Abstract
Self-control is typically defined as choosing a greater, delayed reward over a lesser, more immediate reward. However, in nature, there are other costs besides delay associated with obtaining the greatest outcome including increased effort, potential punishment, and low probability of reward. Effort is an interesting case because it sometimes impairs self-control, by acting as an additional cost, and at other times facilitates self-control, by distracting one from impulsive options. Additionally, different species may perform differently in effortful self-control tasks, based on their natural ecology. To gain insight into these aspects of self-control behavior, we examined capuchin monkeys’ and rhesus monkeys’ self-control in separate working and waiting choice tasks. We hypothesized that capuchins would show greater self-control in the working task, given their naturally higher activity level, whereas rhesus would perform similarly in both tasks. Rhesus performed as predicted, whereas contrary to our hypothesis, capuchins exhibited lesser performance in the working task. Nonetheless, these results may still stem from inherent species differences interacting with details of the methodology. Capuchins, being highly energetic and social monkeys, may have divided their energy and attention between the working task and other elements of the test environment such as visible group mates or manipulanda.
Keywords: Self-control, Delay, Effort, Capuchin monkey, Rhesus monkey
1. Introduction
An individual’s immediate interests and long-term goals can sometimes conflict, resulting in a difficult decision. For example, one might have to reconcile cravings for pizza with a plan to stick to a low-carb diet, or one might have to decide between spending a paycheck on the newest smartphone and saving that money over several months to purchase a car. Metcalfe and Mischel (1999) described these conflicts within a hot/cool system framework in which reflexive and impulsive (hot) urges are at odds with cognitive and strategic (cool) plans. One’s level of self-control can be defined by how one behaves in these situations. In the above examples, individuals who give in to the temptation of pizza or the smartphone would be considered impulsive, whereas those who stick to their diet or savings plan would be considered self-controlled.
Because self-control situations often involve these kinds of dichotomous choices, it is not surprising that psychologists often study impulsivity and self-control in the laboratory using choice tasks. The inter-temporal choice (or ITC) task (also called the delay choice, temporal discounting, or self-control task) involves the choice between two reward options – a lesser reward that is available more immediately (“smaller-sooner”) – and a greater reward that is available after a longer delay (“larger-later;” for a review, see Berns et al., 2007). When presented to human participants, this task is often a verbal exercise involving hypothetical rewards (e.g., Green et al., 1994; Rachlin et al., 1991; but see Forzano and Logue, 1992; Logue and King, 1991). For example, a participant may be asked: “If given the choice between receiving 10 dollars now and receiving 50 dollars in one month, which would you choose?”
The ITC task has also been used to assess self-control in nonhuman animals (hereafter, animals; e.g., Ainslie, 1974; Freeman et al., 2009; Tobin and Logue, 1994; Tobin, Logue et al., 1996; van Haaren et al., 1988). Traditionally, in such tests, the choice options are represented by two arbitrary stimuli (e.g., icons, colored lights, etc.) and the participant learns what each stimulus represents by responding to them in the task. There are some exceptions to this tradition, in which animals respond directly to the different food options (e.g., Addessi et al., 2011; Amici et al., 2008; Rosati et al., 2007), but there is debate as to whether this version of the test measures the same psychological phenomenon as the traditional test (Addessi et al., in press; Genty et al., 2012; Paglieri et al., 2013). In either case, experimenters present the test trials repeatedly within a single session, often while adjusting the delay length or reward amount associated with the greater option, in the interest of characterizing how such variables influence the tested species’ self-control (e.g., Mazur, 1987; Rachlin et al., 1991).
While the majority of self-control research within this paradigm involves choices between differentially delayed reward options, there are other costs associated with self-control besides delay, such as increased effort (Mischel, 1974), the possibility of punishment (Mischel and Grusec, 1967), and low probability of rewards (Rachlin, 1989). With regard to effort, consider the example of a professional runner choosing between competing in a 5K and a full (42 km) marathon. The 5K may be easier to train for, but the top prizes for the marathon are greater. There has been some experimental work conducted to understand the influence of response effort (or work) on self-control in the ITC task. For example, pigeons (Columbia livia) were tested in separate tasks in which they chose between two options: 1. pecking a key a small number of times to receive a small reward versus pecking another key a larger number of times to receive a large reward (the working task); and 2. pecking a key once and waiting a short duration for a small reward versus pecking another key once and waiting a long duration for a larger reward (the waiting task; Grossbard and Mazur, 1986). Pigeons’ choices varied similarly with required effort and required delay, both producing indifference curves that were hyperbolic in shape. In other words, delay and effort seemed to produce similar costs for pigeons’ responding in the ITC paradigm. Humans were reported to behave similarly when tested in tasks involving choices between hypothetical reward options associated with varying levels of delay and effort (Sugiwaka and Okouchi, 2004).
In a related set of studies, two species of nonhuman primates, marmosets (Callithrix jacchus) and tamarins (Saguinus oedipus), were each tested on two versions of the ITC task (Stevens et al., 2005a, 2005b). One task version varied the delay associated with the larger, more delayed, reward option (as in standard ITC tests), whereas the other test varied effort by manipulating the distance the monkeys had to travel to obtain the larger delayed option. Interestingly, self-control performance varied with task type (delay versus effort) and monkey species: marmosets exhibited greater delay tolerance than tamarins, but tamarins were willing to travel further than marmosets. The authors attributed this pattern of results to differences in the two species’ feeding ecology (marmosets often feed on slowly exuding tree sap, whereas tamarins range over large distances to find insect prey).
