Abstract
The hippocampus, which is critical for memory and spatial navigation, contains a proliferating stem cell niche that is especially vulnerable to anti-neoplastic drugs such as cisplatin. Although the damaging effects of cisplatin have recently been recognized, the molecular mechanisms underlying its toxic effects on this vital region are largely unknown. Using a focused apoptosis gene array, we analyzed the early cisplatin-induced changes in gene expression in the hippocampus of adult Sprague-Dawley rats and compared the results to those from the inferior colliculus, a non-mitotic auditory region resistant to cisplatin-induced cell death. Two days after a 12 mg/kg dose of cisplatin, significant increases were observed in five proapoptotic genes Bik, Bid, Bok, Trp53p2 and Card6 and a significant decrease in one antiapoptotic gene Bcl2a1. In contrast, Nol3, an antiapoptotic gene showed a significant increase in expression. The cisplatin-induced increase in Bid mRNA and decrease in Bcl2a1 mRNA was accompanied by a corresponding increase and decrease of their respective proteins in the hippocampus. In contrast, the cisplatin-induced changes in Bcl2a1, Bid, Bik and Bok gene expression in the inferior colliculus were strikingly different from those in the hippocampus consistent with the greater susceptibility of the hippocampus to cisplatin toxicity. Cisplatin also significantly reduced immunolabeling of the cell proliferation marker Ki67 in the subgranular zone (SGZ) of the hippocampus two days post treatment. These results indicate that cisplatin-induced hippocampal cell death is mediated by increased expression of proapoptotic and antiapoptotic genes and proteins that likely inhibit hippocampal cell proliferation.
Keywords: Cisplatin, Apoptosis, Hippocampus, Bid, Bcl2a1
INTRODUCTION
Cancer patients undergoing chemotherapy often develop serious neurological and cognitive side effects, a condition sometimes referred as “chemo brain” (Weiss, 2008). Cisplatin, carboplatin and oxaliplatin, which are widely used to treat a variety of solid and disseminated neoplasms (Abe et al., 2009, Harter et al., 2011) are known to have serious side effects, but their effects on the brain, especially to vulnerable regions such as the hippocampus, are poorly understood (Amptoulach and Tsavaris, 2011, Kannarkat et al., 2007, Whitney et al., 2008). While platinum based chemotherapeutic drugs have dramatically increased the number of cancer survivors, it has also increased the frequency of neurological impairments. In patients that have undergone chemotherapy, estimates of mild cognitive impairment range from 10 to 40% (Matsuda et al., 2005, Soussain et al., 2009). Common neurological complaints of chemotherapy include acute encephalopathy, lukoencephalopathy, vasculopathy, stroke, headache, seizures and neuropathy (Fuse-Nagase et al., 1997, Highley et al., 1992, Soussain et al., 2009, Verstappen et al., 2003). Chemotherapy-induced cognitive dysfunction or “chemobrain” is generally manifested as anxiety, fatigue, pain, inability to concentrate, depression and memory decline (Hurria et al., 2006, Weiss, 2008). Cisplatin, widely used to treat ovarian (Morgan et al., 2012, Yang and Lee, 2012), cervical (Sehouli et al., 2012, Xu et al., 2012) and other solid tumors, is known to cause chemobrain (Whitney et al., 2008). Cisplatin exerts its therapeutic effects on cancers cells by binding with DNA hampering DNA replication and arresting cell division in cancer cells (Ortin et al., 2009); however, cell proliferation normally dividing adult stem cells in skin, bone marrow, muscle and adipose is also suppressed by cisplatin.
