first-strand synthesis system. Relative expression levels were determined by SYBR green real-time PCR. Periostin and GAPDH primers were obtained from SuperArray (Frederick, MD).
Measurement of renal cystic area
Tissue sections (5 μm) of paraffin-embedded kidneys from 20-week old pcy/pcy: Postn+/+ and pcy/pcy: Postn−/− mice were collected 500 μm apart from the largest axial section to avoid redundant measurements of the same cysts. Sections were stained with hematoxylin and eosin and images were collected using a dissecting microscope connected to a digital camera. Total number of cysts and cystic cross-sectional surface area (SA) per kidney section were determined by a blinded observer using a morphometic analysis system.
Measurement of cell proliferation
The number of proliferating cells was determined by staining for PCNA and Ki-67. Briefly, tissue sections were deparaffinized, incubated in 0.3% hydrogen peroxide, rinsed in water and then incubated with an anti-PCNA antibody overnight at 4° C. The antigen was detected using the Zymed SuperPicTure polymer detection kit (Invitrogen) and PCNA-positive cells were visualized using 3,3′-diaminobenzidine. The slides were counter stained with hematoxylin and three images were captured per section for measurement.
Sections were also incubated in anti-Ki-67 antibody (1:100; Abcam, Cambridge, MA) and a secondary anti-rabbit antibody conjugated to AlexaFluor 488 was used to visualize Ki-67 positive nuclei. Nuclei were stained with DAPI in Prolong Gold Antifade mounting reagent (Invitrogen). The number of Ki-67 positive nuclei and total number of nuclei (700–1000 cells/image at 60× magnification) were determined by immunofluorescence using a Nikon Eclipse Ti microscope controlled by Metamorph software.
Measurement of fibrosis
Collagen fibers in kidney sections were stained with Masson trichrome and picrosirus red, as described previously (59, 60). Fibrosis (% SA) was determined by a renal pathologist without prior knowledge of the identity of the specimens (Supplemental Methods). Collagen fibers are highly birefringent and binding of picrosirius red, which is an elongated dye with anisotropic molecular organization, enhances collagen birefringence. Pathogenic collagen fibers appear as thick brilliant red or yellow bundles against a dark background in polarizing light.
Measurement of Blood Urea Nitrogen
Blood was collected at the time of sacrifice and serum was isolated by centrifugation at 1500 × g for 15 min. BUN was determined using an enzymatic conductivity rate method (Beckman Coulter, Brea, CA).
Western Blot Analysis
Kidney lysates were prepared for measurements of periostin, phosphorylated S6 (pS6), total S6 and phosphorylated S6K (pS6K) by immunoblot analysis (61). Proteins (40 μg protein/lane) were separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and incubated overnight at 4° C in primary antibody. Membranes were then washed and incubated with secondary antibody conjugated to horseradish peroxidase. Specific antibody signals were detected using chemiluminescence and band images were quantified using a Fluor-S MAX Multi Imager System (Bio-Rad).
Statistics
Data are expressed as mean and standard error (SE). Statistical significance was determined by unpaired t-test for comparison between Postn+/+ and Postn−/− mice.
Supplementary Material
Acknowledgments
Portions of this work have previously been published in abstract form (J. Am. Soc. Nephrol. 20: 439A, 2009). The authors thank Dr. Jared Grantham for helpful suggestions during the preparation of the manuscript.
GRANTS
This work was supported by a grant from the National Institutes of Diabetes and Digestive and Kidney Diseases (R01DK081579 to D. P. Wallace) and a grant from the Kansas City Area Life Sciences Institute (T.A. Fields).
Footnotes
DISCLOSURES
All the authors declared no competing interests.
