Abstract
Alzheimer disease (AD) is a progressive neurodegenerative disorder characterized by severe cognitive impairment, inability to perform activities of daily living and mood changes. Statins, long known to be beneficial in conditions where dyslipidemia occurs by lowering serum cholesterol levels, also have been proposed for use in neurodegenerative conditions, including AD. However, it is not clear that the purported effectiveness of statins in neurodegenerative disorders is directly related to cholesterol-lowering effects of these agents; rather, the pleiotropic functions of statins likely play critical roles. The aim of this review is to provide an overview on the new discoveries about the effects of statin therapy on the oxidative ad nitrosative stress levels as well as on the modulation of the heme oxygenase/biliverdin reductase (HO/BVR) system in the brain. We propose a novel mechanism of action for atorvastatin which, through the activation of HO/BVR-A system, may contribute to the neuroprotective effects thus suggesting a potential therapeutic role in AD and potentially accounting for the observation of decreased AD incidence with persons on statin.
Keywords: Alzheimer disease, biliverdin reductase, cognition, heme oxygenase, oxidative stress, statin
1. Introduction
Alzheimer disease (AD) is the most common form of dementia among the elderly and is characterized by progressive loss of memory and cognition [1]. With the increased life expectancy and subsequent population aging in developed countries, epidemiological data show the incidence of AD increases with age and doubles every 5 years after 65 years of age with 1275 new cases/100000 persons/year [1]. The Alzheimer Association points out that the financial, emotional, and family costs for care of AD patients are enormous and will increase markedly in the near future in the absence of a therapeutic modality to slow or stop onset. It was estimated that delaying the onset of AD by 5 years would decrease its prevalence by 50%. Given these facts, it is clear that the impact on the financial resources involved in the care of this pathology will be enormous. Therefore, it is incumbent for better understanding of the disease biochemistry, particularly the mechanisms related to the period prior to onset of the symptoms. From a neuropathological point of view, amyloid-β-peptide (Aβ) leads to senile plaques, which, together with hyperphosphorylated tau-based neurofibrillary tangles and synapse loss, are the principal pathological hallmarks of AD. Aβ is associated with the formation of reactive oxygen (ROS) and nitrogen (RNS) species, and induces calcium-dependent excitotoxicity, impairment of cellular respiration, and alteration of synaptic functions associated with learning and memory [1].
Statins, a class of hypolipidemic drugs, have been proposed as potential agents for the treatment or prevention of AD [2, 3]. Data from animal models studies suggest possible mechanisms underlying the beneficial role of atorvastatin in preventing AD, including the reduction of Aβ [4], β-secretase (BACE1) protein levels [5] and oxidative stress [6]. However, the importance of statin treatment in AD is still under debate, given that some randomized clinical trials did not show any significant benefit on cognition as reviewed by [7, 8]. In particular, the concerns regarding the mechanism of action by which statins mediate their potentially beneficial effects remain to be fully clarified. Are these benefits due to the well-known ability of statins to lower cholesterol or to their so called pleiotropic effects [9-11]? Statins in fact, can modulate several cellular pathways, independent of their ability to inhibit HMG-CoA reductase. These processes include effects on oxidative and nitrosative stress levels and modulation of the heme oxygenase/biliverdin reductase (HO/BVR) system which are area of studies ongoing in the Butterfield laboratory and will be the main subject of discussion in this review.
2. Statins’ pharmacokinetic and pharmacodynamics profile
Statins are a family of drugs with pleiotropic functions. To this class belong eight drugs: mevastatin and lovastatin, which were the first developed and studied in humans; pravastatin and simvastatin, which can be considered as derivatives of the parental lovastatin; and atorvastatin, fluvastatin, rosuvastatin and pitavastatin, which are distinct synthetic compounds [12]. Due to their main mechanism of action, namely the inhibition of the hydroxyl-methyl-glutaryl-CoA (HMG-CoA) reductase, statins are widely used for the treatment of dyslipidemias [12]. By inhibiting HMG-CoA reductase, statins block the conversion of HMG-CoA into mevalonate, the first step in cholesterol biosynthesis [12, 13]. As a result of statin administration, low-density lipoprotein (LDL)-cholesterol synthesis decreases in hepatocytes, and this reflects a reduced cholesterol blood level. In addition to this effect, statins have been shown to reduce triglyceride and increase HDL-cholesterol plasma levels. Taken together, the composite effect of statins in reducing triglycerydes and LDL-cholesterol, coupled with the increase in HDL-cholesterol, put these drugs in the arena of cardiovascular agents, due to their ability to counteract hyperlipidemias, the major cause of atherosclerosis which, in turn, is a common pathogenetic mechanism for coronary artery disease, ischemic cerebrovascular disease and peripheral vascular disease [12, 13].
Although all statins share the same main mechanism of action, their pharmacokinetic profile is quite different. All statins are well absorbed by the intestine when given orally, even though they undergo marked first-pass effects in the liver, which reduces the systemic biovailability (5-30%) [12]. With the exception of simvastatin and lovastatim, which are pro-drugs and require hepatic activation, other statins are administered as β-hydroxy-acids. Upon administration, statins reach peak plasma concentration, ranging from 10-448 ng/ml, within 0.5-4 h. In the plasma, statins are bound to albumin (43-99%) and this binding accounts for their variable half-life [12]. Atorvastatin and rosuvastatin are the statins with the longest half-life (15-30 and 20.8 h, respectively), whereas fluvastatin, lovastatin, pravastatin and simvastatin have half-lives around 0.5-3 h [12]. Statins generally are metabolized by the liver through the isoforms 3A4 (atorvastatin, lovastatin and simvastatin) and 2C9 (fluvastatin and rosuvastatin) of the cytochrome-P-450 (CYP) system, whereas pravastatin undergoes sulfation. The primary route of elimination is fecal, and only a minor fraction of statins is eliminated via urine [12, 13].
The main adverse effects of statins are hepatotoxicity and myopathy. A transient elevation of serum transaminases (up to 3-times the baseline value) is a common outcome of statin therapy [13]. However, the incidence of this side effect is low and dose-dependent and does not imply the contraindication of statins in individuals with concomitant liver diseases such as hepatitis C [13]. Myalgia is often associated with statin use and is paralleled by significant elevation in plasma creatine kinase [13]. Rhabdomyolisis is quite rare, and the risk to develop this side effect of statins is correlated to the dose and plasma concentration [13]. About 30 cases of serious hepatic failure and 42 cases of death due to rhabdomyolisis associated with statin administration were reported to the FDA over the last 15 years [13, 14]. In order to reduce the incidence of hepatotoxicity and myopathy, statins should not be taken with inhibitors of CYP3A4 such as azole antifungals, erythromycin, ritonavir and grapefruit juice. Also the association statins and fibrates should be avoided, in particular gemfibrozil [13].
3. Therapeutic use of statins in the treatment of Alzheimer dementia: insights from epidemiological studies and randomized clinical trials
The association between Alzheimer disease and cholesterol levels has grown in the last decade due to population-based studies supporting the observation that hypercholesterolemia in midlife correlates with an increase in the risk to suffer AD in later life [15]. Moreover, polymorphisms in apolipoprotein E (apoE) and other proteins involved in cholesterol metabolism are considered as risk factors for AD. Thus, the treatment of AD with statins, to inhibit cholesterol synthesis and accumulation in the brain, was proposed by several authors as an effective emerging therapy to stop or delay the neurodegenerative process [16]. Over time a number of studies were performed to test the efficacy of statin treatment on cognitive decline, but many discrepancies exist among results obtained from preclinical studies, epidemiological studies and randomized clinical trials and so far statin neuroprotective activity is still under debate [17-21] (see table 1 and 2). Several experimental factors, such as the type of statin selected, the study group employed, the stage of the disease and the duration of treatment could in part explain the divergence among different studies [22, 23] and should be taken in account to evaluate statins effectiveness on AD onset and progression.
Table 1.
Epidemiological studies on AD subjects using statins
| Reference | Statins | Cohort size | follow up | Results |
|---|---|---|---|---|
| Jick et al. 2000 [3] | atorvastatin, cerivastatin, fluvastatin, pravastatin and simvastatin analyzed together | 1364 subjects | 6 years | lower risk of developing dementia |
| Wolozin et al. 2000 [24] | lovastatin, pravastatin analyzed together and lovastatin, pravastatin and simvastatin analyzed separately | 60349 subjects | N/A | protective effect of lovastatin and pravastatin on AD prevalence |
| Hajjar et al. 2002 [25] | not specified | 655 subjects | 1 year | lower risk of developing AD |
| Rodriguez et al. 2002 [32] | not specified | 845 | 2 years | no effects on elderly people |
| Rockwood et al. 2002 [26] | not specified | 2305 subjects | 1 year | lower risk of developing dementia |
| Zandi et al. 2005 [33] | lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin and fluvastatin analyzed together | 4895 subjects | 3 years | no correlation with the risk of developing any dementia or AD |
| Rea et al. 2005 [34] | lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin and fluvastatin analyzed together | 2798 subjects | ≅5 years | no correlation with the risk of developing any dementia or AD |
| Li et al. 2007 [27] | simvastatin, lovastatin, pravastatin and atorvastatin analyzed together | 110 subjects | ≅12 years | lower Braak stages |
| Sparks et al. 2008 | not specified | 2068 subjects | 3 years | reduced risk of incident AD |
|
ADAPT study [28, Cramer et al. 2008
SALSA study [30] |
atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin analyzed together | 1021 subjects | 5 years | reduction in the incidence of combined dementia and cognitive impairment without dementia |
| Arvanitakis, et al. 2008 [35] | simvastatin, lovastatin, atorvastatin, pravastatin and fluvastatin analyzed together | 929 subjects | ≅ 12 years | decreased amyloid pathological changes, no effects on AD risk or cognitive ability. |
|
Haag et al. 2009
Rotterdam study [31] |
atorvastatin, cerivastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin analyzed together and simvastatin and pravastatin analyzed separately | 6992 subjects | 9.2 years | reduced risk of late-onset AD |
Table 2.