Another common test of self-control that has occasionally incorporated the effort/work component is the delay of gratification (DoG) paradigm. Unlike the ITC task, performance in a DoG task is focused, not on the initial choice of whether or not to delay, but instead on the individual’s behavior during the delay interval (Mischel and Ebbesen, 1970). For example, a subject is provided with a small reward option that can be consumed at any time, but the subject also has the option of continually foregoing that available reward for the length of a delay period in order to receive a greater reward (e.g., Beran et al., 1999). Patterson and Carter (1979) tested children in such a situation in which they had to work (i.e., fulfill a response requirement) to obtain a delayed reward. They also tested children in the typical delay of gratification situation in which children had to wait for the end of the delay period to obtain a reward. In both cases, there was a condition in which the delayed rewards were visible to the children, and a condition in which those rewards were not visible. Patterson and Carter (1979) found no main effect of effort on delay of gratification performance, but they did find an interesting interaction between reward presence and task type: attention paid to the delayed rewards facilitated delay of gratification in the working situation, whereas attention to the rewards in the waiting situation was detrimental to performance. In a related study, a chimpanzee was found to delay gratification for longer when the DoG task required an active work component in order for reward items to accumulate as compared to when the chimpanzee had to wait for the rewards to accumulate with nothing to do (Beran & Evans, 2009). Thus, these studies suggest that effort-based self-control may not always operate in similar fashion to delay-based self-control.
The previous work conducted in this area seems to indicate that the influence of task effort on self-control performance depends largely on intervening variables (e.g., natural history or external cues). To gain a greater understanding of the role that effort plays in self-control behavior, it seems that multiple species will need to be tested in multiple settings. Therefore, to shed further light on this problem, we tested two species of monkeys, capuchin monkeys (Cebus apella) and rhesus monkeys (Macaca mulatta), in working (i.e., effort) and waiting (i.e., delay) versions of a self-control task. Both species had been previously tested in separate delay-based self-control tests (e.g., Evans and Beran, 2007; Evans et al., 2012; Freeman et al., 2009; Freeman et al., 2012; Paglieri et al., 2013; Szalda-Petree et al., 2004), but they had never been directly compared in an effort-based self-control test. We presented both species with computerized versions of the ITC task that used arbitrary digital stimuli to represent the smaller-sooner and larger-later choice options, and these stimuli linked to either delay periods or series of response requirements (sustained joystick movements) resulting in food delivery.
Capuchin monkeys are generally very active monkeys, spending large proportions of time manipulating objects (including tools), foraging destructively for embedded food sources, and moving about (Fragaszy et al., 2004; Ross and Giller, 1988). In contrast, rhesus monkeys tend to forage for nearby, readily-available food sources, and there are few reports of any specialized manual behaviors by these monkeys (Fooden, 2000; Richard et al., 1989). Additionally, as noted above, these two species have shown overlapping levels of performance in self-control tasks involving differentially delayed rewards, such as the accumulation DoG test (Evans and Beran, 2007; Evans et al., 2012a). Therefore, we hypothesized that capuchin monkeys would tolerate greater delay/effort to obtain rewards than rhesus monkeys in the working ITC task (given their higher level of activity and dynamic foraging ecology), and that these two species would show similar delay tolerances to one another in the waiting ITC task (given their similar levels of performance in previous delay-based tests). Following the same logic, we expected capuchin monkeys to perform better in the working condition than they did in the waiting condition (given their more active nature). It also follows that we did not expect the rhesus monkeys to perform any differently in the working task than they performed in the waiting task (since nothing about their behavioral ecology would seem to prepare them more for one task over the other). This performance pattern would lend support to the idea published by Stevens et al. (2005a, 2005b) that differential performance in these kinds of tasks is related to species differences in foraging ecology.
2. Material and Methods
2.1. Participants
We tested nine laboratory-housed capuchin monkeys including five males (Drella: age 22; Gabe: age 14; Griffin: age 15; Liam: age 9; Logan: age 7) and four females (Gambit: age 16; Lily: age 15; Nala: age 10; and Wren: age 10). Capuchin monkeys were group housed but voluntarily shifted into individual 33 x 46 x 61 cm enclosures for testing for a small food reward (e.g., 1 peanut), and they retained visual and auditory access to nearby monkeys that were also separated for testing. We also tested eight laboratory-housed rhesus monkeys, all of which were males (Chewie: age 13; Gale: age 29; Han: age 10; Hank: age 29; Lou: age 19; Luke: age 13; Murph: age 19; and Obi: age 9). Rhesus monkeys were housed individually, but had constant visual and auditory access to other nearby monkeys, in addition to a 24-hour period with access to a compatible social partner once per week. All monkeys had 24-hour access to water and 1–2 enrichment manipulanda and were fed manufactured chow as well as various fruits and vegetables between 1600 and 1800 hours. Most monkeys had participated in at least one prior study of self-control behavior involving a manual or computerized test apparatus (Bramlett et al., 2012; Evans, 2007; Evans and Beran, 2007; Evans et al., 2012; Paglieri et al., 2013).
