Abstract
We hypothesize that semantic memory for object concepts involves both representations of visual feature knowledge in modality-specific association cortex and heteromodal regions that are important for integrating and organizing this semantic knowledge so it can be used in a flexible, contextually appropriate manner. We examined this hypothesis in an fMRI study of mild Alzheimer’s disease (AD). Participants were presented with pairs of printed words and asked whether the words match on a given visual-perceptual feature (e.g. guitar, violin: SHAPE). Stimuli probed natural kinds and manufactured objects, and judgments involved shape or color. We found activation of bilateral ventral temporal cortex and left dorsolateral prefrontal cortex during semantic judgments, with AD patients showing less activation of these regions than healthy seniors. Moreover, AD patients showed less ventral temporal activation relative to healthy seniors for manufactured objects but not natural kinds. We also used diffusion-weighted MRI of white matter to examine fractional anisotropy (FA). Patients with AD showed significantly reduced FA in the superior longitudinal fasciculus and inferior frontal-occipital fasciculus that carry projections linking temporal and frontal regions of this semantic network. Our results are consistent with the hypothesis that semantic memory is supported in part by a large-scale neural network involving modality-specific association cortex, heteromodal association cortex and projections between these regions. The semantic deficit in AD thus arises from gray matter disease that affects the representation of feature knowledge and processing its content, as well as white matter disease that interrupts the integrated functioning of this large-scale network.
A guiding principle in recent theories of semantic memory has been the representation of sensory-motor feature knowledge associated with object concepts in (or near) regions of the brain that are responsible for perceptual and motor functions of the corresponding material (Barsalou, Simmons, Barbey, & Wilson, 2003; Martin, 2007). We refer to this as the sensory-motor account of semantic memory. Under this view, the representation of visual features of object concepts such as shape or color relies on the corresponding areas of visual association cortex, the representation of auditory features of object concepts depends in part on auditory association cortex, and so on. Although Wernicke expressed several of these ideas with remarkable clarity over a century ago (Gage & Hickok, 2005), there has been resurgent interest in recent years as converging behavioral and functional neuroimaging evidence has become available to test this hypothesis (Bonner & Grossman, 2012; Kiefer & Pulvermüller, 2012; Rodriguez, McCabe, Nocera, & Reilly, 2012; Simmons, Martin, & Barsalou, 2005; Simmons et al., 2007). For visual features of object concepts, these findings suggest a particular reliance on ventral temporal-occipital and lateral temporal cortices that support visual-perceptual processing (Martin, 2007).
In addition to modality-specific sensory and motor association cortices, we hypothesize that heteromodal regions play a role in object concepts by integrating information from multiple modalities into a coherent concept and organizing these representations so that they can be applied to various contexts in an appropriate manner (Grossman et al., 2003; Koenig & Grossman, 2007). Two regions which have been suggested to play a role in integrating diverse representations of feature knowledge are the anterior temporal lobe (Patterson, Nestor, & Rogers, 2007) and the angular gyrus (Binder & Desai, 2011; Bonner, Peelle, Cook, & Grossman, 2013). The angular gyrus in particular is an appealing candidate as a semantic “hub” due to its relatively strong connectivity with modality-specific sensory-motor cortices (Bonner, et al., 2013), and its consistent recruitment across a broad range of semantic tasks (Binder, Desai, Graves, & Conant, 2009).
Complementing these integrative regions are heteromodal areas of prefrontal cortex that are also thought to support processing of semantic representations of object concepts (Devlin, Matthews, & Rushworth, 2003; Gough, Nobre, & Devlin, 2005; Grossman et al., 2002; Jefferies, Patterson, & Lambon Ralph, 2008; Koenig et al., 2005; Reilly, Rodriguez, Peelle, & Grossman, 2011; Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997). Hypothesized processes supported by these frontal regions include retrieval of lexical and semantic information, organization of representations in semantic memory so that conceptual knowledge can be applied in a context-sensitive manner, as well as rule-based evaluation of concepts. Processes supported by regions of dorsolateral prefrontal cortex may not be specific to semantic processing, but may also reflect flexible domain-general executive resources (Duncan, 2010; E. E. Smith & Jonides, 1999).
In addition to these cortical regions, it is important to consider the role of the white matter projections that are needed to integrate information across these gray matter areas. Previous work has suggested that both dorsal stream and ventral stream tracts support projections between frontal regions and temporal regions of the large-scale network supporting semantic memory. The dorsal stream includes the superior longitudinal fasciculus/arcuate fasciculus complex (SLF), while the ventral stream includes the inferior frontal-occipital fasciculus (IFO). In a recent fMRI study, we showed that both of these tracts contribute to the semantic network during processing of object concepts (Grossman et al., 2013).
While considerable evidence from fMRI studies of healthy adults has been marshaled to support the approach outlined above, an important caveat is that fMRI activation studies of healthy adults do not indicate whether a brain region is necessary for a particular function. Thus, it is important to obtain converging evidence from other sources, such as investigations of patients with focal disease affecting the central nervous system. In this study, we turn to evidence from patients with mild Alzheimer’s disease (AD). Although the most prominent clinical characteristic of AD is difficulty with episodic memory, AD patients also show consistent deficits on tasks probing semantic memory (Gonnerman, Andersen, Devlin, Kempler, & Seidenberg, 1997; Grossman et al., 1997; Hodges & Patterson, 1995; Hodges, Salmon, & Butters, 1992; Lambon Ralph et al., 2001).