Stem cells are also present in two distinct niches in the adult mammalian brain, the subventricular zone and the subgranular zone (SGZ) of the hippocampus (Jin et al., 2003, Navarro-Quiroga et al., 2006, Tanaka et al., 2007, Valente et al., 2009). The significance of the hippocampal proliferative zone is that this region plays an important role in spatial navigation and the formation of new memories related to recently experienced events (Appleby et al., 2011, Kyd and Bilkey, 2005, Moita et al., 2003, Scoville and Milner, 2000). Roughly 250,000 new cells are born in the hippocampus each month (Cameron and McKay, 2001). Many of these newborn cells differentiate into neurons, integrate into the neural circuitry of the hippocampus and enhance learning and memory (van Praag et al., 1999, van Praag et al., 2002). The hippocampus is especially vulnerable to brain trauma, heavy metal toxicity, inflammation and Alzheimer’s disease. Damage to the hippocampus is associated with memory loss, cognitive impairment, disorientation and mood disorders (Bekinschtein et al., 2007, Blank et al., 2002, Bolouri and Small, 2004, Dawe et al., 2011, Ekdahl et al., 2003, Femenia et al., 2012, Ishida et al., 1997, Ishikawa et al., 1997, Kesner and Williams, 1995, Kraus et al., 2010, Luft et al., 2008, Monje et al., 2003, Niedermeyer and Ghigo, 2011, Pietropaolo et al., 2007, Prestia et al., 2011, Vanderwolf, 2001). Stem cells in the hippocampus are especially vulnerable to the toxic effects of cisplatin such that treatments results in a significant depression in cell proliferation and neurogenesis (Dietrich et al., 2006, James et al., 2008, Piccolini et al., 2012, Rzeski et al., 2004). However, the biological mechanisms underlying cisplatin-induced hippocampal neurotoxicity and cell cycle arrest are largely unknown. Therefore, we used focused gene arrays and immunolabeling to investigate the mechanisms of cisplatin-induced cell cycle arrest and cell death in the hippocampus two days following cisplatin treatment, a period during which cell death is increasing and cell proliferation is decreasing in the hippocampus. For comparison, tissue was also evaluated from the inferior colliculus, a non-proliferative zone in the auditory pathway.
MATERIALS AND METHODS
Animals
Twenty nine male SASCO Sprague-Dawley rats from Charles River Laboratories (Wilmington, MA) were used for this study. The mean weights of the animals were 325 ± 50 gm. Nine rats were used for the qRT-PCR array studies, eight were used for the confirmatory q-RT-PCR studies, six were used for the Western blot studies and six were used for immunohistochemistry studies. The experimental protocol was reviewed and approved by University at Buffalo Institutional Animal Care and Use Committee.
Cisplatin Treatment
Cisplatin (cis-diamminedichloro-platinum (ll) (Sigma-Aldrich) was dissolved in saline at 1 mg/ml concentration. Experimental rats received a single dose of 12 mg/kg cisplatin which was slowly administered by intraperitoneal injection using a perfusion pump at the rate of 150 µl per minute as described in detail in our earlier publication dealing cisplatin ototoxicity and hearing loss (Jamesdaniel et al., 2008). Control rats received an intraperitoneal injection of sterile saline following the same procedures. Cisplatin and saline injections were administered to the rats under isoflurane anesthesia (4% induction, 1.5% maintenance). Afterwards, rats were twice daily subcutaneously injected with 20 ml/kg of normal saline to minimize cisplatin-induced kidney damage. The weight loss ranged from 5–8% in the cisplatin treated rats. The cisplatin treated rats were slightly less active after the treatment than the control rats; however, none of the rats died from the treatments. At two days post treatment, the animals were euthanized by CO2 and decapitated for tissue collection.