2.2. Materials
The monkeys were tested using the Language Research Center’s Computerized Test System comprising a personal computer, digital joystick, color monitor, and pellet dispenser (Evans et al., 2008; Richardson et al., 1990). Monkeys manipulated the joystick with their hands to produce isomorphic movements of a small round cursor on the computer screen. Contacting stimuli with the cursor sometimes resulted in the delivery of different amounts of 45-mg (capuchins) or 94-mg (rhesus) banana-flavored chow pellets (Bio-Serv, Frenchtown, NJ) via a pellet dispenser interfaced to the computer through a digitial I/O board (PDISO8A; Keithley Instruments, Cleveland, OH). Different size pellets were used with each species to account for their different daily caloric needs. The task program was written in source code using Visual Basic 6.0. All monkeys had previously participated in multiple psychological experiments involving this computerized test system (e.g., Beran and Smith, 2011; Beran et al., 2008a, 2008b, 2009; Klein et al., 2011).
2.3. Procedure
2.3.1. General Procedure
The experiment consisted of two trial types – Waiting trials and Working trials. In Waiting trials, monkeys choose between a smaller-sooner reward option and a larger-later reward option, as in most traditional self-control or ITC experiments (e.g., Ainslie, 1974; Freeman et al., 2009; Tobin and Logue, 1994; Tobin et al., 1996; van Haaren et al., 1988). In Working trials, monkeys instead chose between smaller-effortless and larger-effortful reward options within the same self-control framework. Monkeys participated in test sessions consisting of only one of these two trial types until reaching a performance criterion (see below for more details). They then began test sessions involving the other trial type. Approximately half (five capuchins and four rhesus) of the members of each species participated in Waiting trials first and the remainder participated in Working trials first.
Monkeys were tested in 2 to 4 hour sessions during which they could participate in as many (or as few) trials as they chose to complete. Sessions were divided into 14-trial blocks separated by 2-minute inter-block intervals. The first two trials in each block were forced-choice trials (as in Addessi et al., 2011; Amici et al., 2008; Rosati et al., 2007; Stevens et al., 2005a) in which the program presented monkeys with only once choice option, the small-reward stimulus or the large-reward stimulus, which were distinguishable by color and pattern (see Figure 1). Also, as in the previous studies, the remaining trials in a block consisted of 10 free-choice trials, in which the program presented both choice options to the monkey (i.e., the small-reward stimulus and the large-reward stimulus) and 2 additional forced-trials, presented in a random order.
Figure 1.
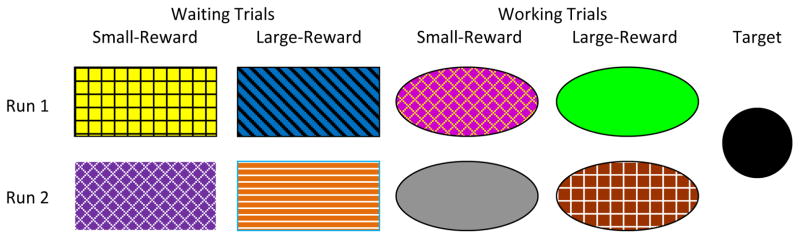
The experimental stimuli arranged according to type and run. In each run, moving the computer cursor to the small-reward stimulus resulted in the immediate delivery of two food pellets, whereas moving the cursor the large-reward stimulus resulted in the delivery of six food pellets following a delay (Waiting) or following a number of joystick responses to a target (Working). These requirements were determined automatically by the computer program according to an adjusting procedure.
In all trials in both the Working and Waiting conditions, contacting the small-reward stimulus with the cursor resulted in the immediate delivery of two food pellets, whereas selecting the large-reward stimulus provided six food pellets after a variable delay or variable number of joystick responses, depending on the task type. Pellets were always dispensed individually at a rate of one pellet per 500 ms. Selecting either stimulus also resulted in the disappearance of the opposing choice stimulus for the remainder of the trial. What happened next depended on the trial type.
In Waiting trials, the selected stimulus and the cursor remained visible until the last food pellet was delivered. The cursor remained affixed to the selected stimulus during the delay period (if applicable) and pellet delivery period, and the monkey could no longer control the movement of the cursor for the remainder of the trial duration. In Working trials, if the monkey selected the small-reward stimulus, the cursor and chosen stimulus remained visible during the brief pellet delivery period (exactly as in Waiting trials). However, if the monkey selected the large-reward stimulus, all stimuli disappeared. The cursor then reappeared in the center of the computer screen and a circular target-stimulus appeared in one of eight positions of a clock-face pattern that surrounding the cursor location (target position was randomly determined by the computer program). The monkey could then move the cursor into contact with the target stimulus, an action that required approximately 5 s (assuming that the monkey moved the cursor in a straight line). After the monkey made contact with this stimulus, additional identical stimuli were presented in new random locations around the clock-face pattern until the current response requirement was fulfilled for the trial. Once the response requirement was fulfilled, the monkey received the delayed reward of six pellets.
2.3.2. Trial Duration
All trials within a session were of a fixed duration, meaning that the period of time between selection of a stimulus in one trial and the presentation of stimuli in the next trial was the same, regardless of which stimulus the monkey chose. This trial duration was determined by the experimenter at the beginning of a session, and it was adjusted if a monkey’s delay/response tolerance approached the trial duration (see below for more details). The use of the fixed trial duration ensured that choosing the smaller-sooner option did not reduce the period of time before the next trial would begin, and thus repeatedly choosing the smaller-sooner option over the course of a multiple block session could not yield a larger total amount of food than consistently choosing the larger-later option (see Genty et al., 2012, for more discussion of the importance of using a fixed trial duration in self-control tests).