Although the specific basis of semantic deficits in AD is a matter of on-going investigation, there is evidence that the semantic memory deficit in patients with AD is associated with both degraded representations of feature knowledge of object concepts and limited processing of information represented in semantic memory (Koenig & Grossman, 2007). Evidence for degraded representations of feature knowledge comes from the observation of category-specific deficits in semantic memory. Thus, patients with AD are thought to have relatively greater difficulty with natural kinds than manufactured artifacts (Garrard et al., 2001; Grossman et al., 1998). This may be due in part to the degraded representation of visual feature knowledge that is though to play a more important role in natural kinds than manufactured objects. Semantic memory judgments of category membership for pictures and words was associated with atrophy in ventral and inferolateral portions of the posterior temporal lobe, a portion of visual association cortex, and angular gyrus (Libon et al., 2013). A follow-up study explicitly assessed the hypothesis that degraded representations of visual feature knowledge contribute to category-specific semantic memory difficulty in AD (Grossman, et al., 2013). In this study, AD patients’ judgments of visual-perceptual features of natural and manufactured object concepts were related to structural imaging studies of gray matter atrophy and white matter fractional anisotropy (FA). AD patients had greater difficulty judging visual-perceptual features of natural kinds than manufactured objects, and regression analyses related this to atrophy in inferolateral temporal-occipital and dorsolateral prefrontal gray matter. These regressions overlapped extensively with temporal and frontal regions activated by controls during judgments of natural kinds, suggesting that disease in areas activated by controls for semantic judgments of natural kinds may limit AD patients’ performance. By comparison, there was less overlap between areas implicated in AD patients’ judgments of manufactured objects and activations seen during healthy controls’ judgments of manufactured artifacts, suggesting that AD patients were able to use other brain regions to help support judgments of manufactured objects. There is also evidence that patients with AD have difficulty with semantic processes. This includes difficulty with the organizational processes that are important for determining whether an object concept is a member of a semantic category (Grossman, et al., 2003; Koenig & Grossman, 2007; Koenig, Smith, & Grossman, 2010).
In the current fMRI study, we directly examined regional cerebral activation in AD patients while they performed a semantic judgment task. Previous fMRI studies of AD during performance of semantic memory tasks are rare. While potentially informative, fMRI studies in patients with neurodegenerative disease are challenging since the task must be easy enough to be performed successfully by patients despite damage to areas important for semantic memory. Here we use the same task as in a previous behavioral study in which AD patients judged shared features of object concepts named by single words (Grossman, et al., 2013). In the task, two words were visually presented on the screen, and subjects were asked to decide whether the words’ associated objects were similar in shape or color. This semantic processing task necessarily places concurrent demands on semantic content (representations of visual-perceptual features of object concepts) and semantically-related process (accessing these semantic concepts and comparing them on a particular feature). While prior work related patients’ judgments to structural imaging of gray matter atrophy in temporal and frontal regions, the current fMRI study was designed to provide converging evidence by showing reduced activation in these temporal and frontal areas. Limited recruitment of visual association cortex in inferolateral temporal-occipital regions during semantic judgments of perceptual features of semantic categories in AD thus would be consistent with degraded representations of visual feature knowledge of object concepts. Limited recruitment of prefrontal cortex during these judgments would be consistent with a deficit in the processes implicated in these semantic judgments. Finally, we use diffusion MRI to investigate the integrity of frontal-temporal white matter pathways in AD in the context of distributed semantic networks.
Methods
Subjects
All subjects were right-handed native English speakers, with normal (or corrected-to-normal) vision. Written informed consent was obtained from all subjects according to a protocol approved by the University of Pennsylvania Institutional Review Board. Demographic information is shown in Table 1.
Table 1.
Mean (± standard deviation) demographic information for patients with Alzheimer’s Disease and healthy controls
| Controls | Alzheimer’s Patients | |
|---|---|---|
| N | 21 | 12 |
| Age (years) | 65.0 | 68.8 (10.18) |
| Education (years) | 15.2 (2.3) | 16.7 (2.99) |
| MMSEa * | 28.0 (1.3) | 22.5 (6.1) |
| Boston Naming | - | 22.4 (6.1) |
| Pyramids and Palm Trees | - | 45.0 (6.0) |
| Animal Fluency | - | 9.9 (5.5) |
| Vegetable Fluency | - | 7.0 (4.7) |
| Tools Fluency | - | 4.8 (3.7) |
MMSE was available for 19 controls
Controls > Alzheimer’s patients, p < .05
We studied 12 patients with AD spectrum disease between the ages of 56–81 years (mean = 68.8; 6 males). Patients were diagnosed in the Department of Neurology at the University of Pennsylvania according to published criteria (McKhann et al., 2011). Exclusion criteria included other neurological disorders such as stroke or hydrocephalus, primary psychiatric disorders such as major depression or schizophrenia, or medical conditions that might interfere with cognitive functioning such as encephalopathy or metabolic disorders. Patients may have been taking a clinically indicated dosage of a medication such as a cholinesterase inhibitor or a small dosage of an anti-depressant, but dosage was stable throughout the entire study and no patients showed evidence of medication-related cognitive side effects.
For 10 of the 12 patients, we also collected a set of neuropsychological measures to assess overall cognitive functioning. This included the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), picture version of the Pyramids and Palm Trees test (Howard & Patterson, 1992), and category fluency for three different category types (animals, vegetables, and tools). For category fluency, patients were asked to name as many members of the category as possible within 60 seconds. We also administered the MMSE (Folstein, Folstein, & McHugh, 1975) as a test of overall cognitive function.
We also studied 21 healthy senior subjects between the ages of 50–78 years (mean = 65.0; 9 males), who served as controls for the AD patients. AD patients and controls were matched in age and education, and had good general health and no history of neurological difficulty, as established by a pre-scan screening form.
Materials
We created pairs of printed nouns, half of which denoted natural kinds (e.g. carrot, banana) and half manufactured artifacts (e.g. baseball, sword). Natural kinds consisted of fruits, vegetables and animals, and manufactured artifacts consisted of instruments, sports equipment and means of transportation. In a preliminary assessment, a large sampling of pairs was evaluated for similarity of color and of shape by nine subjects using a 7-point similarity scale. Pairs with average similarity ratings of 5 or above were considered “same”, and pairs with ratings of 3 or below were considered “different”. From these items, we selected 160 pairs with the most consistent judgments across individuals, where half probed shape and half probed color, and half of the shape and half the color pairs were judged “same” and half “different.” Stimuli were evenly distributed across natural kinds and manufactured artifacts. Frequency scores and familiarity ratings obtained from a different group of 20 young adults were used to match lists of items, and no significant differences were found between the lists for each subcategory, or between the lists for natural kinds and manufactured artifacts, or between the lists for the conditions of shape and color. All stimulus words were highly imageable.