Total RNA Isolation and cDNA Construction
The hippocampus and inferior colliculus were dissected out in an RNase free environment. Since, inferior colliculus is a non-proliferating region; it was used as a negative control. Total RNA was isolated using a RNeasy lipid tissue extraction kit (Qiagen), following the manufacturers protocol. Tissue samples were homogenized in a RNA free 1.7 ml tube containing QIAzol lysis reagent and centrifuged at 12000 g at 4 °C for 15 minutes. The aqueous phase of the sample was transferred to a new tube. An equal volume of 70% ethanol was added and mixed with the sample and transferred to the RNeasy column. The column was incubated with DNase I for 15 minutes at room temperature to avoid genomic DNA contamination and afterwards total RNA was eluted using 30 µl RNase-free water. Total RNA concentration and purity were measured using a spectrophotometer (Beckman Coulter DU 640 or Thermo scientific, NanoDrop 2000). Equal concentrations of total RNA (1 µg) were used to construct cDNA using a RT2 first strand cDNA kit (Cat #: C-03, SA Bioscience Corporation). SuperArray RT qPCR Master Mix (SA Bioscience Corporation) was used for the PCR reaction.
Apoptosis Gene Expression Changes
The Apoptosis RT2 Profiler™ PCR Array – PARN-012A (SuperArray Bio-sciences Corp., MD, USA) was used to investigate the cisplatin-induced changes in apoptotic gene expression in five control and four cisplatin-treated rat hippocampus. Because the hippocampus undergoes apoptosis following cisplatin treatment whereas the inferior colliculus does not, we also used the gene arrays in five control rat inferior colliculus to compare the relative abundance of apoptosis and anti-apoptosis genes in hippocampus. Both brain regions were isolated from same animal for this comparison study. The RT2 Profiler™ PCR array contained 84 apoptotic genes (table II) involved in the intrinsic and extrinsic apoptotic pathway, the calcium-induced cell death pathway and p53 signaling pathway. In addition, the array has primers for 5 housekeeping genes (Hprt, Ldha, Rpl13a, Rplp1 and Actb), a genomic DNA primer, 3 reverse transcription controls and 3 positive PCR controls to facilitate normalization, detect genomic DNA contamination and test the efficiency of cDNA conversion as well as PCR reaction. The PCR reaction was run with a two-step cycling program using Bio-Rad MyiQ Single Color Real Time PCR System The PCR program consisted of one cycle of a hot start (95 °C) for 10 minutes to activate the DNA polymerase and 40 cycles of amplification (95 °C for 15 s, 60 °C for 1 min). Cycle threshold (CT) values were measured for each gene on the array.
Table II.
Apoptosis related genes fold differences (ΔΔCt) relative to control hippocampus at 2 days post cisplatin-treated hippocampus (column 3). Control inferior colliculus (column 4) and hippocampus (column 5) apoptosis related genes mRNA expression (ΔCt) relative to average of five housekeeping (Hprt, Ldha, Rpl13a, Rplp1 and Actb) genes mRNA expression.