Clinical trials on AD subjects using statins
| Reference | Phase | Statins | Cohort size | follow-up | Results |
|---|---|---|---|---|---|
|
Shepherd et al. 2002
PROSPER study [42, 43] |
III | pravastatin 40 mg/day | 5804 subjects | 3.2 years | no evident benefit in cognition |
| MRC/BHF study 2002 [44] | III | simvastatin 40 mg/day | 20536 subjects | 5 years | no differences in cognition |
|
Sparks et al. 2003
ADCLT study [36] |
II | atorvastatin 80 mg/day | 67 subjects | 1 year | positive effect on the ADAS-cog performance was observed in mild-to-moderate AD subjects |
| Rieske et al. 2006 [39, 40] | II | simvastatin 40 mg/day , pravastatin 80 mg/day | 23 subjects | 14 weeks | protective effect on AD development |
| Carlsson et al. 2008 [41] | II | simvastatin 40 mg/day | 57 subjects | 4 months | improved cognitive function in middle-aged adults |
|
Feldman et al. 2010
LEADe study [45, 46] |
II | atorvastatin 80 mg/day | 641 | 18 months | no benefits on AD treatment |
|
Sano et al. 2011
CLASP-AD study [48] |
II | simvastatin 20 mg/day for 6 weeks and then 40 mg/day | 406 | 18 months | no benefits on AD treatment |
Epidemiological evidence on the beneficial effect of statins (atorvastatin, cerivastatin, fluvastatin, pravastatin and simvastatin analyzed together) in lowering the risk of developing dementia were found since 2000 by Jick and colleagues [3] (table 1). In the same year Wolozin et al. [24] showed that lovastatin and pravastatin, but not simvastatin was associated with reduced AD prevalence when compared with the general population or patients taking other medications for hypertension or heart disease. In 2002 Hajjar et al. [25] reported a clinical-based study of 655 patients, and found a lower risk of AD in people who took statins. In another study Rockwood et al. demonstrated that statin use among subjects with incident dementia opposed to cognitively healthy subjects was associated with a lower risk of AD [26]. In a 2007 study by Li et al. [27] a neuropathological assessment on statin users (simvastatin, lovastatin, pravastatin and atorvastatin analyzed together) compared to non-users concluded that subjects had lower Braak stages, but not lowers CERAD scores. Sparks and colleagues [28, 29] performed in 2008 the Alzheimer's Disease Anti-inflammatory Prevention Trial (ADAPT) study, an observational study on elderly individuals at-risk-for-AD that were subjected to statin use, demonstrating a significantly reduced risk of incident AD. In the same year Cramer et al. [30] reported the results from the Sacramento Area Latino Study on Aging (SALSA), a prospective cohort observational study indicating that statins (atorvastatin, cerivastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin analyzed together) were associated with a significant reduction in the incidence of combined dementia and cognitive impairment without dementia (CIND). All the above findings were strongly corroborated by the results of the Rotterdam Study [31], a prospective study involving a cohort of almost 7000 subjects for an average a period of 9.2 years. Haag and colleagues [31] found that statins (atorvastatin, cerivastatin, fluvastatin, pravastatin, rosuvastatin and simvastatin analyzed together and simvastatin and pravastatin analyzed separately) correlates with almost 50 % of reduced risk of late-onset Alzheimer disease, independently of apoE genotype, and their lipophilic properties. However, not all observational studies concluded that statins were associated with beneficial effects in the setting of AD (table 1). In 2002 Rodriguez et al. [32] reported that statins alone did not associate with significant benefit on elderly people from the cross-sectional component of the Pennsylvania-based Monongahela Valley Independent Elders Survey, and in 2005 Zandi et al. [33] showed, on a well-known American cohort study of dementia conducted in Cache County, Utah, that statin (lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin and fluvastatin analyzed together) therapy did not correlate with the risk of developing any dementia or AD, although people taking statins appeared less likely to develop amyloid plaques. In 2005, another study by Rea et al. [34] demonstrated that statin (lovastatin, simvastatin, cerivastatin, atorvastatin, pravastatin and fluvastatin analyzed together) treatment of patients 65 years or older did not vary their risk of developing AD or other forms of dementia, and this result was not dependent on the type of drugs analyzed. Finally, in 2008 Arvanitakis et al. [35] reported that, although statin (simvastatin, lovastatin, atorvastatin, pravastatin and fluvastatin analyzed together) users were less likely to be demented at the time of death and presented decreased amyloid pathological changes, statin use did not associate with AD risk or cognitive ability.
Observational studies generated initial enthusiasm for statins as preventive agents for AD and dementia development and several randomized clinical trials were planned in order to support their neuroprotective activity (table 2). Favorable results were obtained by Sparks et al. [36] who performed the Alzheimer's Disease Cholesterol-Lowering Treatment (ADCLT) study, a randomized double-blind phase II trial in which subjects were administered 80 mg/day of atorvastatin or placebo for 1 year, without dose escalation. A significant positive effect on the ADAS-cog performance was observed in mild-to-moderate AD subjects with high serum cholesterol at baseline, or apolipoprotein E4 allele [37, 38]. Riekse et al. [39, 40] obtained similar outcomes using simvastatin (40 mg/day) or pravastatin (80 mg/day) in hypercholesterolemic subjects without dementia, and the authors suggested that statins may play a preventive role in the risk of disease development. This hypothesis was further supported by the findings of Carlsson et al. [41] who reported that simvastatin (40 mg/day) improved cognitive function in middle-aged adults whose parents suffered from AD.
In contrast with the above-mentioned results, two double-blind, randomized, placebo-controlled phase III trials on statins, PROSPER and the MRC/BHF Heart Protection Study have not confirmed a clinically demonstrable cognitive benefit of statins in the treatment of AD. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) [42, 43] included 5804 patients ranging in age from 70 to 82 years with mean follow-up of 3.2 years. PROSPER patients received pravastatin or placebo, and despite a 34% reduction in the LDL cholesterol concentration there was no evident benefit in cognition. The Medical Research Council / British Heart Foundation Heart Protection Study had the primary goal in evaluating simvastatin ability to prevent heart disease among older 20,536 adults. Participants were followed for up to 5 years and the prevention of cognitive impairment was measured as a secondary outcome [44]. No differences in cognition were observed between the simvastatin-treated and placebo-control groups.
The results from “Lipitor's Effect in AD” (LEADe) trial did not demonstrate significant benefits with atorvastatin in mild to moderate Alzheimer's disease [45, 46]. Treatment in LEADe consisted of 80 mg/day atorvastatin compared to placebo for 72 weeks in patients (mean age 74 years) with mild-to- moderate AD receiving background therapy of donepezil 10 mg daily. Recently, the Cholesterol-lowering Agent to Slow the Progression of AD trial (CLASP-AD), a multicenter, randomized, double-blind, placebo-controlled phase II trial, with the aim to investigate effects of an 18-month simvastatin treatment (20 mg/day for 6 weeks, then 40 mg/day for the remainder of the study period) vs. placebo was completed [46, 47]. Sano et al. [48] found that simvastatin had no positive effect on the results of mild-to-moderate AD patients. Interestingly, a recent work by Padala et al. reported that AD patients under statin regimen experienced an improvement in cognition with discontinuation of statins and worsening with re-challenge [49].
High levels of serum cholesterol are thought to contribute to the pathology of Alzheimer's disease but overall, the neuroprotective value of cholesterol synthesis inhibitors still remains unclear. So far, data obtained from observational studies appear insufficient due to their heterogeneity and the presence of many confounding variables, to define the potential role of statin to improve cognitive decline and to recommend statins for the treatment of Alzheimer's disease or dementia [45]. In addition, phase III randomized clinical trials showed no enhanced benefits in the use of statins on AD patients in contrast with the outcomes described in phase II studies. Therefore, further clinical trials with large cohort, long duration and distinctive stages of disease need to be performed in order to delineate the ambiguous protective properties of different classes of statins associated with cognition in patients at risk of AD-like dementia.
4. Statin-dependent effects on oxidative and nitrosative stress levels in Alzheimer disease
An intriguing aspect related to the pleiotropic effects induced by statins treatment regards the modulation of oxidative stress-related modifications that occur in neurodegenerative disorders, including AD [50]. From a general point of view, statins possess a janus face since both in vitro and in vivo statins were able to modulate several cellular pathways having neuroprotective or neurotoxic effects independent on their ability to lower cholesterol, but, rather, dependent on the kind of statin used [7]. The neuroprotective effects include: (i) the inhibition of endothelial O2−· formation by preventing the isoprenylation of p21 Rac, which is critical for the assembly of NADPH oxidase after activation of PKC [51]; (ii) the increase of SOD3 activity as well as the number of functionally active endothelial progenitor cells [52]; (iii) the increase of the expression of endothelial nitric oxide synthase (eNOS) by inhibition of Rho isoprenylation [53]; (iv) the activation of eNOS via post-translational mechanisms involving activation of the PI3K/Akt pathway [54]. Coversely, the neurotoxic effects include: (i) increased cell death [55]; (ii) decreased CoQ10 levels [56, 57]; (iii) inhibition of Ras-induced ERK1/2 phosphorylation [58]; (iv) decrease neurite outgrowth [59]; (v) reduction of myelin basic protein expression [60].
With regard to AD, over the past two decade, the predominant view regarding the cause of the pathology is embodied in the amyloid cascade hypothesis, which predicts that amyloid pathology is upstream from tau pathology and neuronal loss, and hence, enormous effort was undertaken to research and develop disease-modifying strategies targeting synthesis, aggregation, and clearance of Aβ [1, 61]. Aβ peptides, together with altered mitochondrial function, and the presence of trace metal ions such as iron and copper, have been identified as potential sources of oxidative stress [62-64]. Consistent with the Aβ-induced oxidative stress hypothesis, oxidative stress is the result of Aβ insertion as oligomers into the bilayer causing ROS production and initiating lipid peroxidation and protein oxidation in AD pathology [62, 65-67]. Statins showed positive effects against AD-relevant Aβ-induced oxidative stress in mice models of AD [68, 69] as well as a reduction in CSF tau protein phosphorylation in humans [70]. However, although statins’ treatment appears to provide greater benefits, it is difficult to tease out whether the benefits are really due to lower cholesterol levels or to statin pleiotropy [11].
In 2008, Kurinami et al. reported that pre-treatment with fluvastatin (5 mg/kg/day), but not with simvastatin (5 mg/kg/day), significantly prevented memory impairment induced by Aβ in mice. The beneficial effects of fluvastatin might be explained by the prevention of cholinergic neuronal loss through a significant decrease in Aß accumulation and oxidative stress. However, in this study, Aß was exogenously injected and therefore, the decrease in Aß accumulation by fluvastatin was not through the direct inhibition of Aß production and/or secretion, but possibly through a novel action of statins on Aß metabolism [68].
In 2009, Tong et al. by using 10 month-old mutant amyloid precursor protein transgenic mice (APP mice), showed that simvastatin (20 mg/kg/day, 8 weeks) improved reactivity of cerebral arteries, rescued the blood flow response to neuronal activation, attenuated oxidative stress and inflammation, and reduced cortical soluble Aβ levels and the number of Aβ plaque-related dystrophic neurites. However, at such an advanced stage of the pathology, it failed to reduce Aβ plaque load and normalize cholinergic and memory deficits. These findings demonstrated that low-dose simvastatin treatment in aged APP mice largely salvages cerebrovascular function and has benefits on several AD landmarks, which conceivably could contribute to some of the positive effects of statins reported in AD patients [71].