References
- 1.Grantham JJ. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol. 1993;3:1841–1857. doi: 10.1681/ASN.V3121841. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PD, Hreniuk D, Gabow PA. Abnormal extracellular matrix and excessive growth of human adult polycystic kidney disease epithelia. J Cell Physiol. 1992 Feb;150:360–369. doi: 10.1002/jcp.1041500220. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 4.Harris PC. Molecular basis of polycystic kidney disease: PKD1, PKD2 and PKHD1. Curr Opin Nephrol Hypertens. 2002 May;11:309–314. doi: 10.1097/00041552-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Rossetti S, Strmecki L, Gamble V, et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001 Jan;68:46–63. doi: 10.1086/316939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol. 1999 Jul;19:327–343. [PubMed] [Google Scholar]
- 7.Qin S, Taglienti M, Nauli SM, et al. Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest. 2010 Oct;120:3617–3628. doi: 10.1172/JCI41531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, Boletta A, Chapman A, et al. Prospects for mTOR inhibitor use in patients with polycystic kidney disease and hamartomatous diseases. Clin J Am Soc Nephrol. 2010 Jul;5:1312–1329. doi: 10.2215/CJN.01360210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weimbs T. Regulation of mTOR by polycystin-1: is polycystic kidney disease a case of futile repair? Cell Cycle. 2006 Nov 1;5:2425–2429. doi: 10.4161/cc.5.21.3408. [DOI] [PubMed] [Google Scholar]
- 10.Wahl PR, Serra AL, Le Hir M, et al. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006 Mar;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 11.Wallace DP. Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta. 2011 Oct;1812:1291–1300. doi: 10.1016/j.bbadis.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace DP, Quante MT, Reif GA, et al. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol. 2008 Nov;295:F1463–1471. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006 Jan;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Nagao S, Wallace DP, et al. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003 Jun;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T, Wallace DP, Magenheimer BS, et al. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004 Sep 24;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 16.Belibi F, Ravichandran K, Zafar I, et al. mTORC1/2 and rapamycin in female Han:SPRD rats with polycystic kidney disease. Am J Physiol Renal Physiol. 2011 Jan;300:F236–244. doi: 10.1152/ajprenal.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song X, Di Giovanni V, He N, et al. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet. 2009 Jul 1;18:2328–2343. doi: 10.1093/hmg/ddp165. [DOI] [PubMed] [Google Scholar]
- 18.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011 Oct;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006 Jul;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Norris RA, Damon B, Mironov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007 Jun 1;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao S, Ouyang G, Bai X, et al. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004 Apr;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 22.Gillan L, Matei D, Fishman DA, et al. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 2002 Sep 15;62:5358–5364. [PubMed] [Google Scholar]
- 23.Lindner V, Wang Q, Conley BA, et al. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005 Jan;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Jin R, Norris RA, et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010 Feb;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 2004 May;24:3992–4003. doi: 10.1128/MCB.24.9.3992-4003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morra L, Rechsteiner M, Casagrande S, et al. Relevance of periostin splice variants in renal cell carcinoma. Am J Pathol. 2011 Sep;179:1513–1521. doi: 10.1016/j.ajpath.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorocos K, Kostoulias X, Cullen-McEwen L, et al. Expression patterns and roles of periostin during kidney and ureter development. J Urol. 2011 Oct;186:1537–1544. doi: 10.1016/j.juro.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Tai IT, Dai M, Chen LB. Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis. 2005 May;26:908–915. doi: 10.1093/carcin/bgi034. [DOI] [PubMed] [Google Scholar]
- 29.Kruzynska-Frejtag A, Machnicki M, Rogers R, et al. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001 May;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 30.Stanton LW, Garrard LJ, Damm D, et al. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000 May 12;86:939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 31.Norris RA, Kern CB, Wessels A, et al. Identification and detection of the periostin gene in cardiac development. Anat Rec A Discov Mol Cell Evol Biol. 2004 Dec;281:1227–1233. doi: 10.1002/ar.a.20135. [DOI] [PubMed] [Google Scholar]