| S.No | Gene Symbol | Fold different in hippocampus at 2 days post cisplatin treatment (ΔΔCt) |
Inferior Colliculus (ΔCt) |
Hippocampus (ΔCt) |
|---|---|---|---|---|
| 1 | Apaf1 | 1.06 | 6.39 | 6.67 |
| 2 | Api5 | 1.03 | 1.83 | 2.55 |
| 3 | Aven | 1.16 | 4.74 | 6.26 |
| 4 | Bad | 1.52 | 3.05 | 4.63 |
| 5 | Bag1 | 1.00 | 1.79 | 2.99 |
| 6 | Bak1 | 1.26 | 6.22 | 7.31 |
| 7 | Bax | 1.25 | 3.67 | 4.85 |
| 8 | Bcl10 | −1.41 | 4.27 | 4.81 |
| 9 | Bcl2 | −1.10 | 5.96 | 6.17 |
| 10 | Bcl2a1d | −1.70 | 8.44 | 8.38 |
| 11 | Bcl2l1 | 1.40 | 2.87 | 4.23 |
| 12 | Bcl2l11 | −1.49 | 13.36 | 13.13 |
| 13 | Bcl2l2 | 1.49 | 3.87 | 5.59 |
| 14 | Bclaf1 | 1.13 | 2.76 | 3.24 |
| 15 | Bid | 1.56 | 6.22 | 7.52 |
| 16 | Bik | 1.67 | 11.09 | 13.19 |
| 17 | Birc3 | 1.50 | 10.12 | 12.16 |
| 18 | Birc5 | −1.20 | 7.24 | 7.15 |
| 19 | Bnip1 | 1.21 | 4.66 | 5.33 |
| 20 | Bnip2 | −1.01 | 5.54 | 5.98 |
| 21 | Bnip3 | 1.14 | 0.56 | 1.54 |
| 22 | Bok | 1.81 | 3.37 | 4.18 |
| 23 | Card10 | 1.98 | 8.62 | 10.67 |
| 24 | Card6 | 1.70 | 7.66 | 9.54 |
| 25 | Casp1 | −1.07 | 5.70 | 7.67 |
| 26 | Casp12 | −1.22 | 8.50 | 9.61 |
| 27 | Casp14 | 1.01 | 13.50 | 13.85 |
| 28 | Casp2 | 1.30 | 6.82 | 7.94 |
| 29 | Casp3 | −1.01 | 4.13 | 5.02 |
| 30 | Casp4 | −1.19 | 5.37 | 6.33 |
| 31 | Casp6 | −1.02 | 5.50 | 7.44 |
| 32 | Casp7 | 1.11 | 8.43 | 9.20 |
| 33 | Casp8 | −1.29 | 10.26 | 10.08 |
| 34 | Casp8ap2 | 1.30 | 4.52 | 6.79 |
| 35 | Casp9 | 1.39 | 5.63 | 5.88 |
| 36 | Cd40 | −1.11 | 8.99 | 9.81 |
| 37 | Cd40lg | 1.09 | 13.50 | 13.83 |
| 38 | Cflar | 1.14 | 3.49 | 4.45 |
| 39 | Cidea | 1.07 | 7.42 | 8.26 |
| 40 | Cideb | 1.68 | 10.82 | 12.06 |
| 41 | Cradd | 1.10 | 5.67 | 6.40 |
| 42 | Dad1 | 1.08 | 1.46 | 2.01 |
| 43 | Dapk1 | 2.00 | 6.54 | 6.51 |
| 44 | Dffa | 1.52 | 6.43 | 8.14 |
| 45 | Dffb | 1.07 | 12.49 | 11.95 |
| 46 | Fadd | 1.80 | 6.47 | 7.61 |
| 47 | Faim | −1.12 | 3.90 | 4.63 |
| 48 | Fas | −1.09 | 10.58 | 11.59 |
| 49 | Faslg | −1.38 | 7.51 | 8.91 |
| 50 | Gadd45a | −1.25 | 4.87 | 5.85 |
| 51 | Hrk | 1.49 | 6.85 | 6.83 |
| 52 | Il10 | −1.12 | 11.32 | 13.52 |
| 53 | Lhx4 | 4.00 | 9.68 | 10.93 |
| 54 | Lta | 1.82 | 13.23 | 13.79 |
| 55 | Ltbr | 2.77 | 6.24 | 8.75 |
| 56 | Mapk8ip1 | 1.36 | 0.43 | 1.58 |
| 57 | Mcl1 | 1.32 | 3.33 | 3.83 |
| 58 | Naip2 | 1.29 | 10.96 | 11.44 |
| 59 | Nfkb1 | 1.58 | 5.23 | 7.17 |
| 60 | Nol3 | 1.47 | 4.23 | 6.36 |
| 61 | Polb | 1.49 | 4.34 | 6.34 |
| 62 | Prdx2 | 1.12 | −0.62 | 0.52 |
| 63 | Prlr | 4.02 | 12.58 | 11.01 |
| 64 | Prok2 | 1.05 | 10.03 | 9.14 |
| 65 | Pycard | −1.30 | 5.15 | 5.58 |
| 66 | Ripk2 | 1.04 | 3.98 | 5.04 |
| 67 | Sphk2 | 1.11 | 3.96 | 4.51 |
| 68 | Tnf | −1.37 | 9.33 | 9.89 |
| 69 | Tnfrsf10b | 1.41 | 10.23 | 12.38 |
| 70 | Tnfrsf11b | 1.04 | 4.77 | 6.85 |
| 71 | Tnfrsf1a | 1.55 | 6.63 | 8.34 |
| 72 | Tnfrsf1b | 1.46 | 9.13 | 10.39 |
| 73 | Tnfsf10 | −1.30 | 7.58 | 9.29 |
| 74 | Tnfsf12 | 1.24 | 6.35 | 7.91 |