Procedure
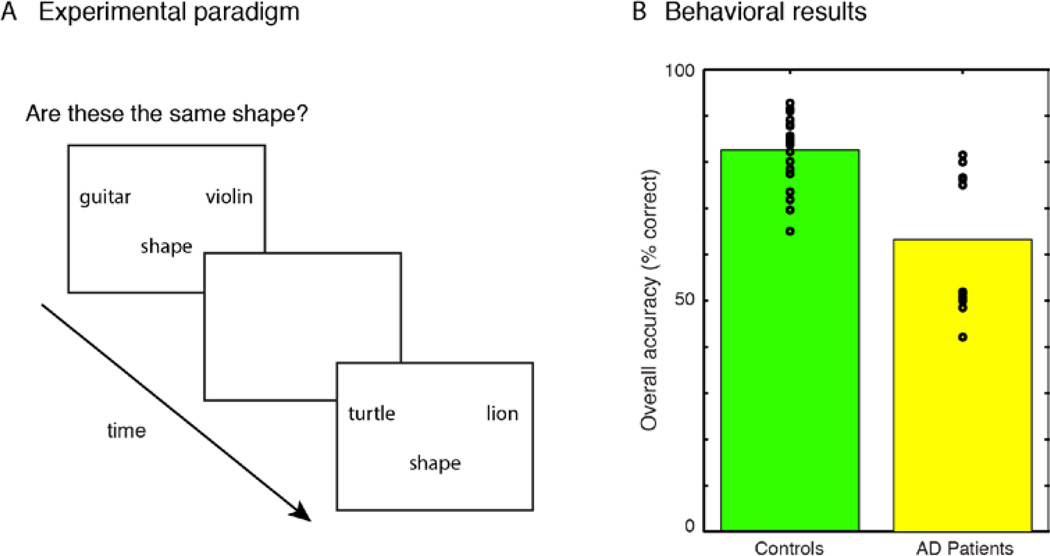
The trial procedure is shown schematically in Figure 1A. Each trial began with a 500 ms crosshair followed by presentation of a pair of nouns, pseudowords or unnamable visual forms. Pairs remained on the screen for 2.5 sec or until subjects responded using a keypad to indicate “same” or “different.” Between each trial, there was an interval of 0, 3, 6, 9 or 12 seconds, during which time a blank, white screen was displayed. The various intertrial intervals were distributed throughout each scanning session. Subjects were trained in advance on the experimental method with several practice items, and all subjects appeared to understand the task and the procedure for indicating their judgments.
Figure 1.
(A) Schematic of semantic association task. (B) Accuracy on semantic judgments for all stimuli for controls and patients with Alzheimer’s disease. Performance for each subject is indicated by a circle, with bars representing group means.
Presentation was blocked by task in order to minimize executive control demands associated with switching between materials or between probes. The first set of stimulus blocks involved the word-judgment task, which asked subjects to compare pairs of written object nouns based on a perceptual attribute. Blocks began with a question for 3 sec indicating the attribute to be compared during the block (e.g. “Are these the same color?” or “Are these the same shape?”), and the relevant property (i.e. “color”/”shape”) was written below each word pair during presentation of the remainder of the stimuli for a block. An event-related design was used, and 80 word pairs (40 pairs of natural kinds and 40 pairs of manufactured objects) were presented in a fixed pseudorandom order, for the block probing each perceptual attribute.
Following the word-judgment blocks, subjects performed a form-judgment task, comparing pairs of unnamable visual geometric forms based on a given perceptual feature (color or shape). The perceptual feature to be compared was indicated by the presentation of a question (e.g. “Are these the same shape?”), and the perceptual feature of interest (e.g. “shape”) was printed below each pair of forms during the remainder of the block, as during the probes of object noun pairs. The geometric forms were systematically varied for the relevant perceptual feature, so that there were 8 same and 8 different pairs for each perceptual feature.
Subjects also performed a pseudoword-judgment task, in which they judged letter similarity in 20 pairs of pronounceable pseudowords with no English meaning, according to the American Heritage Dictionary. Pseudowords were matched for syllable length and letter length to the noun stimuli in the word-judgment task. Subjects were asked to read the pseudowords silently, and judge whether the pairs matched for constituent letters. The pairs ended in the same or different letters, and there were equal numbers of same and different pairs.
Because semantic processing was our primary interest, our analyses focused on group differences in activity during the color and shape judgment tasks.
MRI data acquisition and analysis
Neuroimaging data were acquired on a Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) at 3T, beginning with acquisition of a T1-weighted structural volume using a MPRAGE sequence (repetition time [TR] = 1620 ms, echo time [TE] = 3 ms, flip angle = 15°, 1 mm slice thickness, 192 × 256 matrix, voxel size = 0.98 × 0.98 × 1 mm). Blood oxygenation level-dependent functional MRI images were acquired with 3 mm isotropic voxels, flip angle = 15°, TR = 3 s, TEeff = 30 ms, and a 64 × 64 matrix.
We analyzed the fMRI data using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). For each subject, images were realigned to the first image, coregistered to the structural image, and normalized to Montreal Neurological Institute (MNI) space using unified segmentation (Ashburner & Friston, 2005), including resampling to 2 × 2 × 2 mm voxels, and spatially smoothed with a 9 mm full-width at half maximum (FWHM) Gaussian kernel. We modeled responses to events using a canonical hemodynamic response function, and included movement parameters as covariates of no interest. We consider only activity related to correct responses (incorrect responses were modeled using a separate condition). The parameter estimates from single-subject analyses were brought to second-level random effects analyses for making group inferences, thresholded at a voxelwise threshold of p < .001 (uncorrected), and FDR corrected for multiple comparisons at the cluster level (q < .05) using random field theory (Chumbley & Friston, 2009).
Diffusion-weighted data acquisition and analysis
Diffusion imaging acquisition parameters were: FOV = 240 mm; matrix size = 128 × 128; number of slices = 70; imaging resolution = 1.9 × 1.9 × 2 mm; TR = 8000 ms; TE = 82 ms; fat saturation. In total, 31 volumes were acquired per subject, one without diffusion weighting (b = 0 s/mm2) and 30 with diffusion weighting (b = 1000 s/mm2) along 30 non-collinear directions.
Diffusion-weighted images were pre-processed using ANTS and the Camino toolkit (Cook et al., 2006) using the PipeDream processing pipeline. Motion and distortion artifacts were removed by affine co-registration of each diffusion-weighted image to the unweighted (b = 0) image. Diffusion tensors were computed using a linear least squares algorithm (Salvador et al., 2005) implemented in Camino.
Using the T1 template described above, DTI images from each subject were relocated to the T1 template space by PipeDream. Any distortion between the subject's T1 and DTI image was corrected by registering the fractional anisotropy (FA) to the T1 image. The DTI image was then warped to template space by applying both the intra-subject (FA to subject T1) and intersubject (subject T1 to template) warps. We also reoriented the tensors using the preservation of principal directions algorithm (Alexander, Pierpaoli, Basser, & Gee, 2001).