- 32.Baril P, Gangeswaran R, Mahon PC, et al. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007 Mar 29;26:2082–2094. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 33.Riener MO, Fritzsche FR, Soll C, et al. Expression of the extracellular matrix protein periostin in liver tumours and bile duct carcinomas. Histopathology. 2010 Apr;56:600–606. doi: 10.1111/j.1365-2559.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki H, Lo KM, Chen LB, et al. Expression of Periostin, homologous with an insect cell adhesion molecule, as a prognostic marker in non-small cell lung cancers. Jpn J Cancer Res. 2001 Aug;92:869–873. doi: 10.1111/j.1349-7006.2001.tb01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tischler V, Fritzsche FR, Wild PJ, et al. Periostin is up-regulated in high grade and high stage prostate cancer. BMC Cancer. 2010;10:273. doi: 10.1186/1471-2407-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kudo Y, Ogawa I, Kitajima S, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 2006 Jul 15;66:6928–6935. doi: 10.1158/0008-5472.CAN-05-4540. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi H, Calvet JP, Dittemore-Hoover D, et al. A hereditary model of slowly progressive polycystic kidney disease in the mouse. J Am Soc Nephrol. 1991 Jan;1:980–989. doi: 10.1681/ASN.V17980. [DOI] [PubMed] [Google Scholar]
- 38.Olbrich H, Fliegauf M, Hoefele J, et al. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet. 2003 Aug;34:455–459. doi: 10.1038/ng1216. [DOI] [PubMed] [Google Scholar]
- 39.Wallace DP, Hou YP, Huang ZL, et al. Tracking kidney volume in mice with polycystic kidney disease by magnetic resonance imaging. Kidney Int. 2008 Mar;73:778–781. doi: 10.1038/sj.ki.5002771. [DOI] [PubMed] [Google Scholar]
- 40.Rios H, Koushik SV, Wang H, et al. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005 Dec;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattone VH, 2nd, Sinders RM, Hornberger TA, et al. Late progression of renal pathology and cyst enlargement is reduced by rapamycin in a mouse model of nephronophthisis. Kidney Int. 2009 Jul;76:178–182. doi: 10.1038/ki.2009.147. [DOI] [PubMed] [Google Scholar]
- 42.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006 Apr 4;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnet CS, Aldred M, von Ruhland C, et al. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum Mol Genet. 2009 Jun 15;18:2166–2176. doi: 10.1093/hmg/ddp149. [DOI] [PubMed] [Google Scholar]
- 44.Manning BD, Tee AR, Logsdon MN, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002 Jul;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 45.Lorts A, Schwanekamp JA, Baudino TA, et al. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-beta pathway. Proc Natl Acad Sci U S A. 2012 Jul 3;109:10978–10983. doi: 10.1073/pnas.1204708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010 Aug 10;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito T, Suzuki A, Imai E, et al. Tornado extraction: a method to enrich and purify RNA from the nephrogenic zone of the neonatal rat kidney. Kidney Int. 2002 Sep;62:763–769. doi: 10.1046/j.1523-1755.2002.00533.x. [DOI] [PubMed] [Google Scholar]
- 48.Calvet JP. Molecular genetics of polycystic kidney disease. J Nephrol. 1998;11:24–34. [PubMed] [Google Scholar]
- 49.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008 Dec;9:548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999 Jul;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 51.Oka T, Xu J, Kaiser RA, et al. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007 Aug 3;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snider P, Hinton RB, Moreno-Rodriguez RA, et al. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008 Apr 11;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol. 2011 Oct;7:556–566. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- 54.Satirapoj B, Wang Y, Chamberlin MP, et al. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant. 2012 Jul;27:2702–2711. doi: 10.1093/ndt/gfr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sen K, Lindenmeyer MT, Gaspert A, et al. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol. 2011 Oct;179:1756–1767. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada H, Ban S, Nagao S, et al. Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int. 2000 Aug;58:587–597. doi: 10.1046/j.1523-1755.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 57.Gattone VH, 2nd, Chen NX, Sinders RM, et al. Calcimimetic inhibits late-stage cyst growth in ADPKD. J Am Soc Nephrol. 2009 Jul;20:1527–1532. doi: 10.1681/ASN.2008090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omori S, Hida M, Fujita H, et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol. 2006 Jun;17:1604–1614. doi: 10.1681/ASN.2004090800. [DOI] [PubMed] [Google Scholar]
- 59.Montes GS, Junqueira LC. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 1991;86( Suppl 3):1–11. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, Sun L, Wang W, et al. Quantitative analysis of fibrosis formation on the microcapsule surface with the use of picro-sirius red staining, polarized light microscopy, and digital image analysis. J Biomed Mater Res A. 2006 Jan;76:120–125. doi: 10.1002/jbm.a.30491. [DOI] [PubMed] [Google Scholar]
- 61.Nagao S, Nishii K, Yoshihara D, et al. Calcium channel inhibition accelerates polycystic kidney disease progression in the Cy/+ rat. Kidney Int. 2008 Feb;73:269–277. doi: 10.1038/sj.ki.5002629. [DOI] [PubMed] [Google Scholar]
- 62.Farris AB, Adams CD, Brousaides N, et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011 Jan;22:176–186. doi: 10.1681/ASN.2009091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.