2.3.3. Adjusting Procedure
In Waiting trials, the delay associated with the large-reward stimulus was set to 0 seconds at the beginning of the experiment and it was adjusted according to a monkey’s response pattern after each trial-block (as in Addessi et al., 2011; Amici et al., 2008; Rosati et al., 2007; Stevens et al., 2005a). The computer program tallied the number of small-reward and large-reward responses made by monkeys during the 10 free-choice trials of each block. If the monkey made 6 or 7 large-reward responses in a block, then the delay associated with that stimulus was increased by 5 seconds for the next block. If the monkey made 8, 9, or 10 such large-reward responses, then 10 seconds was added to the delay for the next block. The same adjustments were made, but in the opposite direction, when a monkey made 6–7 or 8–10 responses to the small-reward stimulus in a block. If a monkey chose the small-reward and large-reward stimuli in an equal number of trials in a block, then the delay associated with the large reward remained the same for the subsequent block.
The adjusting procedure worked similarly for Working trials. As in Waiting trials, at the beginning of the experiment, the response requirement was set to 0 target responses, and was automatically adjusted according to the number of large- and small-reward stimulus selections a monkey made in a previous block. To match the delays associated with large-reward selection in Waiting trials, the response requirement of Working trials was adjusted by either 1 or 2 targets, which required 5 s and 10 s of additional joystick movement by the monkey in order to obtain the large reward.
The above adjusting procedure continued until the delay or response requirement associated with the large reward became stable across 10 consecutive trial-blocks (as in Addessi et al., 2011; Amici et al., 2008; Rosati et al., 2007; Stevens et al., 2005a). Stability occurred when the mean delay/response requirement of the most recent five blocks differed from the mean of the previous five blocks by less than 10%. Once a monkey met this criterion, we reset the large-reward delay/response requirement to 0 seconds/targets and repeated the experiment with a new set of small-reward and large-reward stimuli within the same trial type (Figure 1). This provided a measure of reliability regarding the delay durations that the monkeys tolerated in both conditions. Once a monkey met the criterion twice with the same type of trials (Waiting trials or Working trials), they began test sessions with the other trial type.
2.4. Analysis
Our data failed to meet some of the assumptions required for parametric statistical tests, so we instead performed nonparametric tests for all analyses. We first conducted a set of analyses to confirm the reliability of the ITC task as a within subjects manipulation. For each species, we conducted a Wilcoxon matched-pairs test (two tailed, α = .05) to compare the number of trial-blocks needed to reach criterion in the two runs of the same trial type. Also, for each species, we conducted a Wilcoxon matched-pairs test (two tailed, α = .05) to compare the mean delay or mean number of targets tolerated by monkeys in the two runs of the same trial type.
Second, we conducted a set of analyses to examine the influence of task type and monkey species on performance level in the self-control task. For these analyses, we used the monkeys’ response time data in the Working task (i.e., the duration for which they moved the cursor into contact with the required number of targets). We performed a Mann Whitney U test for each task type in which we compared the delay tolerance exhibited by each species. We also performed a Wilcoxon matched-pairs test for each monkey species to compare their delay tolerance across task type.
3. Results
Each monkey’s performance in each version of the task is presented in Table 1. In the Waiting task, there was not a significant difference in the number of trial-blocks needed to meet the stability criterion between the two runs for either species (capuchins: Z = .563, n = 9, p = .574; rhesus: Z = 1.192, n = 8, p = .233). Similarly, there was not a significant difference in the mean delay tolerated between runs for either species (capuchins: Z = .652, n = 9, p = .515; rhesus: Z = 1.4, n = 8, p = .161). Overall, collapsing across runs, the capuchin monkeys reached stability criterion in 51.94 trial-blocks (SE = 7.0) and tolerated an average delay of 41.51 seconds (SE = 4.53) to receive the larger reward, and rhesus monkeys reached stability criterion in 37.19 trial-blocks (SE = 5.62) and tolerated an average delay of 62.97 seconds (SE = 12.8) to receive the larger reward.
Table 1.