We used FA as a marker of white matter integrity. Voxelwise comparisons of FA between AD patients and controls was done using the randomise function in FSL 4. Significant results were identified using threshold-free cluster enhancement (S. M. Smith & Nichols, 2009), whole-brain corrected at p < .05.
Results
Behavioral results
Behavioral results across all word categories are shown in Figure 1B. As expected, as a group AD patients performed worse on the behavioral task than the control subjects, t(31) = 4.93, p < .001.
fMRI results
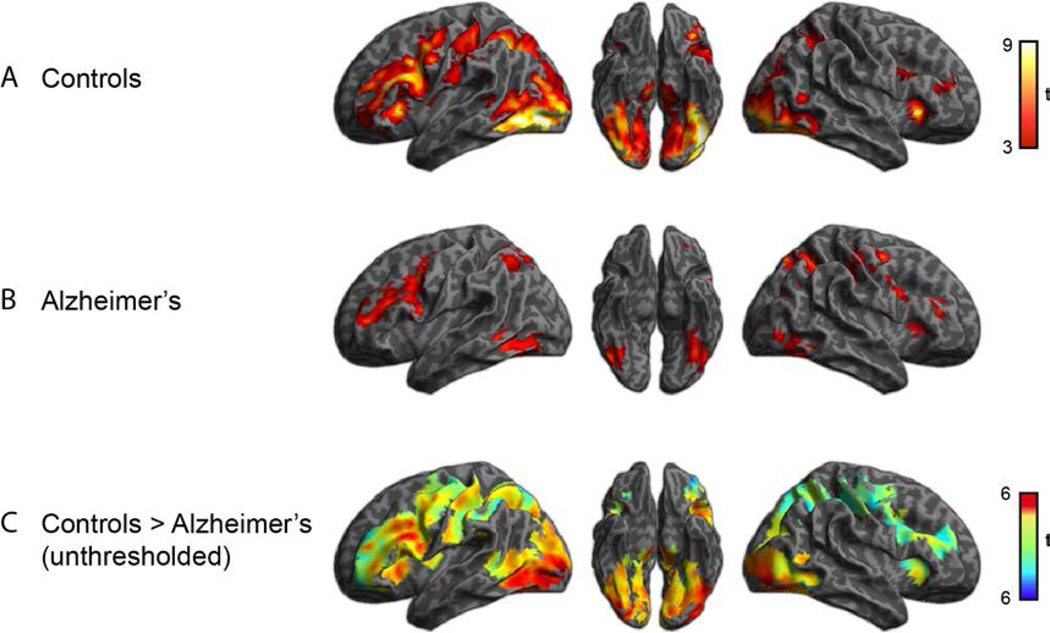
We first examined activity during the semantic processing task for all words, collapsing across word category and visual feature comparison. As noted above, we examined activity during correct responses only. These results are shown in Figure 2 and Tables 2–4. Healthy seniors and AD patients showed qualitatively similar patterns of recruitment encompassing inferior temporal cortex, inferior parietal cortex, and inferior frontal cortex, although these activations were not as robust in AD patients. We then directly compared the two groups of subjects within this common semantic network by first finding the regions that were recruited on average in both groups, and then within this mask looking for any group differences. We show the unthresholded t statistics from this comparison in Figure 2C. AD patients showed significantly less activation in left temporal-occipital cortex compared to healthy seniors, with a suggestion of reduced activity (at p < .001, uncorrected) in AD in left inferior frontal cortex. There were no regions in which AD patients showed significantly more activity than controls, although there were some clusters in right inferior frontal gyrus that were present at p < .001 uncorrected (see Table 4).
Figure 2.
Activity associated with associative semantic processing compared to rest. (A) Activity for healthy older adults. (B) Activity for patients with Alzheimer’s disease. (C) Direct comparison between controls and patients within the region showing a response when averaged for both groups. Warmer colors indicate greater activity in controls than patients, cool colors greater activity in patients than in controls.
Table 2.
Maxima for fMRI activity related to semantic processing task for healthy adults
| Cluster size (µl) | Region | Peak Coordinate | Z score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Controls | |||||
| 317600 | Left inferior occipital cortex | −40 | −74 | −4 | 6.10 |
| Left inferior occipital cortex | −38 | −84 | −10 | 6.01 | |
| Left inferior occipital cortex | −44 | −62 | −10 | 6.00 | |
| 7072 | Right anterior insula/frontal operculum | 32 | 22 | 0 | 5.72 |
| Right middle frontal gyrus | 44 | 44 | 20 | 3.99 | |
| Right inferior frontal gyrus | 40 | 30 | 14 | 3.94 | |
| 8008 | Supplemental motor area | −8 | 16 | 46 | 4.71 |
| Supplemental motor area | 6 | 14 | 44 | 4.37 | |
| Supplemental motor area | −6 | 6 | 54 | 4.22 | |
| 4416 | Right precentral gyrus | 48 | 12 | 34 | 4.17 |
| Right precentral gyrus | 42 | 2 | 30 | 4.13 | |
Table 4.
Maxima for fMRI activity related to semantic processing task that differ between healthy adults and Alzheimer’s patients
| Cluster size (µl) | Region | Peak Coordinate | Z score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Controls > AD Patients | |||||
| 5432 | Left occipital cortex | −30 | −94 | −2 | 4.02 |
| Left occipital cortex | −32 | −76 | −6 | 3.71 | |
| Left occipital cortex | −36 | −86 | −10 | 3.70 | |
| 1304a | Right occipital cortex | 34 | −80 | −12 | 3.78 |
| 174a | Right calcarine sulcus | 18 | −66 | 8 | 3.51 |
| 184a | Brainstem | 2 | −24 | −12 | 3.45 |
| 208a | Left anterior insula | −34 | 20 | 4 | 3.45 |
| 616a | Left calcarine sulcus | −12 | −78 | 14 | 3.43 |
| 448a | Left inferior frontal gyrus | −38 | 10 | 26 | 3.42 |
| AD Patients > Controls | |||||
| 240a | Right middle frontal gyrus | 32 | 20 | 44 | 3.40 |
| 192a | Right middle frontal gyrus | 34 | 34 | 38 | 3.27 |
cluster not significant
One possible factor contributing to the difference in activity between the healthy adults and AD patients is that we expect AD patients to have widespread reductions in gray matter volume. That is, our observation of reduced activity in AD patients may be a direct consequence of morphological differences between groups. To investigate this possibility, we extracted both parameter estimates and gray matter volume from the peak voxels showing group differences (Table 4). For each peak, we performed a regression analysis of the fMRI parameter estimates against gray matter volume, and then repeated the group comparisons by performing two sample t-tests on the residuals (which reflect neural activity, having factored out the effect of gray matter volume). Results were consistent for all of these peaks, all t values > 2.8, all p values ≤ .003. Thus, group differences in gray matter volume do not seem to underlie our finding differences in activity between healthy adults and AD patients.