Subsequently, in 2011, Kurata et al. reported on the beneficial effects of atorvastatin (30 mg/kg/day, p.o.) and pitavastatin (3mg/kg/day, p.o.) in APP transgenic mice treated from 5 months to 20 months of age. These researchers showed improved behavioral memory and reduced the numbers of senile plaques and phosphorylated tau-positive dystrophic neurites at 15 and 20 months of age [4]. These protective effects of statins took 10 months from the beginning of treatment to demonstrate an improvement, and sensitivity to the statin treatment was linked to behavioral memory, senile plaques and phosphorylated tau-positive dystrophic neurites in this order [4]. These findings suggest that early treatment with both atorvastatin and pitavastatin prevented subsequent worsening of cognitive function and the amyloidogenic process, probably due to pleiotropic effects, consistent with a therapeutic potential for AD [4]. In the same year, Piermartiri et al. showed that atorvastatin (10 mg/kg/day, 7 days) treatment was neuroprotective against cell degeneration induced by Aβ(1-40), reducing inflammatory and oxidative responses and increasing the expression of glutamatergic transporters [72].
The different effects obtained in the above-cited studies arise from a number of aspects including (i) the experimental model used; (ii) the kind of statin used whose effects in turn are dependent on both their pharmacokinetics and pharmacodynamics properties; (iii) the scheme of treatment employed; and (iv) the duration of the observations. In particular, the lipophilic or hydrophilic nature of statins represents an important aspect. Nevertheless, because cholesterol and cholesterol synthesis intermediates and derivatives are important in the brain and because it is difficult to determine the beneficial statin dose, currently hydrophilic statins use could be more secure for investigational purposes. In fact, more lipophilic statins when prescribed as a treatment for hypercholesterolemia, had a higher incidence of neurological side effects than others [73]. For this reason it becomes clear that several variables have to be taken into consideration when a statin treatment is planned. In addition, rodent models have a significant limitation because long-term treatment with statins leads to an up-regulation of HMG-CoA reductase, thereby preventing any stable, long-term reduction in cholesterol levels [74]. This fact leads to difficulties in conducting long term studies in rodents with extensive behavioral testing but additionally leads to doses of statins that are physiologically excessive relative to human clinical trials. Thus, translating outcomes from rodent studies to humans is limited.
Based on this limitation in rodents, an animal model that (i) could better mimic the development of AD pathology observed in humans, and (ii) could represent a good model to evaluate the long-term effects of statins is required. Aged beagles, represent a good pre-clinical model to study AD because they deposit endogenous levels of Aβ of identical sequence to human Aβ [75] as they age and thus are a natural higher mammalian model of aging. The canine β-amyloid precursor protein (APP) is virtually identical to human APP (~98% homology). Most of the deposits in the canine brain are of the diffuse subtype, but are fibrillar at the ultrastructural level which models early plaque formation in humans [76-78]. Moreover, in terms of the pattern and severity of cognitive decline, the aged canine parallels mild cognitive impairment in humans [79]. Aged beagles can be used to conduct long-term studies using statins and extensive behavioral testing, as HMG-CoA reductase is not up-regulated over time in these animals.
Murphy et al. in 2010 showed that long-term atorvastatin (80 mg/day for 14.5 months) did not have any effect on Aβ levels, despite a significant reduction in β-secretase 1 (BACE1) protein levels and activity in the brain of aged beagles [5]. The lack of change in Aβ levels in canines treated for over one year, in spite of substantial changes in circulating cholesterol and lipid profiles, strongly suggested that changes in preexisting Aβ pathology may not be the mechanism related to some reports of clinical benefits of statin treatment. It is possible that reductions in the activity of BACE1, may lead to reduced risk in dementia through other mechanisms [5].
In support of this idea is the overwhelming rate of drugs failing clinical trials over the past decade indicating that single target therapies in patients with AD, even in those with milder stages of the disease, may be insufficient because of the numerous pathways and resultant damage triggered by accumulation of Aβ [73]. As patients who already have dementia also have extensive neuronal loss, in addition to the presence of plaques and tangles, it is unlikely that removing the existing pathologies will be beneficial without a simultaneous strategy to also help the brain recover from the extensive neuronal damage. Our view is that researchers must focus attention on understanding how the brain responds to extensive neuronal loss and find ways to promote functional recovery. The increased levels of oxidative and nitrosative stress and the resultant neurotoxic effects observed in the brain of AD subjects [80-82] may represent good targets to evaluate in this sense.
The Butterfield laboratory was the first to use aged beagles in order to evaluate oxidative and nitrosative stress levels in the brain following long-term administration of a statin. In 2010, Barone et al. found that although no change in Aβ levels occur [5], long-term atorvastatin (80 mg/day for 14.5 months) significantly reduced lipoperoxidation, protein oxidation and nitration, and increased GSH levels in parietal cortex of aged beagles [6]. This effect was cholesterol- and Aβ-independent and specific for brain [6] suggesting that an additional benefit of atorvastatin is possibly based on its induced antioxidant properties. Furthermore, the significant correlations found among decreased levels of oxidative stress markers and decreased size discrimination error score - reflecting improved cognition - observed in aged dogs after atorvastatin treatment, suggested that the effect on cognition could be due to the reduced oxidative stress instead of the ability of atorvastatin to reduce cholesterol levels.
Another main point with regard to the effects produced by statins on oxidative and nitrosative stress levels is that cholesterol itself can be oxidized with likely loss of its functions, despite its lower levels. Cholesterol can undergo oxidative modifications at least by two mechanisms: a direct radical attack involving ROS or RNS (non-enzymatic mechanism), or by the activity of a specific enzymes (enzymatic mechanism) [83]. Cholesterol oxidation leads to the formation of oxysterols. These latter moieties are major regulators of cholesterol homeostasis in the central nervous system [83]. Among oxysterols, 7-ketocholesterol (7-K) and 25-hydroxycholesterol (25-OH) have been shown to cause apoptotic neuronal death by inducing mitochondrial dysfunction [84], Ca2+ influx and perturbation of intracellular ionic homeostasis [85, 86]. Although some evidence suggests the importance of cholesterol oxidation products both as in vivo markers of oxidative stress [27, 87, 88], as well as for their pro-oxidant features [84-86, 89], few studies exist regarding the effect of statins on cholesterol oxidation products in vivo [27, 90, 91]. In 2010, our laboratory showed for the first time that atorvastatin can have two independent effects on cholesterol and cholesterol oxidation products, since a reduction of cholesterol was not associated with a reduction of 7-K or 25-OH and vice versa. In fact, the levels of both 7-K and 25-OH were reduced in brain (where no change in cholesterol levels were observed), while 7-K levels were significantly increased in serum (where a significant reduction of cholesterol levels was observed) in dogs receiving atorvastatin [6]. In our opinion, these results support the idea that atorvastatin possesses pleiotropic functions that are responsible of the effects observed in the brain. While for the brain a conceivable conclusion could be that, despite no changes in cholesterol levels, the decrease of 7-K and 25-OH could be due to the atorvastatin-dependent or -mediated antioxidant effects as described below in this review, whereas for the serum other possibilities have to be explored. Indeed, it seems that despite a reduction of cholesterol levels in serum [5], an increase of its oxidative metabolism occurs. It would not be surprising to find out that 7-K, due to its pro-oxidant features, is responsible, at least in part, for the lack of atorvastatin-mediated antioxidant effects observed in serum [5] . These concerns suggests that an in depth analysis of the effects mediated by statin therapy should be performed in order to avoid possible risks related to the production of cholesterol-dependent toxic species, such as 7-K, despite cholesterol reduction.
Last, but not least, side effects of long-term statin treatment include a decrease in CoQ10 levels resulting in impairment of energy metabolism in heart, skeletal muscle, and liver [92]. CoQ10 is a mitochondrial electron transporter vital for ATP production and a powerful mitochondrial and cellular antioxidant found in all cells [93]. CoQ10 is a potent gene regulator, involved in the expression of hundreds of genes, including those involved in optimal mitochondrial function and inflammatory processes [94]. HMG-CoA reductase is a key enzyme in CoQ10 biosynthesis. Inhibition of HMG-CoA reductase by statins is associated with lower circulating levels of CoQ10 in rodents [95], canines [96] and humans [97]. CoQ10 deficiency results in decreased mitochondrial activity and mitochondrial degradation and increased ROS and inflammation [98]. CoQ10 levels tend to naturally decline with age and may play a role in both AD-related mitochondrial dysfunction and inflammation [99]. Further, aged canines show impaired mitochondrial function and may be particularly vulnerable to reduced CoQ10 [100].
Our group demonstrated that atorvastatin (80 mg/day for 14.5 months) treatment reduced CoQ10 in the parietal cortex of aged beagles even if not in a significant manner [101]. Conversely, as expected, CoQ10 was significantly reduced in serum of statin-treated dogs compared with controls [101]. Interestingly, we found that reversal learning error scores were inversely correlated with parietal cortex CoQ10, but not serum CoQ10. Thus, lower levels of CoQ10 in the parietal cortex, but not serum, are associated with deficits in reversal learning ability (impaired cognition) [101].
These results suggest that statins can exert antioxidant/pro-oxidant effects depending on the site of action and on the mechanisms modulated. Due to duration of statin treatment, it would be interesting to carry out in vivo studies to analyze in the brain changes that occur to cholesterol oxidation products and CoQ10. Can these changes to be correlated? Do statins decrease CoQ10 in the brain? Is reduction/increase of CoQ10 associated with different levels of oxysterols? Supplementation of the diet with CoQ10 was reported to reverse many of these alterations [102]. Hence, CoQ10 supplementation may respresent a potential therapeutic strategy to improve statin therapeutic approaches in AD.
Based on this evidence, we opine that the rationale for statin therapy in AD should be expanded beyond simple cholesterol lowering to include the wide range of mechanisms in which statins can exert an influence. Since human brain analysis is only possible post-mortem, in the absence of a surrogate peripheral marker for brain changes secondary to statin therapy, this suggestion above remains a difficult proposition. Such a surrogate marker would permit temporal monitoring of effectiveness of statin therapy in humans.
5. Statin-dependent effects on the heme oxygenase/biliverdin reductase system in CNS and their relevance for Alzheimer disease
The up-regulation of the heme oxygenase-1/biliverdin reductase-A (HO-1/BVR-A) system is one of the earliest events in the adaptive response to stress [103-105] . Heme oxygenase exists in two main isoforms named HO-1 and HO-2. Heme oxygenase-1 is the inducible isoform and is up-regulated in response to oxidative and nitrosative stress or some pharmacological treatments. Conversely, HO-2 is the constitutive isoform and is involved in the physiologic turnover of heme. Both isoforms catalyze the same reaction [103, 104]. Similar to HO, two isoforms of BVR were described and named BVR-A and BVR-B [106-108]. Both these enzymes generate bilirubin (BR), but only BVR-A reduces BV-α into the powerful antioxidant and antinitrosative molecule BR-IX-alpha [109, 110], whereas BVR-B (the fetal isoform) refers the other BV isoforms, such as BV-β, BV-γ and BV-δ [106-108].