Monkeys’ performance in the Waiting and Working ITC tasks
| Monkey | Waiting trials | Working trials | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Blocks to stability | Delay tolerance | Blocks to stability | Target tolerance | ||||||
|
| |||||||||
| Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | Run 1 | Run 2 | ||
| Capuchin | Drella | 25 | 25 | 19.5 | 93.0 | 10 | 115 | 3.1 | 3.6 |
| Gabe | 70 | 35 | 58.5 | 32.5 | 90 | 30 | 2.3 | 2.1 | |
| Gambit | 75 | 20 | 49.5 | 37.5 | 105 | 10 | 2.5 | 3 | |
| Griffin | 15 | 30 | 44.0 | 40.5 | 15 | 10 | 2.4 | 2.4 | |
| Liam | 45 | 25 | 26.0 | 34.0 | 110 | 20 | 6.8 | 5.9 | |
| Lily | 100 | 55 | 32.5 | 32.7 | 60 | 30 | 2.5 | 3 | |
| Logan | 75 | 25 | 71.5 | 45.0 | 25 | 45 | 4.8 | 5.4 | |
| Nala | 70 | 70 | 28.0 | 23.0 | 15 | 15 | 5 | 4.2 | |
| Wren | 30 | 110 | 26.0 | 27.5 | 70 | 15 | 4 | 2.9 | |
|
| |||||||||
| Mean | 50.88 | 40.51 | 43.89 | 3.66 | |||||
|
| |||||||||
| Rhesus | Chewie | 20 | 90 | 26 | 33.5 | 25 | 15 | 6.9 | 9 |
| Gale | 30 | 30 | 116.5 | 58.5 | 20 | 45 | 14.9 | 18.9 | |
| Han | 30 | 30 | 73 | 89.5 | 35 | 65 | 6.9 | 9.4 | |
| Hank | 10 | 15 | 5 | 58.5 | 50 | 10 | 19.7 | 9.8 | |
| Lou | 75 | 35 | 128 | 138 | 20 | 25 | 19.1 | 29.7 | |
| Luke | 20 | 40 | 27 | 52 | 50 | 25 | 8.8 | 7.5 | |
| Murph | 20 | 40 | 62 | 65.5 | 20 | 55 | 14 | 15.3 | |
| Obi | 50 | 60 | 39.5 | 40 | 25 | 25 | 5.3 | 5.5 | |
|
| |||||||||
| Mean | 37.19 | 63.28 | 31.88 | 12.54 | |||||
|
| |||||||||
In the Working task, there also was not a significant difference in the number of trial-blocks needed to meet the stability criterion in each run for either species (capuchins: Z = 1.120, n = 8, p = .263; rhesus: Z = .254, n = 8, p = .799). Similarly, there was not a significant difference in the mean response requirement tolerated in each run for either species (capuchins: Z = .563, n = 9, p = .574; rhesus: Z = 1.192, n = 8, p = .233). Overall, collapsing across runs, the capuchin monkeys reached the stability criterion in 43.89 trial-blocks (SE = 6.6) and tolerated an average response requirement of 3.66 targets (SE = .47) to receive the larger reward, and rhesus monkeys reached stability criterion in 31.88 trial-blocks (SE = 3.46) and tolerated an average response requirement of 12.54 targets (SE = 2.23) to receive the larger reward. Because no difference was found in performance between runs of either trial type, we collapsed the data across runs (within trial type) for all remaining analyses.
As noted above, in order to compare monkeys’ performance between the two task types we utilized monkeys’ mean response time data from the Working task (the duration required to perform the required target responses) as opposed to their mean target tolerance. These values are illustrated in Figure 2. Within-species analyses revealed that capuchins monkeys exhibited a higher delay tolerance in the Waiting task as compared to the Working task (Z = 2.547, n = 9, p = .011), whereas rhesus monkeys showed no difference in delay tolerance between the two tasks (Z = 1.40, n = 8, p = .889). Between-species analyses revealed that, in the Waiting task, capuchin monkeys and rhesus monkeys did not differ significantly in performance (Z = 1.155, n = 17, p = .248), whereas in the Working task, capuchin monkeys exhibited lower delay tolerance than rhesus monkeys (Z = 3.464, n = 17, p = .001).
Figure 2.
Monkeys’ ITC performance as a function of species and task type. Delay tolerance values in the Working task were calculated by multiplying monkeys’ target tolerance by 5 s (the duration of sustained joystick movement required to contact each target). Error bars represent 95% confidence intervals for the means. An asterisk represents a statistically significant difference between indicated bars, whereas an “ns” represents the lack of a statistically significant difference between indicated bars.
4. Discussion
Our results were consistent across runs of the titration protocol, indicating that the performance exhibited in these tasks was a result of the monkeys’ tolerance for an average delay period or number of responses, rather than a potential artifact of the experimental design. This indicates that these computerized inter-temporal choice tests are reliable and useful for measuring self-control in nonhuman primates, and perhaps other species, in much the same way that the pioneering studies in this area produced robust and consistent results with other laboratory species in choice and learning experiments (e.g., Ainslie, 1974; Chung and Hernnstein, 1967; Green and Myerson, 2004; Navarick and Fantino, 1976; Szalda-Petree et al., 2004; Tobin et al., 1996; van Haaren et al., 1988). It follows that the general outcome of this study – that these monkeys tolerated delays and work periods of several seconds in order to obtain a larger supply of food pellets – is reliable as well. Moreover, this general outcome is consistent with multiple previous studies involving these and related species illustrating that nonhuman primates are capable of self-control and delay of gratification within certain temporal and reward parameters (e.g., Addessi and Rossi, 2011; Beran, 2002; Dufour et al., 2007; Evans et al., 2012a, 2012b; Freeman et al., 2009; Stevens et al., 2005a, 2005b; Tobin et al., 1996).
Despite the generally successful performance of monkeys in this study, our more specific predictions regarding how monkey species and task type would influence self-control performance within this paradigm were only partially supported. We expected rhesus monkeys to exhibit the same level of self-control in both versions of the task because nothing about their behavioral ecology seemed to prepare them better for one task over the other. Consistent with this hypothesis, rhesus monkeys showed no difference in delay tolerance (or converted effort tolerance) between task types. However, we expected capuchin monkeys to exhibit greater self-control in the Working task in comparison to the Waiting task. What we actually found was the opposite pattern. Nonetheless, there are a few potential explanations for this unexpected outcome related to differences in species-typical behavior. First, the specific design of our Working task may have influenced the capuchin monkeys’ performance differently than expected. This task required monkeys to sustain the movement of a cursor in a single direction for approximately 5 seconds to touch a target item on the screen, and this action had to be repeated multiple times in most cases. We intended this to be an active, working task that we hypothesized would better engage the capuchin monkeys’ inherently energetic behavior. However, given our unexpected results, it may be the case that sustaining a single motor movement for a relatively long duration may have actually impaired capuchin monkeys’ performance because of their active nature. Informal observations of capuchin monkeys in our laboratory give the impression that, while these monkeys are apparently almost always active, they do not seem to sustain attention on one particular activity for very long. Thus, engaging in long sustained joystick deflections may possibly have worked against these monkeys’ nature. It is possible that their energetic demeanor would have been better engaged in a motor task involving quicker responses with more frequent changes in direction, such as using the cursor to chase down multiple moving targets that ‘bounce’ off of the screen edges. Future research is necessary to investigate this possibility.