It is noteworthy that behavioral performance of the AD patients was somewhat variable. Poorer behavioral performance could simply indicate less efficient semantic processing, but it could also indicate that a subgroup of the patients were not performing the semantic task. To investigate this possibility we performed a median split on the AD patients based on overall accuracy, identifying 6 patients with better performance and 6 patients with worse performance. To identify regions involved in the semantic task we looked at where there was task-related activity for the entire group (i.e., a [1 1] contrast of healthy adults and AD patients). We then extracted parameter estimates for the AD patients from the maxima, and conducted independent samples t-tests to test for differences between better and worse performing patients. Of the 21 maxima tested, only one showed an uncorrected p value of < .05. This was [−40 28 16], in left inferior frontal gyrus, t(10) = 3.0, p = .013. All other t values were ≤ 1.0. Thus, we do not see any strong evidence for clear subgroups in our AD patients. However, this issue would certainly be worth examining in a larger cohort of patients.
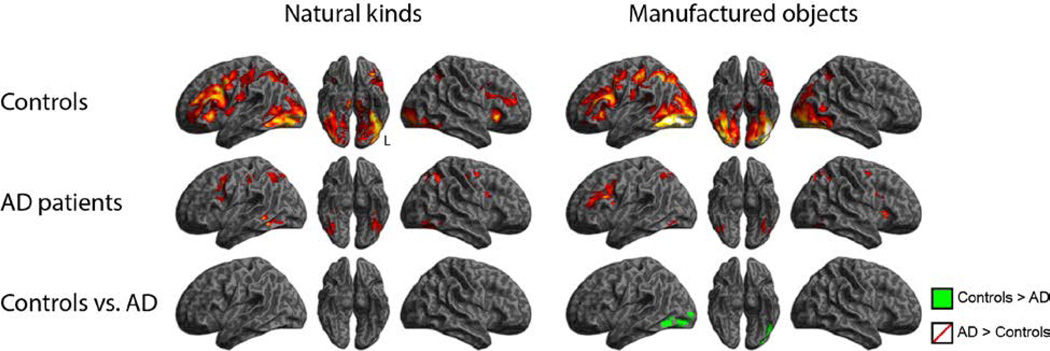
We next compared patterns of activity for judgments about natural kinds and manufactured objects separately, shown in Figure 3. There were no whole-brain corrected differences between controls and AD patients for natural kinds; however, for manufactured objects, AD patients showed significantly reduced activation in left lateral temporal-occipital regions. There were no regions in which AD patients showed more activation than controls. In addition, there were no differences between groups for judgments of shape or color.
Figure 3.
Activity during associative semantic processing for natural kinds and manufactured objects.
There were no significant differences between healthy adults and AD patients for either the perceptual or pseudoword conditions.
Diffusion imaging results
We next examined the diffusion imaging data to see whether there were differences in white matter tract integrity (indexed by FA) between AD patients and controls. The results of this analysis are shown in Figure 4, with maxima listed in Table 5. We found several regions in which AD patients showed significantly reduced FA compared to controls, falling within SLF and IFO.
Figure 4.
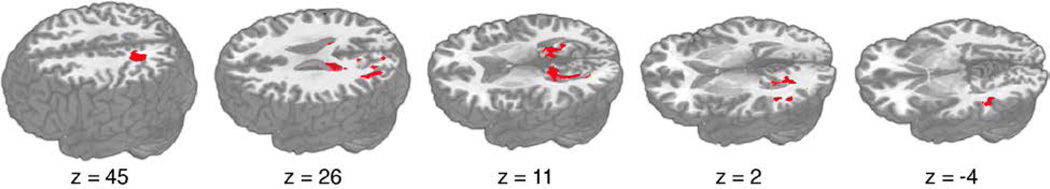
Voxelwise comparison of fractional anisotropy (FA) between patients with Alzheimer’s disease and controls overlaid on a canonical T1-weighted image. Clusters indicate significantly reduced FA in patients relative to controls, cluster-corrected at a whole brain level p < .05. MNI coordinates are displayed below each axial slice.
Table 5.
Clusters showing reduced fractional anisotropy in AD patients relative to controls
| Cluster size (µl) |
Region | Peak Coordinate | P- value |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 5607 | Splenium of corpus callosum | −17 | −37 | 8 | 0.007 |
| LEFT SUPERIOR LONGITUDINAL/ARCUATE FASCICULUS COMPLEX | |||||
| 2848 | Left superior parietal lobule white matter | −23 | −45 | 45 | 0.015 |
| 2507 | Left lateral occipital gyrus white matter | −38 | −59 | 28 | 0.002 |
| 50 | Left lateral occipital gyrus white matter | −39 | −74 | 6 | 0.039 |
| 1034 | Left superior temporal gyrus white matter | −52 | −44 | −4 | 0.011 |
| 267 | Left middle temporal gyrus white matter | −52 | −56 | 3 | 0.030 |
| 13 | Left supramarginal gyrus white matter | −47 | −33 | 37 | 0.048 |
| LEFT INFERIOR FRONTAL-OCCIPITAL FASCICULUS | |||||
| 73 | Left fusiform gyrus white matter | −35 | −66 | −10 | 0.042 |
| RIGHT SUPERIOR LONGITUDINAL/ARCUATE FASCICULUS COMPLEX | |||||
| 204 | Right angular gyrus white matter | 44 | −47 | 29 | 0.023 |
| 15 | Right angular gyrus white matter | 35 | −53 | 38 | 0.048 |
Discussion
Patients with AD have difficulty judging visual features of manufactured and natural object concepts. During these judgments, we observed reduced activation in AD patients compared to healthy seniors in a large-scale neural network involving inferolateral temporal and prefrontal regions. This suggests that semantic memory deficits in AD are due in part to both degraded representations of visual feature knowledge of object concepts and impoverished processes that operate on this knowledge. We also found significantly reduced recruitment of inferolateral temporal cortex during judgments of manufactured artifacts in AD compared to controls, implicating degradation of the representation of visual feature knowledge of manufactured object concepts. However, there were no differences between patients and controls for natural kinds. Finally, the dorsal stream white matter projections in SLF and ventral stream projections in IFO between these frontal and temporal regions appear to be compromised in AD. Thus, although our findings need to be considered in the context of our modestly-sized cohort of AD patients, they suggest that semantic memory deficits in AD are due in part to several levels of disruption of a large-scale neural network for semantic memory. We consider each of these findings below.