The HO-1/BVR-A system reduces the intracellular levels of pro-oxidant heme and generates equimolar amounts of the free radical scavengers biliverdin-IX alpha (BV)/bilirubin-IX alpha (BR) as well as the pleiotropic gaseous neuromodulator carbon monoxide (CO) and iron [Fe(II)] [104, 109, 111-113] (Figure 1). Specifically, the up-regulation of the HO-1/BVR-A system was proposed as a useful mechanism to counteract AD-induced oxidative/nitrosative damage [66, 105, 114-118]. However, BVR-A not only reduces BV into BR, but it is also a serine/threonine/tyrosine kinase that belongs to the insulin receptor substrate family [106, 107]. Interestingly, BVR-A stimulates its own reductase activity through the autophosphorylation of specific serine/threonine residues [119]. In addition, phosphorylated BVR-A interacts with members of the mitogen activated protein kinase family, in particular, the extracellular signal-regulated kinases 1/2 (ERK1/2), and regulates the expression of oxidative-stress-responsive genes such as HO-1 or inducible nitric oxide synthase (iNOS) [106, 120-123].
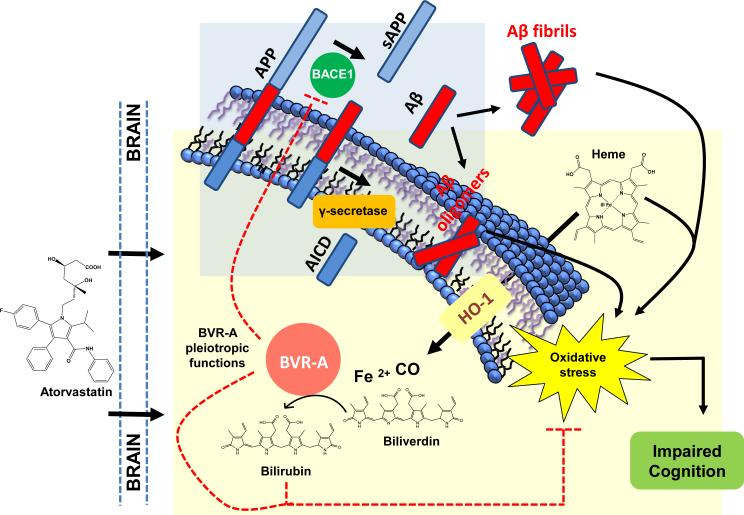
Figure 1. Schematic representation of atorvastatin-induced HO/BVR-A system-dependent neuroprotective effects in the brain.
Alzheimer disease (AD) is characterized by an increase of beta amyloid production (Aβ) following beta (BACE1) and gamma secretase (γ-secretase)-dependent cleavage of amyloid precursor protein (APP). Accumulation of Aβ oligomers is responsible for the observed increased oxidative stress levels in the brain. In order to counteract increased oxidative stress levels, cells promote the up-regulation of the heme oxygenase-1/biliverdin reductase-A (HO-1/BVR-A) system that is one of the earliest events in the adaptive response to stress. The HO-1/BVR-A system reduces the intracellular levels of pro-oxidant heme and generates equimolar amounts of the free radical scavengers biliverdin/bilirubin as well as the pleiotropic gaseous neuromodulator carbon monoxide (CO) and ferrous iron [Fe(II)]. Atorvastatin administration was able to increases (i) HO-1 protein levels and (ii) both BVR-A protein levels and activity (increased bilirubin (BR) production). Either BVR-A and BR possesses antioxidant features responsible of the reduction of oxidative stress, as demonstrated by the negative correlations found between oxidative stress biomarkers levels and (i) BVR-A protein levels or (ii) BVR activity in the brain [147]. Furthermore the up-regulation of the HO-1/BVR-A system is associated with an improvement of cognitive functions (learning) following atorvastatin treatment [147]. Finally, BVR-A protein levels and activity were significantly associated with decreased BACE1 protein levels suggesting a role for BVR-A in Aβ production [147]. All these effects contribute to the neuroprotective role of the HO-1/BVR-A system in the brain. Arrows, stimulation; dotted lines, inhibition.
There is a notable degree of sequence similarity between the primary structure of BVR-A and the kinase domain of the insulin receptor and insulin receptor substrates (IRS) [119], and the organization of the protein is similar to that of protein kinase C (PKC) isozymes [124]. The N terminus contains the catalytic domain, whereas the bulk of consensus signaling motifs are crowded in the C terminus . The secondary structure of BVR-A is a prominent factor in its signaling activity. The C-terminal half of BVR-A consists of a large six-stranded β-sheet, which is likely to be the platform for protein–protein interactions [125]. Tyrosine residues in the two consensus Src homology (SH2)-binding motifs (Tyr198 in YMKM and Tyr228 in YLSF) are targets of insulin receptor kinase (IRK), whereas those in the catalytic domain are autophosphorylated [119]. The basic-leucine-zipper (bZip) motif is a DNA interactive structure found in all stress-activated transcription factors including c-Jun, c-Fos, activating transcription factor 2 (ATF2; also known as cAMP-response element binding protein [CREB]), and myelocytomatosis viral oncogene (cMyc). BVR-A in dimeric form binds to DNA [126]. The consensus sequences of the nuclear-localization signal (NLS) and nuclear-export signal (NES) are essential for BVR-A-facilitated nuclear transport of activated signaling components such as extracellular signal-regulated kinase (ERK1/2) [120] and for cytoplasm-nuclear transport of heme, the transcriptional regulator of gene expression, including that of HO-1 [120, 121]. The β-sheet features two consensus MAPK binding sites, the C- and D(d)-box, which are conserved sequences that provide, respectively, high- and low-affinity binding sites for kinases in the MAPK pathway [127] and are key elements in BVR-A regulation of MEK (MAPK–ERK kinase)–ERK1/2–Elk (eukaryotic-like protein kinase) signaling [120]. The dual specific kinase activity of BVR-A is characterized by the ability of this protein to recognize both the hydroxyl groups of Ser/Thr, and Tyr [128]. The substrates known, so far, to be phosphorylated by BVR-A include (i) IRS-1 and (ii) the members of conventional and atypical groups of PKC isozymes: PKC-βII and PKC-ζ and PKC-δ, respectively [106, 129]. Critically for signaling activity, BVR-A also interacts with several protein kinase such as ERK1/2, MEK, Elk1; this interaction aids in the activation of these kinases, as well as playing a role in their translocation to the site of activity. The experiments used to characterize these sequences relied on site-directed mutagenesis and siRNA studies to disrupt each function [120, 121, 126, 130-132]. More recent studies have focused on the use of peptides based on the BVR-A sequence as a means of disrupting protein/protein interactions, and/or modulating the enzyme activities of BVR-A or its binding partners (reviewed in [129]).
Recent studies raised the questions about the activation of the HO-1/BVR-A system in neurodegenerative disorders, opening a debate on its real pathophysiological and clinical significance [105]. Given that up-regulation of HO-1 is widely accepted as a sensitive and fairly ubiquitous marker of oxidative stress, two main schools of thought exist with regard to the role of HO-1/BVR-A system in AD. One of these, accounts for a detrimental activity of HO-1 suggesting that iron deposition and attendant neuronal dysfunction in AD may represent downstream effects of sustained HO-1 over-activity within the astrocyte compartment [133-136]; whereas the other, coming from our group, proposed the up-regulation of HO-1/BVR-A system as a neuroprotective mechanism aimed to counteract the rise of oxidative stress observed during the onset and the progression of AD [62, 66, 111, 117, 137]. However, despite what appears at a first glance to be two completely different hypotheses, since they propose two opposite effects, an in-depth analysis based on our novel findings [138-141] suggest that they complement each other based on the pathophysiological conditions relevant to AD [105]. In particular, our group reported alterations in both HO-1 and BVR related to increased oxidative/nitrosative post-translational modifications in the brain of AD and MCI subjects [138-141]. Thus, we suggest that the neuroprotective effects mediated by the HO/BVR-A system can be obtained only if the fine balance between the activity of HO-1 or HO-2 and that of BVR-A are maintained [105]. Also the fetal isoform BVR-B [108] was recently proposed as a serum biomarker in AD individuals [142], but the presence and pathophysiological role of this isoform in brain tissue needs further elucidation. In order to complete this intricate puzzle, the role of BVR-B has to be also carefully considered. Whether the presence of BVR-B will be confirmed in AD brain, the differential contribution of both BVR isoforms to the cell stress response should be further explored. Indeed, it is not possible to single out the differential contribution of BVR-A and BVR-B to the generation of BR and the improvement of the cell adaptive response. Although it is possible to specifically measure BVR-A activity by using the alpha isomer of BV, it is not possible to specifically measure BVR-B activity because BV-β, BV-δ and BV-γ are also substrates for BVR-A [143].
As explained throughout this review, the HO/BVR-A axis is a major signal transduction pathway involved in the regulation of important cell functions. The multiple effects produced by CO and BR in several experimental systems have prompted some authors to propose the HO/BVR-A system as a potential target for new drugs. In support of this proposal, these investigators have cited evidence that many drugs with seemingly well-established mechanisms of action also interact with the HO/BVR-A system and that this interaction may potentiate the drug's therapeutic effects [144]. These drugs include opioids, anti-inflammatory, antineoplastic, cardiovascular, and immunosuppressive drugs as well as drugs actin on the central nervous system (reviewd in [104]). However, the prospect of therapeutic manipulation of the HO/BVR system also raises a number of very legitimate concerns, mainly related to the dual nature of heme, CO, and bilirubin. In particular, uncontrolled activation of this axis can lead to toxic effects stemming from heme depletion or the accumulation of CO and bilirubin. Furthermore, all these drugs were shown to have an effect on HO without any information about BVR.
The challenge will be to find any possible pharmacological treatment that might conceivably be capable of overcoming or at least reducing these obstacles related to the neurotoxic effects.
Particularly interesting with regard to this is the ability of statins to modulate the HO/BVR-A axis. Statins’ effects on HO-1 expression and HO activity have both proved to be drug- and tissue-type specific. A single dose of simvastatin, lovastatin, atorvastatin, and rosuvastatin increased HO activity in rat heart, lung, and liver tissues [145, 146]. This effect appears to be protective since the administration of zinc protoporphyrin-IX (Zn-PP-IX), an inhibitor of HO activity, increases simvastatin toxicity, as manifested by elevated alanine transaminase levels [145, 146]. However, despite a number of studies that over the years addressed the ability of statins to induce HO-1 in several tissues both in vitro and in vivo [104, 144], few reports account for their role in the central nervous system (CNS). Furthermore, the analyses were limited to the only HO-1, missing any kind of information with regard to the effects on BVR-A. Indeed, the activation of BVR-A is required for the production of BR, whose antioxidant and antinitrosative properties were associated with reduced oxidative and nitrosative stress levels following statins treatment [147]. Due to the topic of this review, we will limit our discussion to the effects in the CNS, highlighting the progress obtained with regard to AD.