Another possible explanation for the performance pattern exhibited by the capuchin monkeys is the social nature of this species combined with the fact that they are tested in close visual proximity to other working individuals of their social group. Members of this species form strong social bonds with various group members (Fragaszy et al., 2004), and having close visual access to these group-mates during testing may have had differential effects on the two tasks. They may have engaged in more social interaction (via facial expressions and vocalizations), when they did not have to continually respond with a joystick to earn delayed rewards, and this may have facilitated longer delays in the Waiting condition. Conversely, engaging in this kind of social activity during the Working task would have been difficult to do without causing trials to be timed-out as a result (trials were of a fixed duration, and if they were not completed within the allowable time, they were cancelled without the delivery of reward). Although rhesus monkeys were also tested with visual access to conspecifics, the same social effects on task performance would not be expected for two reasons: rhesus monkeys are generally a less socially-tolerant species than capuchin monkeys (Matsumura, 1999); and our rhesus monkeys are primarily singly-housed with occasional access to a social partner that is (in most cases) not one of the conspecifics that are in view during testing. Although we did not record behavioral data, we were able to perform an exploratory analysis of uncompleted trials in the Working task, which showed that capuchin monkeys left 0 – 1% of trials unfinished and rhesus monkeys left 0 – 5% of trials unfinished. Further, if capuchin monkeys were more distracted by nearby conspecifics in the Working task, it was not evident in their response time data, as capuchin monkeys and rhesus monkeys required similar amounts of time to contact each required target (mean capuchin monkeys: 5.50 s; mean rhesus monkeys: 5.07 s). Therefore, this alternative explanation does not seem to be supported by these data. However, additional testing with both species in different social environments will be necessary to confirm or deny this possible explanation.
In addition to analyzing monkeys’ performance between conditions of the present study, it is also interesting to compare the performance of these species to other groups of monkeys previously tested in ITC tasks. In particular, our estimate of the typical duration at which capuchin monkeys are indifferent between a smaller, sooner reward and a larger, later reward using a computerized ITC task (approximately 42 seconds) fell between two rather discrepant estimates from previous studies that made use of real food items as choice stimuli (albeit somewhat closer to one estimate than the other; Amici et al., 2008: 22 seconds; Addessi et al., 2011: 81 seconds). The difference in performance between these capuchin ITC studies may parallel methodological differences between the three studies, such as the use of different food amounts or different trial timing algorithms, providing different numbers of trials per session (see Addessi et al., 2011 for more discussion on these two points), and/or the way in which the food amounts were represented in the tasks (visible, and thus prepotent, food amounts vs. representative stimulus). The latter explanation has been specifically investigated in a recent study involving another nonhuman primate species (Genty, et al., 2012). Those authors directly compared monkeys’ performance in the ITC task as a function of whether foods were visible or were concealed by different colored containers at choice time, and they found that monkeys were significantly more likely to respond to the larger-later option when it consisted of visible food rewards, therefore inflating their actual delay tolerance level. These results suggested that the monkeys were pointing to the bigger amount of food because it was bigger, and thus more prepotent, and not because it was a delayed but better outcome (for more discussion of this point, see Addessi et al., in press; Paglieri et al., 2013).
Animal models of choice are valuable not only for understanding what evolutionary pressures or environmental contexts impact how individuals and species choose between options, but also for establishing the similarities or differences across species in areas of research such as self-control behavior. Capuchin monkeys and rhesus monkeys will likely play a valuable role in understanding the evolutionary foundations of primate (including human) self-control behavior, and the impulsivity that precludes such self-control. The ITC task plays a critical role in this research agenda, as do other tests such as the accumulation task (e.g., Evans et al., 2012), the reverse-reward contingency task (in which one must choose the lesser of two options in order to obtain the greater option, e.g., Anderson, Hattori, & Fujita, 2008), and other tasks of self-control (e.g., Bramlett et al., 2012; Evans and Westergaard, 2006). We look forward to the time when performance can be examined within the same individuals, but across multiple self-control tasks, as this approach will be especially beneficial in allowing for a fuller understanding of these species’ capacity for self-control to emerge.
Highlights.