Degraded representation of visual feature knowledge in ventral temporal-occipital cortex in Alzheimer’s disease
There is considerable evidence suggesting that visual feature knowledge of object concepts is represented in part in or near areas of visual association cortex that are important for processing the corresponding visual-perceptual attributes. This is consistent with the sensory-motor approach to semantic memory. Most of the evidence in support of this model has come from fMRI studies of healthy adults. fMRI studies provide crucial information about the association of a brain region with task performance, but do not indicate the subset of regions that are necessary for a specific cognitive function. Further, in studies of sensory-motor features of object knowledge, there is also the potential confound between the representation of a sensory-motor feature and perceiving the same material.
One additional way to circumvent problems such as these involves converging evidence from other sources. In particular, several studies of patients with focal brain damage in modality-specific association cortex also have shown difficulty with representations of conceptual knowledge that depend in part on the modality associated with the compromised brain region. For example, patients with disease in auditory association cortex appear to have difficulty with concepts like “thunder” that depend on auditory feature knowledge (Bonner & Grossman, 2012), and patients with disease in motor brain regions appear to be compromised in their knowledge of concepts that depend in part on action knowledge (Bak & Chandran, 2012; Bak, O'Donovan, Xuereb, Boniface, & Hodges, 2001; Grossman et al., 2008). Importantly, these patients do not have a deficit perceiving the material that is being probed that could confound performance.
Patients with AD have disease in visual association cortex in inferolateral portions of the temporal lobe. This raises the possibility that semantic memory difficulty in AD is due in part to degraded representations of visual feature knowledge about object concepts. Previous work has suggested this possibility as well. These previous investigations involved judgments of object concepts and used regression analyses to relate patient performance to gray matter atrophy in visual association cortex. For example, one recent study asked AD patients to judge whether a familiar word or a picture is a member of a superordinate category such as “tool” or “vegetable” (Libon, et al., 2013). Patients were significantly impaired on this task relative to healthy seniors, and their performance was related to lateral and ventral portions of the posterior temporal lobe including visual association cortex. A follow-up investigation used materials identical to those used in the present study to explicitly examine the role of visual association cortex in judgments of visual feature knowledge of object concepts (Grossman, et al., 2013). We found that AD patients are significantly impaired in their judgments of visual feature knowledge. Regression analyses related performance at least in part to inferolateral portions of temporal-occipital cortex. In the present study, we used functional imaging to demonstrate that AD patients’ impaired judgments of visual feature knowledge of objects is due in part to limited recruitment of these inferolateral portions of temporal-occipital cortex, particularly for manufactured objects. Since we used words as stimuli, it is unlikely that deficits were due to difficulty perceiving the visual-perceptual attributes of a pictured stimulus. While it may be possible that these patients have difficulty with mental imagery that is limiting their performance, this implies a deficit in generating the semantic representation needed to evaluate the mental image. Reading involves areas of temporal-occipital cortex near these regions of reduced activation in AD, but these mild AD patients did not have any evidence of alexia. Deficits associated with reduced temporal-occipital activation relative to controls could not be attributed easily to imagery or reading difficulty when this effect was found only for manufactured objects but not natural kinds.
Previous studies have shown category-specific deficits in semantic memory judgments of patients with AD. While there is some variability in demonstrating this deficit in individual AD patients, possibly related to individual differences in the anatomic distribution of disease, patients with AD often have greater difficulty judging natural kinds than manufactured objects. Several possible explanations have been forwarded to explain this observation. For example, it has been claimed that natural kinds are more dependent on visual feature knowledge than manufactured objects, and therefore natural kinds may be more vulnerable to disease in visual association cortex (Saffran et al, 1994). When we examined this hypothesis, we found considerable overlap between areas of temporal-occipital cortex activated by healthy adults during judgments of natural kinds and AD patients’ disease in temporal-occipital cortex (Grossman, et al., 2013). Moreover, regression analyses related judgments of natural kinds to this area as well, suggesting that patients were trying to recruit diseased brain regions to perform a task in the way that it may have been performed prior to their disease. By comparison, areas recruited by healthy adults during judgments of manufactured artifacts had less overlap with areas implicated in AD patients’ judgments of the same stimuli. This suggested that other brain regions could help support judgments of manufactured artifacts in the face of disease in inferolateral temporal-occipital cortex, and provided an explanation for a category-specific deficit with greater difficulty for natural than manufactured objects.
The results of the present study are largely consistent with this account. Thus, during judgments of natural kinds, AD patients did not differ from controls in their recruitment of inferolateral temporal-occipital cortex. By comparison, during judgments of manufactured objects, we observed reduced activation of inferolateral temporal-occipital cortex in AD than controls. Inspection of Figure 3 shows that AD patients had additional activation of right frontal cortex that was not evident in healthy controls. While direct comparison of AD patients and healthy controls did not yield a robust difference between groups, this may represent one area of “compensatory activation” used by AD patients to help support judgments of manufactured objects in the face of temporal-occipital disease. Motor regions of the brain have been associated with the representation of manufactured objects, presumably to represent actions associated with tools, sports equipment and the like, and these features may be activated when visual feature knowledge is degraded. However, action knowledge associated with natural kinds like vegetables is relatively limited and thus may not be informative enough to warrant similar compensatory frontal recruitment. Additional work is needed with larger groups of subjects to assess this hypothesis.
Compromised semantic processing in frontal cortex in Alzheimer’s disease
In our model of semantic memory, the sensory-motor account plays a crucial role in the representation of feature knowledge about object concepts. However, it appears that additional components of semantic memory are needed to integrate these features into coherent concepts and to organize these features so that they can be applied in a context-sensitive manner to the real world. Many studies of semantic memory in healthy adults recruit prefrontal brain regions (Binder, et al., 2009), and disease in this area compromises semantic judgments (Jefferies, et al., 2008).