5.1 Statin-induced effects on HO in the CNS
In 2008, Hsieh et al. reported that simvastatin (50-100 µM, for 24 h) induced HO-1 in glucose-deprived Neuro2A cells [148]. However, this effect was paralleled by an increase in apoptotic cell death perhaps due to the HO-1-dependent increase of Fe(II) release, since both the HO inhibitor Zn-PP-IX and the iron chelator desferrioxamine resulted in blockade of the elevated apoptosis [148]. The same group reported that simvastatin (50-100 μM, for 24 h)-induced HO-1 led to increased NF-kappaB activation and superoxide production in Neuro 2A cells when exposed to LPS, and that Fe(II) production may play a role in such a response [149].
In 2010, Kannan et al. showed that mevastatin (300 nM/day, for 34 days) was able to promote HO-1 induction, but also to accelerate loss of synaptic proteins and neurite degeneration in aging cortical neurons in a heme-independent manner. Comparison of mevastatin-treated and heme-deficient neurons showed that inhibition of heme synthesis had a similar damaging effect on neurite integrity and NMDR receptor expression and function [150].
The above-cited reports clearly account for a detrimental role of statin administration in neurons, showing the up-regulation of HO-1 as a risk factor associated with the neurotoxic effects. However, in our opinion, the evaluation of the only HO-1 expression is not enough to explain its role in mediating the observed effects. In addition, the same concerns with regard to the kind of statin, the scheme of the treatment as well as the experimental model used have to be taken into consideration for an overall evaluation of the results.
The Butterfield group reported about the effects of atorvastatin long-term administration (80 mg/day for 14.5 months) in the brain of aged canine, thus proposing novel in vivo data which support the idea that a statin promotes beneficial effects in the brain through the modulation of the HO/BVR-A system[147, 151] (Figure 1). In a first set of experiments, we evaluated the effects of atorvastatin on brain HO-1 and HO-2, thus extending the neurobiological benefits of atorvastatin to include potentiation of the cell stress response. We found that atorvastatin was able to significantly up-regulate HO-1 in parietal cortex, a well-known area associated with cognitive function [151]. More interesting was the observation that also HO-2, usually considered as constitutive isoform, was up-regulated in the cerebellum of the same animals, thus suggesting regional- and isoform-specific effects for atorvastatin in the brain [151]. Atorvastatin-induced HO-1 up-regulation was associated with a significant reduction of oxidative stress biomarkers, 4-hydroxy-2-nonenal (HNE) and 7-K, in parietal cortex [151], highlighting a potential antioxidant effect. Interestingly, the significant correlations found between HO-1 overexpression and lower size discrimination error scores (improved cognition), observed in aged dogs after treatment with atorvastatin, led us to speculate that the effect on cognition could be due not only to an HO-1 mediated reduction of oxidative stress but also to the generation of carbon monoxide, one of the by-products of HO activity, which plays an important role in the maintenance of synaptic plasticity [152]. Another intriguing correlation was found between higher HO-1 levels and higher GSH intracellular levels. These results were not surprising as the expression of both HO-1 and γ-glutamylcysteine synthetase, a key enzyme in GSH synthesis, is mediated by the transcription factor Nuclear Factor (erythroid-derived 2)-like 2 (Nrf2) [153]. In turn, statins stimulate Nrf2 in several experimental systems, including cultured neurons [148]. Thus, the neuroprotective effect of atorvastatin could be attributable to the coordinated increase of both HO-1 and GSH synthesis. Furthermore, Collinson et al. in 2011 provided a new point of view about the mechanism of action of HO-1 which functions more than a catabolic and antioxidant enzyme [154]. In particular, these researchers showed that antioxidant activity of HO-1 is dependent on the up-regulation of several genes encoding for antioxidant enzymes such as γ -glutamylcysteine synthetase, glutathione peroxidase, catalase, and methionine sulfoxide reductase [154]. In light of this report, the coordinated increase of HO-1 and GSH observed following atorvastatin treatment could be explained not only by the effect of statins on Nrf-2, but also by the HO-1-induced up-regulation of γ-glutamylcysteine synthetase [151].
Another novel finding was the observation about atorvastatin-induced differential modulation of HO isoforms in the brain. While HO-1 was up-regulated in parietal cortex, HO-2 was increased in the cerebellum [151]. This pattern of expression seems to be specific for brain, since no difference was observed in the liver, the main target organ for statins [151]. However, increased HO-2 in the cerebellum was not paralleled by a concomitant reduction of oxidative stress biomarkers in this brain area. A possible explanation for this discrepency, could reside in the different degree of deposition of the pro-oxidant Aβ [155].
5.2 Statin-induced effects on BVR-A in the CNS
As explained above, before any conclusion with regard to the potential neuroprotective or neurotoxic effects mediated by the HO/BVR-A system can be drawn, it is necessary that the expression and activity of the entire system be evaluated. Our group was the first to propose BVR-A as a novel drug-target for atorvastatin in the brain [7,147]. The importance of our findings rests in the fact that atorvastatin, in a good pre-clinical model of AD such as aged beagles, was able to rescue the impaired phenotype with regard to the HO/BVR-A system [147]. We observed a similar phenotype in AD and MCI brain [138, 139, 141]. Indeed, we previously reported BVR-A's involvement in both AD and MCI, by showing phosphorylation (decreased) and oxidative modification (increased) along with the decreased levels and activity of BVR-A in brain of both AD and MCI subjects [138, 139].
In aged beagles, long-term atorvastatin treatment increased BVR-A protein levels and was associated with increased BVR-A phosphorylation and decreased 3-NT-BVR-A in parietal cortex, a brain area involved in cognitive function [147]. These changes led to an increased activation of the HO/BVR-A system as demonstrated by the higher levels of BR produced in parietal cortex of atorvastatin-treated dogs [147].
Our results are innovative in that for the first time in a higher mammalian in vivo model of AD not only the protein levels, but also the post-translational modifications that affect BVR-A's activities following atrovastatin treatement have been analyzed. We posit that the analysis of expression levels alone is not sufficient to explain BVR-A's contribution to cell signaling networks [106, 138, 139]. Significant correlations were found among BVR-A protein levels, activity or phosphorylation with decreased levels of oxidative/nitrosative stress as well as decreased size discrimination error score (reflecting improved cognition) observed in aged dogs after treatment with atorvastatin. These results led us to speculate that the effects on oxidative stress and cognition could be mediated by the activation of the HO-1/BVR-A system. We believe that increased BVR-A protein levels together with its improved functioning could trigger a cell stress response and thus improve cognitive behavior by the following mechanisms: (i) Activation of both conventional and atypical protein kinase C isoforms [106] whose involvement in memory function is now well established [156]; (ii) Interaction with members of the MAPK family, such as ERK1/2-Mek-Elk1, through which BVR-A regulates important metabolic pathways as well as the expression of oxidative-stress-responsive genes including HO-1 or inducible nitric oxide synthase (iNOS) [106, 120-122]; (iii) Production of the powerful antioxidant BR as result of BVR-A's reductase activity. Since the phosphorylation of BVR-A on Tyr residues is required to interact with ERK-Mek-Elk1 [120], the increase of pTyr-BVR-A in the parietal cortex following atorvastatin treatment, coupled with the negative correlation between pTyr-BVR-A and size discrimination error scores, could suggest an activation of the MAPK-related signal transduction pathways that, in turn, promotes a robust cell stress response [106]. At the same time, the significant correlations found among BVR activity and decreased total PC and 3-NT levels suggest an indirect antioxidant role for BR, consistent with prior studies [110, 157-159] (Figure 1). Furthermore, as previously demonstrated, BR increased neuronal NOS expression and nitric oxide formation in both primary cultures of cerebellar granule neurons and neurotrophin-sensitive PC12 cells [160]. And NO plays a key role in the long-term potentiation and synaptic plasticity [114]. In addition, in PC12 cells BR upregulated CREB [160], which is considered an important transcription factor regulating both short- and long- term memory [161]. However, from a mechanistic point of view, it is not known exactly how atorvastatin regulates the enzymatic properties of BVR-A and research to gain insights on this topic is ongoing in our laboratory.
These results together with those obtained for HO and oxidative/nitrosative stress levels strongly support the notion that removing pre-existing Aβ may not be enough to recover impaired neuronal functions observed in AD, and that other mechanisms have to be taken into account. Despite that no changes for Aβ levels were observed in the brain of aged beagles [5], significant association among decreased oxidative/nitrosative stress levels, HO/BVR-A activation, and decreased size discrimination error scores (reflecting improved cognition) was found. These results suggest that atorvastatin independent of its ability to lower cholesterol and thus Aβ (as was initially supposed [9-11]) can promote beneficial effects by modulating other pathways (Figure 1). This shift in thinking implies that statin therapy for AD could be used in support of other treatments aimed to reduce Aβ/tau-induced pathology. In this regard, due to their pharmacokinetic and pharmacodynamics properties, the effects produced by more lipophilic statins [which theoretically should cross better the blood brain barrier (BBB)] such as simvastatin, would need to be evaluated. One limitation of our studies in fact could be that atorvastatin does not possess a high ability to cross the BBB, even if robust CNS effects are consistent with: (i) decreased oxidative and nitrosative stress levels; (ii) HO protein levels and (iii) BVR-A protein levels, post-translational modifications and activity outcome measures.
6. Future Directions
An interesting finding coming from our studies was the association between BVR-A and BACE1. BVR-A protein levels and phosphorylation were negatively associated with BACE1 protein levels in the parietal cortex of atorvastatin-treated aged beagles. In particular, the increased phosphorylation of BVR-A following atorvastatin treatment was associated with a reduction of BACE1 protein levels (Figure 1). These correlations may be related to the increased kinase activity of BVR-A, which in turn could either directly or indirectly promote BACE1 degradation in lysosomes [162-164], which would have an effect on Aβ production. In other words, atorvastatin likely is not able to promote the clearance of pre-existing Aβ, but, rather, would inhibit the production of new Aβ. This mechanism could represent a good topic to address in order to rule out any possible involvement of statins in the primary prevention of AD.
Another direction for statin basic and clinical research in stages of AD concern their effects in preclinical AD (PCAD), a stage in which AD-related pathology, but normal cognition, exists. Such studies could evaluate the potential of statins to slow progression of PCAD to amnestic MCI and AD and on Aβ clearance vs production.