Monkeys perform reliably in computerized inter-temporal choice self-control tests
Rhesus monkeys perform equally in self-control tests involving effort and delay
Capuchin monkeys exhibit more self-control in delay-based than effort-based tests
Performance differences between tests may relate to species’ natural history
Acknowledgments
The research was supported by National Institute of Child Health and Development (grant HD-060563). Bonnie Perdue was supported, in part, by the Duane M. Rumbaugh Fellowship from Georgia State University, and Audrey Parrish was supported, in part, by the 2CI University Doctoral Fellowship from Georgia State University. The authors thank Jessica Bramlett and Daniel Rice for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addessi E, Paglieri F, Beran MJ, Evans TA, Macchitella L, De Petrillo F, Focaroli V. Delay choice versus delay maintenance: Different measures of delayed gratification in capuchin monkeys (Cebus apella) Journal of Comparative Psychology. doi: 10.1037/a0031869. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Addessi E, Rossi S. Tokens improve capuchin performance in the reverse–reward contingency task. Proceedings of the Royal Society B: Biological Sciences. 2011;278:849–854. doi: 10.1098/rspb.2010.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Impulse control in pigeons. Journal of the Experimental Analysis of Behavior. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Hattori Y, Fujita K. Quality before quantity: Rapid learning of reverse-reward contingency by capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2008;122:445–448. doi: 10.1037/a0012624. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) Journal of General Psychology. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Delay of gratification by chimpanzees (Pan troglodytes) in working and waiting situations. Behavioural Processes. 2009;80:177–181. doi: 10.1016/j.beproc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Harris EH, Evans TA, Klein ED, Chan B, Flemming TM, Washburn DA. Ordinal Judgments of Symbolic Stimuli by Capuchin Monkeys (Cebus apella) and Rhesus Monkeys (Macaca mulatta): The Effects of Differential and Nondifferential Reward. Journal of Comparative Psychology. 2008a;122:52–61. doi: 10.1037/0735-7036.122.1.52. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Klein ED, Evans TA, Chan B, Flemming TM, Harris EH, Washburn DA. Discrimination reversal learning in capuchin monkeys (Cebus apella) The Psychological Record. 2008b;58:3–14. [Google Scholar]
- Beran MJ, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees (Pan troglodytes) Developmental Psychobiology. 1999;34:119–127. doi: 10.1002/(sici)1098-2302(199903)34:2<119::aid-dev5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Smith JD. Information seeking by rhesus monkeys (Macaca mulatta) and capuchin monkeys (Cebus apella) Cognition. 2011;120:90–105. doi: 10.1016/j.cognition.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Smith JD, Coutinho MVC, Couchman JJ, Boomer J. The psychological organization of “uncertainty” responses and “middle” responses: A dissociation in capuchin monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35:371–381. doi: 10.1037/a0014626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Laibson D, Loewenstein G. Intertemporal choice – toward an integrative framework. Trends in Cognitive Sciences. 2007;11:482–488. doi: 10.1016/j.tics.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Bramlett J, Perdue B, Evans T, Beran M. Capuchin monkeys (Cebus apella) let lesser rewards pass them by to get better rewards. Animal Cognition. 2012;15:963–969. doi: 10.1007/s10071-012-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Herrnstein RJ. Choice and delay of reinforcement. Journal of the Experimental Analysis of Behavior. 1967;10:67–74. doi: 10.1901/jeab.1967.10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour V, Pelé M, Sterck EHM, Thierry B. Chimpanzee (Pan troglodytes) anticipation of food return: Coping with waiting time in an exchange task. Journal of Comparative Psychology. 2007;121:145–155. doi: 10.1037/0735-7036.121.2.145. [DOI] [PubMed] [Google Scholar]
- Evans TA. Performance in a computerized self-control task by rhesus macaques (Macaca mulatta): The combined influence of effort and delay. Learning and Motivation. 2007;38:342–357. doi: 10.1016/j.lmot.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Delay of gratification and delay maintenance in rhesus macaques (Macaca mulatta) Journal of General Psychology. 2007;134:199–216. doi: 10.3200/GENP.134.2.199-216. [DOI] [PubMed] [Google Scholar]
- Evans TA, Westergaard GC. Self-control and tool-use in tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 2006;120:163–166. doi: 10.1037/0735-7036.120.2.163. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Chan B, Klein ED, Menzel CR. An efficient computerized testing method for the capuchin monkey (Cebus apella): Adaptation of the LRC-CTS to a socially housed nonhuman primate species. Behavior Research Methods, Instruments, & Computers. 2008;40:590–596. doi: 10.3758/brm.40.2.590. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Paglieri F, Addessi E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): when quantity is salient, symbolic stimuli do not improve performance. Animal Cognition. 2012a;15:539–548. doi: 10.1007/s10071-012-0482-1. [DOI] [PubMed] [Google Scholar]
- Evans TA, Perdue BM, Parrish AE, Menzel EC, Brosnan SF, Beran MJ. How Is Chimpanzee Self-Control Influenced by Social Setting? Scientifica. 2012b;2012:9. doi: 10.6064/2012/654094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooden J. Systematic review of the rhesus macaque. Macaca mulatta. Fieldiana Zoology. 2000;96:1–180. [Google Scholar]
- Forzano LB, Logue AW. Predictors of adult humans’ self-control and impulsiveness for food reinforcers. Appetite. 1992;19:33–47. doi: 10.1016/0195-6663(92)90234-w. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The complete capuchin. Cambridge University Press; Cambridge: 2004. p. 339. [Google Scholar]