The present study confirms the contribution of prefrontal regions to semantic memory. We observed activation of prefrontal and inferior frontal regions during judgments of visual feature knowledge about object concepts. We also observed reduced frontal activation in AD patients who have semantic deficits. We do not think that this activation is reflecting a nonspecific effect of disease that may be seen in any such judgment task since the task itself was quite simple. Moreover, our previous work showed that AD patients have some prefrontal atrophy, and this was correlated with semantic memory performance (Grossman, et al., 2013). The findings of the present report are consistent with a prior fMRI study of healthy young adults using materials identical to those of the present study that demonstrated prefrontal activation, presumably to support judgments of feature knowledge associated with object concepts (Grossman, et al., 2013). This previous work also examined patients with AD, and regression analyses implicated prefrontal areas in the semantic deficits observed in these patients. We interpreted our findings as consistent with a large-scale semantic memory network involving both modality-specific association cortex in the representation of feature knowledge about objects, and heteromodal association cortex in processes crucial for semantic memory.
There has been some debate about the potential role of prefrontal cortex in semantic processing. A strong body of work supports the hypothesis that frontal brain regions such as inferior frontal cortex appear to contribute to processes such as selective retrieval of information from semantic memory (Thompson-Schill, et al., 1997; Wagner, Maril, Bjork, & Schacter, 2001). Others have argued that the organization of feature knowledge about object concepts depends in part on dorsolateral prefrontal cortex (Grossman, et al., 2008; Koenig & Grossman, 2007; Peelle, Troiani, & Grossman, 2009). Here, knowledge about object concepts is organized so that it is sensitive to the context in which it is being used. Turning a pail upside down to use it as a footrest, for example, requires reorganizing the features of the object so that it can be used appropriately in its context. The functions of this organizational component in semantic memory are similar to many of the organization roles supported by prefrontal cortex in other cognitive domains (Duncan, 2010; Jefferies, et al., 2008).
In the previous fMRI study using these materials in healthy young adults (Grossman, et al., 2013), we observed that both inferior frontal cortex and dorsolateral prefrontal cortex are recruited. Thus, it was difficult to discriminate in the previous study between possible functions of selective retrieval and feature organization that frontal cortex may play in semantic memory. The present study may be useful in this regard because we observed activation of dorsolateral frontal cortex but not inferior frontal cortex during task performance in AD. Minimal recruitment of inferior frontal cortex would appear to implicate a compromised selective retrieval mechanism in AD patients’ semantic memory difficulties. However, a direct comparison of AD patients and controls revealed relatively reduced activation in AD in both dorsolateral prefrontal and inferior frontal regions, suggesting that an organizational component also may play a role in the semantic memory deficits of AD patients. Additional work is needed to define further the role of prefrontal disease in AD patients’ impaired semantic memory.
Large-scale integration of semantic memory network in Alzheimer’s disease
fMRI studies show that both temporal-occipital and prefrontal regions are recruited during judgments of visual feature knowledge of object concepts. While both of these regions appear to be recruited during these judgments, performance presumably also must be coordinated between regions so that they function harmoniously during semantic judgments. Some evidence about the integrated functioning of these brain regions in semantic memory came from a study assessing healthy seniors using these identical materials (Peelle, Chandrasekaran, Powers, Smith, & Grossman, 2013). We found reduced activation in these regions in healthy seniors with poorer performance compared to healthy seniors whose performance equaled that of young adults. Moreover, we observed gray mater atrophy only in frontal regions of the poorer performing seniors, but there was no atrophy of the temporal-occipital region. We argued that reduced temporal-occipital activation in the poorer-performing seniors was due in part to reduced recruitment of this area into the semantic network by prefrontal cortex. This interaction between brain regions in a large-scale neural network is highly likely to be mediated by white matter projections between these components of the semantic memory network.
Previous work in healthy young adults suggests that prefrontal and temporal-occipital components of the semantic memory network are integrated by at least two white matter projections: There appears to be a dorsal white matter projection through the superior longitudinal fasciculus, and a ventral projection through the inferior frontal-occipital fasciculus (Grossman, et al., 2013). Damage to these pathways may contribute to interrupting projections between the functional gray matter components of the semantic memory network.
Our previous assessment of AD using structural imaging suggested that frontal-temporal white matter projections are compromised in AD, particularly for the gray matter regions subserving semantic memory aspects of natural kinds. This appeared to be most evident in the ventral projections, but was also evident in the dorsal projections. In the present study, we found reduced fractional anisotropy in the superior longitudinal fasciculus, that is, the dorsal projection between frontal and posterior temporal brain regions, as well as the ventral projection through the inferior frontal-occipital fasciculus. This may serve to compromise the interaction between these brain regions and further interfere with semantic memory in AD.
Table 3.