Determining the fate of oxysterols and CoQ10 in brain at different stages of AD may provide new insights of action of statins in neurodegeneration. Given the enormous burden on worldwide population that AD currently presents, with even more burden in the near future, gaining new insight into molecular action and usefulness of statin in AD may offer a promising strategy for progress.
Acknowledgements
This work was supported in part by an NIH grant to DAB [AG-05119].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. The New England journal of medicine. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Kandiah N, Feldman HH. Therapeutic potential of statins in Alzheimer's disease. J Neurol Sci. 2009;283:230–4. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- 3.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 4.Kurata T, Miyazaki K, Kozuki M, Panin VL, Morimoto N, Ohta Y, et al. Atorvastatin and pitavastatin improve cognitive function and reduce senile plaque and phosphorylated tau in aged APP mice. Brain Res. 2011;1371:161–70. doi: 10.1016/j.brainres.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP, Morales J, Beckett TL, Astarita G, Piomelli D, Weidner A, et al. Changes in cognition and amyloid-beta processing with long term cholesterol reduction using atorvastatin in aged dogs. J Alzheimers Dis. 2010;22:135–50. doi: 10.3233/JAD-2010-100639. [DOI] [PubMed] [Google Scholar]
- 6.Barone E, Cenini G, Di Domenico F, Martin S, Sultana R, Mancuso C, et al. Long-term high-dose atorvastatin decreases brain oxidative and nitrosative stress in a preclinical model of Alzheimer disease: a novel mechanism of action. Pharmacol Res. 2011;63:172–80. doi: 10.1016/j.phrs.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: Perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64:180–6. doi: 10.1016/j.phrs.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuinness B, O'Hare J, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2010:CD007514. doi: 10.1002/14651858.CD007514.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterfield DA. Atorvastatin and Abeta(1-40): not as simple as cholesterol reduction in brain and relevance to Alzheimer disease. Exp Neurol. 2011;228:15–8. doi: 10.1016/j.expneurol.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Bersot TP. Drug therapy for hypercholesterolemia and dyslipidemia. In: Laurence LB, editor. Goodman and Gilman's, The pharmacological basis of therapeutics. McGraw-Hill; 2011. pp. 877–908. [Google Scholar]
- 14.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Ricciarelli R, Canepa E, Marengo B, Marinari UM, Poli G, Pronzato MA, et al. Cholesterol and Alzheimer's disease: a still poorly understood correlation. IUBMB Life. 2012;64:931–5. doi: 10.1002/iub.1091. [DOI] [PubMed] [Google Scholar]
- 16.Silva T, Teixeira J, Remiao F, Borges F. Alzheimer's disease, cholesterol, and statins: the junctions of important metabolic pathways. Angew Chem Int Ed Engl. 2013;52:1110–21. doi: 10.1002/anie.201204964. [DOI] [PubMed] [Google Scholar]
- 17.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol Level and Statin Use in Alzheimer Disease II. Review of Human Trials and Recommendations. Arch Neurol-Chicago. 2011;68:1385–92. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol Level and Statin Use in Alzheimer Disease I. Review of Epidemiological and Preclinical Studies. Arch Neurol-Chicago. 2011;68:1239–44. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Ferro A, Benito-Leon J, Mitchell AJ, Bermejo-Pareja F. A review of the potential therapeutic role of statins in the treatment of Alzheimer's disease: current research and opinion. Neuropsych Dis Treat. 2013;9:55–63. doi: 10.2147/NDT.S29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparks DL. Alzheimer Disease Statins in the Treatment of Alzheimer Disease. Nat Rev Neurol. 2011;7:662–3. doi: 10.1038/nrneurol.2011.165. [DOI] [PubMed] [Google Scholar]
- 21.Rockwood K. Epidemiological and clinical trials evidence about a preventive role for statins in Alzheimer's disease. Acta Neurol Scand. 2006;114:71–7. doi: 10.1111/j.1600-0404.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: Perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64:180–6. doi: 10.1016/j.phrs.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandiah N, Feldman HH. Therapeutic potential of statins in Alzheimer's disease. Journal of the Neurological Sciences. 2009;283:230–4. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- 24.Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–43. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 25.Hajjar L, Schumpert J, Hirth V, Wieland D, Eleazer GP. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J Gerontol a-Biol. 2002;57:M414–M8. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 26.Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–7. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 27.Arca M, Natoli S, Micheletta F, Riggi S, Di Angelantonio E, Montali A, et al. Increased plasma levels of oxysterols, in vivo markers of oxidative stress, in patients with familial combined hyperlipidemia: reduction during atorvastatin and fenofibrate therapy. Free Radic Biol Med. 2007;42:698–705. doi: 10.1016/j.freeradbiomed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Sparks DL, Kryscio RJ, Connor DJ, Sabbagh MN, Sparks LM, Lin YS, et al. Cholesterol and Cognitive Performance in Normal Controls and the Influence of Elective Statin Use after Conversion to Mild Cognitive Impairment: Results in a Clinical Trial Cohort. Neurodegener Dis. 2010;7:183–6. doi: 10.1159/000295660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparks DL, Kryscio RJ, Sabbagh MN, Connor DJ, Sparks LM, Liebsack C. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr Alzheimer Res. 2008;5:416–21. doi: 10.2174/156720508785132316. [DOI] [PubMed] [Google Scholar]
- 30.Cramer C, Haan MN, Galea S, Langa KM, Kalbfleisch JD. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008;71:344–50. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haag MD, Hofman A, Koudstaal PJ, Stricker BH, Breteler MM. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009;80:13–7. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez EG, Dodge HH, Birzescu MA, Stoehr GP, Ganguli M. Use of lipid-lowering drugs in older adults with and without dementia: a community-based epidemiological study. J Am Geriatr Soc. 2002;50:1852–6. doi: 10.1046/j.1532-5415.2002.50515.x. [DOI] [PubMed] [Google Scholar]
- 33.Zandi PP, Sparks DL, Khachaturian AS, Tschanz J, Norton M, Steinberg M, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–24. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 34.Rea TD, Breitner JC, Psaty BM, Fitzpatrick AL, Lopez OL, Newman AB, et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol. 2005;62:1047–51. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 35.Arvanitakis Z, Schneider JA, Wilson RS, Bienias JL, Kelly JF, Evans DA, et al. Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology. 2008;70:1795–802. doi: 10.1212/01.wnl.0000288181.00826.63. [DOI] [PubMed] [Google Scholar]
- 36.Sparks DL, Lopez J, Connor D, Sabbagh M, Seward J, Browne P, et al. A position paper: based on observational data indicating an increased rate of altered blood chemistry requiring withdrawal from the Alzheimer's Disease Cholesterol-Lowering Treatment Trial (ADCLT). J Mol Neurosci. 2003;20:407–10. doi: 10.1385/JMN:20:3:407. [DOI] [PubMed] [Google Scholar]
- 37.Sparks DL, Connor DJ, Sabbagh MN, Petersen RB, Lopez J, Browne P. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer's disease: results of the Alzheimer's Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol Scand Suppl. 2006;185:3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 38.Sparks DL, Petanceska S, Sabbagh M, Connor D, Soares H, Adler C, et al. Cholesterol, copper and Abeta in controls, MCI, AD and the AD cholesterol-lowering treatment trial (ADCLT). Curr Alzheimer Res. 2005;2:527–39. doi: 10.2174/156720505774932296. [DOI] [PubMed] [Google Scholar]
- 39.Riekse RG, Li G, Petrie EC, Leverenz JB, Vavrek D, Vuletic S, et al. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis. 2006;10:399–406. doi: 10.3233/jad-2006-10408. [DOI] [PubMed] [Google Scholar]
- 40.Riekse RG, Hazzard W, Leverenz J, Kelly W, Boorkman P, Lash B, et al. Effects of statins on Abeta in cerebrospinal fluid. Journal of the American Geriatrics Society. 2005;53:S175–S. [Google Scholar]
- 41.Carlsson CM, Gleason CE, Hess TM, Moreland KA, Blazel HM, Koscik RL, et al. Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer's disease. J Alzheimers Dis. 2008;13:187–97. doi: 10.3233/jad-2008-13209. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 43.Trompet S, van Vliet P, de Craen AJ, Jolles J, Buckley BM, Murphy MB, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 44.Heart Protection Study Collaborative G MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 45.Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–64. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 46.McGuinness B, O'Hare J, Craig D, Bullock R, Malouf R, Passmore P. Statins for the treatment of dementia. Cochrane Db Syst Rev. 2010 doi: 10.1002/14651858.CD007514.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Reiss AB, Wirkowski E. Statins in neurological disorders: mechanisms and therapeutic value. ScientificWorldJournal. 2009;9:1242–59. doi: 10.1100/tsw.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sano M, Bell KL, Galasko D, Galvin JE, Thomas RG, van Dyck CH, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–63. doi: 10.1212/WNL.0b013e318228bf11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padala KP, Padala PR, McNeilly DP, Geske JA, Sullivan DH, Potter JF. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer's dementia: a prospective withdrawal and rechallenge pilot study. Am J Geriatr Pharmacother. 2012;10:296–302. doi: 10.1016/j.amjopharm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Orr JD. Statins in the spectrum of neurologic disease. Curr Atheroscler Rep. 2008;10:11–8. doi: 10.1007/s11883-008-0003-5. [DOI] [PubMed] [Google Scholar]
- 51.Wallerath T, Poleo D, Li H, Forstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. J Am Coll Cardiol. 2003;41:471–8. doi: 10.1016/s0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- 52.Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–63. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 53.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 54.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–10. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miron VE, Rajasekharan S, Jarjour AA, Zamvil SS, Kennedy TE, Antel JP. Simvastatin regulates oligodendroglial process dynamics and survival. Glia. 2007;55:130–43. doi: 10.1002/glia.20441. [DOI] [PubMed] [Google Scholar]
- 56.Littarru GP, Langsjoen P. Coenzyme Q10 and statins: biochemical and clinical implications. Mitochondrion. 2007;7(Suppl):S168–74. doi: 10.1016/j.mito.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–7. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 58.Cerezo-Guisado MI, Garcia-Roman N, Garcia-Marin LJ, Alvarez-Barrientos A, Bragado MJ, Lorenzo MJ. Lovastatin inhibits the extracellular-signal-regulated kinase pathway in immortalized rat brain neuroblasts. The Biochemical journal. 2007;401:175–83. doi: 10.1042/BJ20060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu MS, Pitts AF, Winters TR, Green SH. ras isoprenylation is required for ras-induced but not for NGF-induced neuronal differentiation of PC12 cells. The Journal of cell biology. 1991;115:795–808. doi: 10.1083/jcb.115.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maier O, De Jonge J, Nomden A, Hoekstra D, Baron W. Lovastatin induces the formation of abnormal myelin-like membrane sheets in primary oligodendrocytes. Glia. 2009;57:402–13. doi: 10.1002/glia.20769. [DOI] [PubMed] [Google Scholar]