- Freeman K, Nonnemacher J, Green L, Myerson J, Woolverton W. Delay discounting in rhesus monkeys: Equivalent discounting of more and less preferred sucrose concentrations. Learning & Behavior. 2012;40:54–60. doi: 10.3758/s13420-011-0045-3. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behavioural Processes. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty E, Karpel H, Silberberg A. Time preferences in long-tailed macaques (Macaca fascicularis) and humans (Homo sapiens) Animal Cognition. 2012:15. doi: 10.1007/s10071-012-0540-8. [DOI] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of Delayed Rewards: A Life-Span Comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossbard CL, Mazur JE. A comparison of delays and ratio requirements in self-control choice. Journal of the Experimental Analysis of Behavior. 1986;45:305–315. doi: 10.1901/jeab.1986.45-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ED, Evans TA, Beran MJ. An investigation of prospective and retrospective coding in capuchin monkeys and rhesus monkeys. Zeitschrift für Psychologie/Journal of Psychology. 2011;219:85–91. [Google Scholar]
- Logue AW, King GR. Self-control and impulsiveness in adult humans when food is the reinforcer. Appetite. 1991;17:105–120. doi: 10.1016/0195-6663(91)90066-2. [DOI] [PubMed] [Google Scholar]
- Matsumura S. The evolution of “egalitarian” and “despotic” social systems among macaques. Primates. 1999;40:23–31. doi: 10.1007/BF02557699. [DOI] [PubMed] [Google Scholar]
- Mazur JE, Commons ML, Mazur JE, Nevin JA, Rachlin H. An adjusting procedure for studying delayed reinforcement, The effect of delay and of intervening events on reinforcement value. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1987. [Google Scholar]
- Metcalfe J, Mischel W. A hot/cool system analysis of delay of gratification: Dynamics of willpower. Psychological Review. 1999;106:3–19. doi: 10.1037/0033-295x.106.1.3. [DOI] [PubMed] [Google Scholar]
- Mischel W. Processes in delay of gratification. Advances in Experimental Social Psychology. 1974;7:249–292. [Google Scholar]
- Mischel W, Ebbesen EB. Attention in delay of gratification. Journal of Personality and Social Psychology. 1970;16:329–337. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Mischel W, Grusec J. Waiting for rewards and punishments: Effects of time and probability on choice. Journal of Personality and Social Psychology. 1967;5:24–31. doi: 10.1037/h0024180. [DOI] [PubMed] [Google Scholar]
- Navarick DJ, Fantino E. Self-control and general models of choice. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:75–87. [Google Scholar]
- Paglieri F, Focaroli V, Bramlett J, Tierno V, McIntyre JM, Addessi E, Evans TA, Beran MJ. The hybrid delay task: Can capuchin monkeys (Cebus apella) sustain a delay after an initial choice to do so? Behavioural Processes. 2013;94:45–54. doi: 10.1016/j.beproc.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CJ, Carter DB. Attentional Determinants of Children’s Self-Control in Waiting and Working Situations. Child Development. 1979;50:272–275. [Google Scholar]
- Rachlin H. Judgment, decision, and choice: A cognitive/behavioral synthesis. WH Freeman; New York: 1989. [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AF, Goldstein SJ, Dewar RE. Weed macaques: the evolutionary implications of macaque feeding ecology. International Journal of Primatology. 1989;10:569–594. [Google Scholar]
- Richardson WK, Washburn DA, Hopkins WD, Savage-Rumbaugh SE, Rumbaugh DM. The NASA/LRC computerized test system. Behavior Research Methods, Instruments, & Computers. 1990;22:127–131. doi: 10.3758/bf03203132. [DOI] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, Hauser MD. The Evolutionary Origins of Human Patience: Temporal Preferences in Chimpanzees, Bonobos, and Human Adults. Current Biology. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Ross R, Giller P. Observations on the activity patterns and social interactions of a captive group of blackcapped or brown capuchin monkeys (Cebus apella) Primates. 1988;29:307–317. [Google Scholar]
- Stevens JR, Hallinan EV, Hauser MD. The ecology and evolution of patience in two New World monkeys. Biology Letters. 2005a;1:223–226. doi: 10.1098/rsbl.2004.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Rosati AG, Ross KR, Hauser MD. Will travel for food: Spatial discounting in two New World monkeys. Current Biology. 2005b;15:1855–1860. doi: 10.1016/j.cub.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiwaka H, Okouchi H. Reformative self-control and discounting of reward value by delay or effort. Japanese Psychological Research. 2004;46:1–9. [Google Scholar]
- Szalda-Petree AD, Craft BB, Martin LM, Deditius-Island HK. Self-control in rhesus macaques (Macaca mulatta): Controlling for differential stimulus exposure. Perceptual and Motor Skills. 2004;98:141–146. doi: 10.2466/pms.98.1.141-146. [DOI] [PubMed] [Google Scholar]
- Tobin H, Logue AW. Self-control across species (Columbia livia, Homo sapiens, and Rattus norvegicus) Journal of Comparative Psychology. 1994;108:126–133. doi: 10.1037/0735-7036.108.2.126. [DOI] [PubMed] [Google Scholar]
- Tobin H, Logue AW, Chelonis JJ, Ackerman KT, May JGI. Self-control in the monkey Macaca Fascicularis. Animal Learning & Behavior. 1996;24:168–174. [Google Scholar]
- van Haaren F, van Hest A, van de Poll NE. Self-control in male and female rats. Journal of the Experimental Analysis of Behavior. 1988;49:201–211. doi: 10.1901/jeab.1988.49-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlamings PHJM, Uher J, Call J. How the great apes (Pan troglodytes, Pongo pygmaeus, Pan paniscus, and Gorilla gorilla) perform on the reversed contingency task: The effects of food quantity and food visibility. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:60–70. doi: 10.1037/0097-7403.32.1.60. [DOI] [PubMed] [Google Scholar]