Maxima for fMRI activity related to semantic processing task for Alzheimer’s patients
| Cluster size (µl) | Region | Peak Coordinate | Z score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| 5080 | Right anterior insula/frontal operculum | 24 | 26 | 10 | 4.61 |
| Right inferior frontal gyrus | 36 | 30 | 18 | 4.48 | |
| Right anterior insula/frontal operculum | 30 | 20 | 4 | 3.59 | |
| 34424 | Vermis | 0 | −68 | −22 | 4.49 |
| Right cerebellum | 16 | −70 | −20 | 4.37 | |
| Vermis | −4 | −60 | −12 | 4.09 | |
| 21808 | Left inferior frontal gyrus | −40 | 24 | 30 | 4.24 |
| Left inferior frontal gyrus | −48 | 8 | 10 | 4.21 | |
| Left precentral gyrus | −34 | 2 | 38 | 4.15 | |
| 9472 | Right precentral gyrus | 28 | −4 | 48 | 4.24 |
| Right postcentral gyrus | 38 | −22 | 34 | 4.10 | |
| Right postcentral gyrus | 56 | −26 | 50 | 3.93 | |
| 10704 | Right superior parietal cortex | 18 | −70 | 52 | 4.15 |
| Right inferior parietal cortex | 26 | −50 | 42 | 4.14 | |
| Right inferior parietal cortex | 34 | −62 | 48 | 4.06 | |
| 8312 | Left superior parietal cortex | −28 | −60 | 52 | 4.00 |
| Left inferior parietal cortex | −30 | −50 | 36 | 3.97 | |
| Left superior parietal cortex | −16 | −74 | 52 | 3.64 | |
| 3960 | Right inferior frontal gyrus | 38 | 8 | 26 | 3.88 |
| Right putamen | 28 | 0 | 14 | 3.81 | |
| Right precentral cortex | 58 | 2 | 36 | 3.58 | |
| 2248 | Left putamen | −22 | −4 | 12 | 3.78 |
| Left putamen | −24 | −16 | 4 | 3.60 | |
Acknowledgments
We are grateful to Keerthi Chandrasekaran for assistance with data collection and processing. We thank the radiographers at the Hospital of the University of Pennsylvania for their assistance with data collection, and volunteers for their participation. Research reported in this publication was supported in part by the National Institutes on Aging and Neurological Disorders and Stroke of the National Institutes of Health under award numbers R01AG038490, R01AG015116, R01AG017586, R01AG032953, R01NS044266, and R01NS053488. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Partial transformations of diffusion tensor magnetic resonance images. IEEE Transactions on Medical Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bak TH, Chandran S. What wires together dies together: Verbs, actions and neurodegeneration in motor neuron disease. Cortex. 2012;48:936–944. doi: 10.1016/j.cortex.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Bak TH, O'Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease–dementia–aphasia syndrome. Brain. 2001;124:103–120. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Barsalou LW, Simmons WK, Barbey AK, Wilson CD. Grounding conceptual knowledge in modadlity-specific systems. Trends in Cognitive Sciences. 2003;7(2):84–91. doi: 10.1016/s1364-6613(02)00029-3. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in primary progressive aphasia. Journal of Neuroscience. 2012;32:7986–7991. doi: 10.1523/JNEUROSCI.6241-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Peelle JE, Cook PA, Grossman M. Heteromodal conceptual processing in the angular gyrus. NeuroImage. 2013;71:175–186. doi: 10.1016/j.neuroimage.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage. 2009;44:62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-source diffusion-MRI reconstruction and processing; Paper presented at the International Society for Magnetic Resonance in Medicine; 2006. [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cotex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental state: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gage N, Hickok G. Multiregional cell assemblies, temporal binding and the representation of conceptual knowledge in cortex: A modern theory by a "classical" neurologist, Carl Wernicke. Cortex. 2005;41:823–832. doi: 10.1016/s0010-9452(08)70301-0. [DOI] [PubMed] [Google Scholar]
- Garrard P, Lambon Ralph MA, Watson PC, Powis J, Patterson K, Hodges JR. Longitudinal profiles of semantic impairment for living and nonliving concepts in dementia of Alzheimer's type. Journal of Cognitive Neuroscience. 2001;13:892–909. doi: 10.1162/089892901753165818. [DOI] [PubMed] [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer's disease. Brain and Language. 1997;57:254–279. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cotex with transcranial magnetic stimulation. Journal of Neuroscience. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, ELman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, Glosser G, DeVita C, Moore P, Rhee J, Gee J. Neural basis for semantic memory difficulty in Alzheiemer's disease: an fMRI study. Brain. 2003;126:292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Grossman M, Payer F, Onishi K, D'Esposito M, Morrison D, Sadek A, Alavi A. Language comprehension and regional cerebral defects in frontotemporal degeneration and Alzheimer's disease. Neurology. 1998;50(1):157–163. doi: 10.1212/wnl.50.1.157. [DOI] [PubMed] [Google Scholar]
- Grossman M, Payer F, Onishi K, White-Devine T, Morrison D, D'Esposito M, Alavi A. Constraints on the cerebral basis for semantic processing from neuroimaging studies of Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Peelle JE, Smith EE, McMillan CT, Cook PA, Powers J, Burkholder L. Category-specific semantic memory: Converging evidence from BOLD fMRI and Alzheimer's disease. NeuroImage. 2013;68:263–274. doi: 10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Smith EE, Koenig P, Glosser G, DeVita C, Moore P, McMillan C. The neural basis for categorization in semantic memory. NeuroImage. 2002;17:1549–1561. doi: 10.1006/nimg.2002.1273. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–459. doi: 10.1016/0028-3932(94)00127-b. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer's disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramids and palm trees: A test of semantic access form pictures and words. Bury St Edmunds: Thames Valley; 1992. [Google Scholar]
- Jefferies E, Patterson K, Lambon Ralph MA. Deficits of knowledge versus executive control in semantic cognition: Insights from cued naming. Neuropsychologia. 2008;46:649–658. doi: 10.1016/j.neuropsychologia.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: Theoretical developments, curent evidence and future directions. Cortex. 2012;48:805–825. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Process and content in semantic memory. In: Hart J Jr, Kraut MA, editors. Neural basis of semantic memory. Cambridge: Cambridge University Press; 2007. pp. 247–264. [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Grossman M. The neural basis for novel semantic categorization. NeuroImage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Grossman M. Categorization of novel tools by patients with Alzheimer's disease: Category-specific content and process. Neuropsychologia. 2010;48:1877–1885. doi: 10.1016/j.neuropsychologia.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and demantia of Alzheimer's type: a comparative neuropsychological study and literature review. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Rascovsky K, Powers J, Irwin DJ, Boller A, Weinberg D, Grossman M. Comparative semantic profiles in semantic dementia and Alzheimer's disease. Brain. 2013;136:2497–2509. doi: 10.1093/brain/awt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Reviews of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's and Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Chandrasekaran K, Powers J, Smith EE, Grossman M. Age-related vulnerability in the neural systems supporting semantic processing. Frontiers in Aging Neuroscience. 2013;5:46. doi: 10.3389/fnagi.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Grossman M. Interaction between process and content in semantic memory: An fMRI study of noun feature knowledge. Neuropsychologia. 2009;47:995–1003. doi: 10.1016/j.neuropsychologia.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly J, Rodriguez AD, Peelle JE, Grossman M. Frontal lobe damage impairs process and content in semantic memory: Evidence from category specific effects in progressive nonfluent aphasia. Cortex. 2011;47:645–658. doi: 10.1016/j.cortex.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AD, McCabe ML, Nocera JR, Reilly J. Concurrent word generation and motor performance: Further evidence for language-motor interaction. PLOS One. 2012;7:e37094. doi: 10.1371/journal.pone.0037094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran E, Schwartz MF, Umilta C, Moscovitch M. Attention and performance XV: Conscious and nonconscious information processing. Cambridge: MIT Press; 1994. Of cabbages and things: Semantic memory from a neuropsychological perspective -- A tutorial review; pp. 507–536. [Google Scholar]
- Salvador R, Peña A, Menon DK, Carpenter TA, Pickard JD, Bullmore ET. Formal characterization and extension of the linearized diffusion tensor model. Human Brain Mapping. 2005;24:144–155. doi: 10.1002/hbm.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15(10):1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]