- 61.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 62.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 63.Clark TA, Lee HP, Rolston RK, Zhu X, Marlatt MW, Castellani RJ, et al. Oxidative Stress and its Implications for Future Treatments and Management of Alzheimer Disease. Int J Biomed Sci. 2010;6:225–7. [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Z, Zhao B, Ratka A. Oxidative stress and beta-amyloid protein in Alzheimer's disease. Neuromolecular Med. 2011;13:223–50. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 65.Axelsen PH, Komatsu H, Murray IV. Oxidative stress and cell membranes in the pathogenesis of Alzheimer's disease. Physiology (Bethesda) 2011;26:54–69. doi: 10.1152/physiol.00024.2010. [DOI] [PubMed] [Google Scholar]
- 66.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–60. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 67.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–77. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurinami H, Sato N, Shinohara M, Takeuchi D, Takeda S, Shimamura M, et al. Prevention of amyloid beta-induced memory impairment by fluvastatin, associated with the decrease in amyloid beta accumulation and oxidative stress in amyloid beta injection mouse model. Int J Mol Med. 2008;21:531–7. [PubMed] [Google Scholar]
- 69.Tong XK, Nicolakakis N, Fernandes P, Ongali B, Brouillette J, Quirion R, et al. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol Dis. 2009;35:406–14. doi: 10.1016/j.nbd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Riekse RG, Li G, Petrie EC, Leverenz JB, Vavrek D, Vuletic S, et al. Effect of statins on Alzheimer's disease biomarkers in cerebrospinal fluid. J Alzheimers Dis. 2006;10:399–406. doi: 10.3233/jad-2006-10408. [DOI] [PubMed] [Google Scholar]
- 71.Tong XK, Nicolakakis N, Fernandes P, Ongali B, Brouillette J, Quirion R, et al. Simvastatin improves cerebrovascular function and counters soluble amyloid-beta, inflammation and oxidative stress in aged APP mice. Neurobiol Dis. 2009;35:406–14. doi: 10.1016/j.nbd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Piermartiri TC, Figueiredo CP, Rial D, Duarte FS, Bezerra SC, Mancini G, et al. Atorvastatin prevents hippocampal cell death, neuroinflammation and oxidative stress following amyloid-beta(1-40) administration in mice: evidence for dissociation between cognitive deficits and neuronal damage. Exp Neurol. 2010;226:274–84. doi: 10.1016/j.expneurol.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Archives of neurology. 2011;68:1385–92. doi: 10.1001/archneurol.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thelen KM, Rentsch KM, Gutteck U, Heverin M, Olin M, Andersson U, et al. Brain cholesterol synthesis in mice is affected by high dose of simvastatin but not of pravastatin. J Pharmacol Exp Ther. 2006;316:1146–52. doi: 10.1124/jpet.105.094136. [DOI] [PubMed] [Google Scholar]
- 75.Johnstone EM, Chaney MO, Norris FH, Pascual R, Little SP. Conservation of the sequence of the Alzheimer's disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 76.Torp R, Head E, Cotman CW. Ultrastructural analyses of beta-amyloid in the aged dog brain: Neuronal beta-amyloid is localized to the plasma membrane. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2000;24:801–10. doi: 10.1016/s0278-5846(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 77.Torp R, Head E, Milgram NW, Hahn F, Ottersen OP, Cotman CW. Ultrastructural evidence of fibrillar β–amyloid associated with neuronal membranes in behaviorally characterized aged dog brains. Neuroscience. 2000;93:495–506. doi: 10.1016/s0306-4522(99)00568-0. [DOI] [PubMed] [Google Scholar]
- 78.Torp R, Ottersen OP, Cotman CW, Head E. Identification of neuronal plasma membrane microdomains that colocalize beta-amyloid and presenilin: implications for beta-amyloid precursor protein processing. Neuroscience. 2003;120:291–300. doi: 10.1016/s0306-4522(03)00320-8. [DOI] [PubMed] [Google Scholar]
- 79.Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- 80.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxidants & redox signaling. 2012;17:1590–609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butterfield DA, Reed T, Sultana R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer's disease. Free radical research. 2011;45:59–72. doi: 10.3109/10715762.2010.520014. [DOI] [PubMed] [Google Scholar]
- 82.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochimica et biophysica acta. 2010;1801:924–9. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–37. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 84.Han JH, Kim YJ, Han ES, Lee CS. Prevention of 7-ketocholesterol-induced mitochondrial damage and cell death by calmodulin inhibition. Brain Res. 2007;1137:11–9. doi: 10.1016/j.brainres.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 85.Panini SR, Yang L, Rusinol AE, Sinensky MS, Bonventre JV, Leslie CC. Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J Lipid Res. 2001;42:1678–86. [PubMed] [Google Scholar]
- 86.Trousson A, Bernard S, Petit PX, Liere P, Pianos A, El Hadri K, et al. 25- hydroxycholesterol provokes oligodendrocyte cell line apoptosis and stimulates the secreted phospholipase A2 type IIA via LXR beta and PXR. J Neurochem. 2009;109:945–58. doi: 10.1111/j.1471-4159.2009.06009.x. [DOI] [PubMed] [Google Scholar]
- 87.Kathir K, Dennis JM, Croft KD, Mori TA, Lau AK, Adams MR, et al. Equivalent lipid oxidation profiles in advanced atherosclerotic lesions of carotid endarterectomy plaques obtained from symptomatic type 2 diabetic and nondiabetic subjects. Free Radic Biol Med. 2010;49:481–6. doi: 10.1016/j.freeradbiomed.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Larsson H, Bottiger Y, Iuliano L, Diczfalusy U. In vivo interconversion of 7beta-hydroxycholesterol and 7-ketocholesterol, potential surrogate markers for oxidative stress. Free Radic Biol Med. 2007;43:695–701. doi: 10.1016/j.freeradbiomed.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 89.Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessede G, et al. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004;11:897–905. doi: 10.1038/sj.cdd.4401434. [DOI] [PubMed] [Google Scholar]
- 90.Kumar BS, Chung BC, Lee YJ, Yi HJ, Lee BH, Jung BH. A GC/MS-based Simultaneous Quantitative Analytical Method for Urinary Oxysterols and Bile Acids in Rats. Anal Biochem. 2010 doi: 10.1016/j.ab.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 91.Thelen KM, Laaksonen R, Paiva H, Lehtimaki T, Lutjohann D. High-dose statin treatment does not alter plasma marker for brain cholesterol metabolism in patients with moderately elevated plasma cholesterol levels. J Clin Pharmacol. 2006;46:812–6. doi: 10.1177/0091270006289851. [DOI] [PubMed] [Google Scholar]
- 92.Bliznakov EG, Wilkins DJ. Biochemical and clinical consequences of inhibiting coenzyme Q(10) biosynthesis by lipid-lowering HMG-CoA reductase inhibitors (statins): A critical overview. Adv Ther. 1998;15:218–28. [Google Scholar]
- 93.James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Archives of biochemistry and biophysics. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 94.Schmelzer C, Lindner I, Rimbach G, Niklowitz P, Menke T, Doring F. Functions of coenzyme Q10 in inflammation and gene expression. Biofactors. 2008;32:179–83. doi: 10.1002/biof.5520320121. [DOI] [PubMed] [Google Scholar]
- 95.Willis RA, Folkers K, Tucker JL, Ye CQ, Xia LJ, Tamagawa H. Lovastatin decreases coenzyme Q levels in rats. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8928–30. doi: 10.1073/pnas.87.22.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Satoh K, Yamato A, Nakai T, Hoshi K, Ichihara K. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on mitochondrial respiration in ischaemic dog hearts. British journal of pharmacology. 1995;116:1894–8. doi: 10.1111/j.1476-5381.1995.tb16679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rundek T, Naini A, Sacco R, Coates K, DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Archives of neurology. 2004;61:889–92. doi: 10.1001/archneur.61.6.889. [DOI] [PubMed] [Google Scholar]
- 98.Spindler M, Beal MF, Henchcliffe C. Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatric disease and treatment. 2009;5:597–610. doi: 10.2147/ndt.s5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochimica et biophysica acta. 2004;1660:171–99. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Head E, Nukala VN, Fenoglio KA, Muggenburg BA, Cotman CW, Sullivan PG. Effects of age, dietary, and behavioral enrichment on brain mitochondria in a canine model of human aging. Exp Neurol. 2009;220:171–6. doi: 10.1016/j.expneurol.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martin SB, Cenini G, Barone E, Dowling AL, Mancuso C, Butterfield DA, et al. Coenzyme Q10 and cognition in atorvastatin treated dogs. Neuroscience letters. 2011;501:92–5. doi: 10.1016/j.neulet.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Langsjoen PH, Langsjoen JO, Langsjoen AM, Lucas LA. Treatment of statin adverse effects with supplemental Coenzyme Q10 and statin drug discontinuation. Biofactors. 2005;25:147–52. doi: 10.1002/biof.5520250116. [DOI] [PubMed] [Google Scholar]
- 103.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annual review of pharmacology and toxicology. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 104.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Current drug metabolism. 2009;10:579–94. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 105.Barone E, Di Domenico F, Mancuso C, Butterfield DA. The Janus face of the heme oxygenase/biliverdin reductase system in Alzheimer disease: It's time for reconciliation. Neurobiol Dis. 2013 doi: 10.1016/j.nbd.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends in pharmacological sciences. 2009;30:129–37. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Maines MD. New insights into biliverdin reductase functions: linking heme metabolism to cell signaling. Physiology (Bethesda) 2005;20:382–9. doi: 10.1152/physiol.00029.2005. [DOI] [PubMed] [Google Scholar]
- 108.Pereira PJ, Macedo-Ribeiro S, Parraga A, Perez-Luque R, Cunningham O, Darcy K, et al. Structure of human biliverdin IXbeta reductase, an early fetal bilirubin IXbeta producing enzyme. Nature structural biology. 2001;8:215–20. doi: 10.1038/84948. [DOI] [PubMed] [Google Scholar]
- 109.Stocker R. Antioxidant activities of bile pigments. Antioxidants & redox signaling. 2004;6:841–9. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 110.Barone E, Trombino S, Cassano R, Sgambato A, De Paola B, Di Stasio E, et al. Characterization of the S-denitrosylating activity of bilirubin. J Cell Mol Med. 2009;13:2365–75. doi: 10.1111/j.1582-4934.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59:478–93. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 112.Mancuso C, Navarra P, Preziosi P. Roles of nitric oxide, carbon monoxide, and hydrogen sulfide in the regulation of the hypothalamic-pituitary-adrenal axis. J Neurochem. 2010;113:563–75. doi: 10.1111/j.1471-4159.2010.06606.x. [DOI] [PubMed] [Google Scholar]
- 113.Mancuso C, Pani G, Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11:207–13. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 114.Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature reviews Neuroscience. 2007;8:766–75. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 115.Mancuso C, Scapagini G, Curro D, Giuffrida Stella AM, De Marco C, Butterfield DA, et al. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–23. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- 116.Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, Bella R, et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxidants & redox signaling. 2006;8:1975–86. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- 117.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic Biol Med. 1997;23:134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 118.Smith MA, Perry G, Pryor WA. Causes and consequences of oxidative stress in Alzheimer's disease. Free Radic Biol Med. 2002;32:1049. doi: 10.1016/s0891-5849(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 119.Lerner-Marmarosh N, Shen J, Torno MD, Kravets A, Hu Z, Maines MD. Human biliverdin reductase: a member of the insulin receptor substrate family with serine/threonine/tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7109–14. doi: 10.1073/pnas.0502173102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6870–5. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tudor C, Lerner-Marmarosh N, Engelborghs Y, Gibbs PE, Maines MD. Biliverdin reductase is a transporter of haem into the nucleus and is essential for regulation of HO-1 gene expression by haematin. Biochem J. 2008;413:405–16. doi: 10.1042/BJ20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maines MD. Biliverdin reductase: PKC interaction at the cross-talk of MAPK and PI3K signaling pathways. Antioxidants & redox signaling. 2007;9:2187–95. doi: 10.1089/ars.2007.1805. [DOI] [PubMed] [Google Scholar]
- 123.Di Domenico F, Perluigi M, Barone E. Biliverdin Reductase-A correlates with inducible nitric oxide synthasein in atorvastatin treated aged canine brain. Neural Reg Res. 2013;8:1925–37. doi: 10.3969/j.issn.1673-5374.2013.21.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chemical reviews. 2001;101:2353–64. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 125.Whitby FG, Phillips JD, Hill CP, McCoubrey W, Maines MD. Crystal structure of a biliverdin IXalpha reductase enzyme-cofactor complex. Journal of molecular biology. 2002;319:1199–210. doi: 10.1016/S0022-2836(02)00383-2. [DOI] [PubMed] [Google Scholar]
- 126.Ahmad Z, Salim M, Maines MD. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. The Journal of biological chemistry. 2002;277:9226–32. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 127.Myers MG, Jr., Zhang Y, Aldaz GA, Grammer T, Glasheen EM, Yenush L, et al. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Molecular and cellular biology. 1996;16:4147–55. doi: 10.1128/mcb.16.8.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hunter T, Cooper JA. Protein-tyrosine kinases. Annual review of biochemistry. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- 129.Gibbs PE, Tudor C, Maines MD. Biliverdin reductase: more than a namesake - the reductase, its Peptide fragments, and biliverdin regulate activity of the three classes of protein kinase C. Frontiers in pharmacology. 2012;3:31. doi: 10.3389/fphar.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lerner-Marmarosh N, Miralem T, Gibbs PE, Maines MD. Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:3949–62. doi: 10.1096/fj.07-8544com. [DOI] [PubMed] [Google Scholar]
- 131.Miralem T, Gibbs PE, Revert F, Saus J, Maines MD. Human biliverdin reductase suppresses Goodpasture antigen-binding protein (GPBP) kinase activity: the reductase regulates tumor necrosis factor-alpha-NF-kappaB-dependent GPBP expression. The Journal of biological chemistry. 2010;285:12551–8. doi: 10.1074/jbc.M109.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibbs PE, Miralem T, Lerner-Marmarosh N, Tudor C, Maines MD. Formation of ternary complex of human biliverdin reductase-protein kinase Cdelta-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-kappaB, and inducible nitric-oxidase synthase (iNOS). The Journal of biological chemistry. 2012;287:1066–79. doi: 10.1074/jbc.M111.279612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hascalovici JR, Vaya J, Khatib S, Holcroft CA, Zukor H, Song W, et al. Brain sterol dysregulation in sporadic AD and MCI: relationship to heme oxygenase-1. J Neurochem. 2009;110:1241–53. doi: 10.1111/j.1471-4159.2009.06213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takeda A, Itoyama Y, Kimpara T, Zhu X, Avila J, Dwyer BE, et al. Heme catabolism and heme oxygenase in neurodegenerative disease. Antioxidants & redox signaling. 2004;6:888–94. doi: 10.1089/ars.2004.6.888. [DOI] [PubMed] [Google Scholar]
- 135.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94:9866–8. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schipper HM. Heme oxygenase-1 in Alzheimer disease: a tribute to Moussa Youdim. J Neural Transm. 2011;118:381–7. doi: 10.1007/s00702-010-0436-1. [DOI] [PubMed] [Google Scholar]
- 137.Di Domenico F, Owen JB, Sultana R, Sowell RA, Perluigi M, Cini C, et al. The wheat germ agglutinin-fractionated proteome of subjects with Alzheimer's disease and mild cognitive impairment hippocampus and inferior parietal lobule: Implications for disease pathogenesis and progression. Journal of neuroscience research. 2010;88:3566–77. doi: 10.1002/jnr.22528. [DOI] [PubMed] [Google Scholar]
- 138.Barone E, Di Domenico F, Cenini G, Sultana R, Coccia R, Preziosi P, et al. Oxidative and nitrosative modifications of biliverdin reductase-A in the brain of subjects with Alzheimer's disease and amnestic mild cognitive impairment. J Alzheimers Dis. 2011;25:623–33. doi: 10.3233/JAD-2011-110092. [DOI] [PubMed] [Google Scholar]
- 139.Barone E, Di Domenico F, Cenini G, Sultana R, Cini C, Preziosi P, et al. Biliverdin reductase--a protein levels and activity in the brains of subjects with Alzheimer disease and mild cognitive impairment. Biochim Biophys Acta. 2011;1812:480–7. doi: 10.1016/j.bbadis.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Di Domenico F, Barone E, Mancuso C, Perluigi M, Cocciolo A, Mecocci P, et al. HO-1/BVR-a system analysis in plasma from probable Alzheimer's disease and mild cognitive impairment subjects: a potential biochemical marker for the prediction of the disease. J Alzheimers Dis. 2012;32:277–89. doi: 10.3233/JAD-2012-121045. [DOI] [PubMed] [Google Scholar]
- 141.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, et al. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mueller C, Zhou W, Vanmeter A, Heiby M, Magaki S, Ross MM, et al. The heme degradation pathway is a promising serum biomarker source for the early detection of Alzheimer's disease. J Alzheimers Dis. 2010;19:1081–91. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Franklin EM, Browne S, Horan AM, Inomata K, Hammam MA, Kinoshita H, et al. The use of synthetic linear tetrapyrroles to probe the verdin sites of human biliverdin-IXalpha reductase and human biliverdin-IXbeta reductase. The FEBS journal. 2009;276:4405–13. doi: 10.1111/j.1742-4658.2009.07148.x. [DOI] [PubMed] [Google Scholar]
- 144.Li C, Hossieny P, Wu BJ, Qawasmeh A, Beck K, Stocker R. Pharmacologic induction of heme oxygenase-1. Antioxidants & redox signaling. 2007;9:2227–39. doi: 10.1089/ars.2007.1783. [DOI] [PubMed] [Google Scholar]
- 145.Hsu M, Muchova L, Morioka I, Wong RJ, Schroder H, Stevenson DK. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun. 2006;343:738–44. doi: 10.1016/j.bbrc.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 146.Lai IR, Chang KJ, Tsai HW, Chen CF. Pharmacological preconditioning with simvastatin protects liver from ischemia-reperfusion injury by heme oxygenase-1 induction. Transplantation. 2008;85:732–8. doi: 10.1097/TP.0b013e3181664e70. [DOI] [PubMed] [Google Scholar]
- 147.Barone E, Mancuso C, Di Domenico F, Sultana R, Murphy MP, Head E, et al. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. J Neurochem. 2012;120:135–46. doi: 10.1111/j.1471-4159.2011.07538.x. [DOI] [PubMed] [Google Scholar]
- 148.Hsieh CH, Rau CS, Hsieh MW, Chen YC, Jeng SF, Lu TH, et al. Simvastatin-induced heme oxygenase-1 increases apoptosis of Neuro 2A cells in response to glucose deprivation. Toxicol Sci. 2008;101:112–21. doi: 10.1093/toxsci/kfm258. [DOI] [PubMed] [Google Scholar]
- 149.Hsieh CH, Jeng SF, Hsieh MW, Chen YC, Rau CS, Lu TH, et al. Statin-induced heme oxygenase-1 increases NF-kappaB activation and oxygen radical production in cultured neuronal cells exposed to lipopolysaccharide. Toxicological sciences : an official journal of the Society of Toxicology. 2008;102:150–9. doi: 10.1093/toxsci/kfm298. [DOI] [PubMed] [Google Scholar]
- 150.Kannan M, Steinert JR, Forsythe ID, Smith AG, Chernova T. Mevastatin accelerates loss of synaptic proteins and neurite degeneration in aging cortical neurons in a heme-independent manner. Neurobiology of aging. 2010;31:1543–53. doi: 10.1016/j.neurobiolaging.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 151.Butterfield DA, Barone E, Di Domenico F, Cenini G, Sultana R, Murphy MP, et al. Atorvastatin treatment in a dog preclinical model of Alzheimer's disease leads to up-regulation of haem oxygenase-1 and is associated with reduced oxidative stress in brain. Int J Neuropsychopharmacol. 2012;15:981–7. doi: 10.1017/S1461145711001118. [DOI] [PubMed] [Google Scholar]
- 152.Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–50. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 153.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Collinson EJ, Wimmer-Kleikamp S, Gerega SK, Yang YH, Parish CR, Dawes IW, et al. The yeast homolog of heme oxygenase-1 affords cellular antioxidant protection via the transcriptional regulation of known antioxidant genes. J Biol Chem. 2011;286:2205–14. doi: 10.1074/jbc.M110.187062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 156.Sacktor TC. How does PKMzeta maintain long-term memory? Nat Rev Neurosci. 2011;12:9–15. doi: 10.1038/nrn2949. [DOI] [PubMed] [Google Scholar]
- 157.Stocker R, Glazer AN, Ames BN. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987;84:5918–22. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 159.Dore S, Takahashi M, Ferris CD, Zakhary R, Hester LD, Guastella D, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–50. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mancuso C, Capone C, Ranieri SC, Fusco S, Calabrese V, Eboli ML, et al. Bilirubin as an endogenous modulator of neurotrophin redox signaling. J Neurosci Res. 2008;86:2235–49. doi: 10.1002/jnr.21665. [DOI] [PubMed] [Google Scholar]
- 161.Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31:8786–802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–94. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wahle T, Prager K, Raffler N, Haass C, Famulok M, Walter J. GGA proteins regulate retrograde transport of BACE1 from endosomes to the trans-Golgi network. Mol Cell Neurosci. 2005;29:453–61. doi: 10.1016/j.mcn.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 164.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–37. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]