| 75 | Tp53 | 1.02 | 6.85 | 7.17 |
| 76 | Tp53bp2 | 1.59 | 4.42 | 4.55 |
| 77 | Tp73 | 1.63 | 10.79 | 10.07 |
| 78 | Tp73l | −1.21 | 13.50 | 13.56 |
| 79 | Tradd | 1.20 | 5.49 | 7.70 |
| 80 | Traf1 | 1.22 | 9.66 | 10.36 |
| 81 | Traf2 | 1.53 | 5.92 | 7.30 |
| 82 | Traf3 | 1.29 | 4.50 | 5.98 |
| 83 | Traf4 | 1.38 | 5.48 | 6.16 |
| 84 | Xiap | 1.09 | 2.99 | 4.12 |
The initial results of the apoptosis gene array analysis form the hippocampus revealed large changes in the expression of four Bcl2 genes, Bcl2a1, Bid, Bik and Bok. Based on the initial hippocampal results, we carried out additional qRT-PCR experiments to determine if these four Bcl2 genes showed similar changes in expression in the inferior colliculus and hippocampus two days post-cisplatin. To accomplish this, we designed primers for these four Bcl2 genes plus beta actin, a housekeeping gene (Invitrogen); the forward and reverse primers for these five genes are shown in table 1. Four cisplatin-treated rats (two days post cisplatin) and four control rats were used from this experiment. Isolation of total RNA from the hippocampus and cDNA construction is described above. The PCR reaction was run with same program which had been used to run apoptosis RT2 Profiler™ PCR Array using SYBR Green fluorescence (SABiosciences) technology with 25 µl per reaction in Bio-Rad MyiQ Single Color Real Time PCR System.
Table 1.
Gene symbol, accession number and primer sequences for genes selected for validation by RT-PCR
| Symbol | Accession No. | Forward | Reverse |
|---|---|---|---|
| Bok | NM_017312 | ACGGACGTCCTCAAGTGTGTG | TCTCTCTGGCAACAGGAGGAAGA |
| BiK | NM_053704 | AGGCGAGACTAATGGCCAGAGA | CCAGGCACCTCATGAAATCCAAG |
| Bid | NM_022684 | AGGTCAGCAATGGCTCAGGC | CTGCAGCTCGTCTTCACGGT |
| Bcl2a1 | NM_133416 | ATGGAGGCTGGGAAGATGGC | TCTCAAGGGAGCCAGGGTTCT |
| Beta Actin | NM_031144 | AGCCATGTACGTAGCCATCC | ACCCTCATAGATGGGCACAG |
Data Analysis
The CT values of five housekeeping genes (Hprt, Ldha, Rpl13a, Rplp1 and Actb) were averaged and used to normalize the CT values of apoptotic genes. The statistical analysis of cisplatin-induced changes in mRNA expression levels, in the hippocampus, were calculated using SA bioscience online data analysis resource (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). The software automatically performs all ΔΔCt based fold-change calculations from uploaded raw threshold cycle data. The fold changes with p-value less than 0.05 were considered statistically significant. In addition, the expression levels of the 84 apoptotic genes relative to the housekeeping genes were compared in hippocampus and inferior colliculus. The relative abundance of the 84 apoptosis genes in the hippocampus and inferior colliculus were compared using linear regression analysis (GraphPad Prism software, version 5.01).
Western Blot
Expression of Bcl2a1 and Bid proteins were evaluated in the hippocampus of normal control rats (n=3) and cisplatin-treated rats (n=3; 12 mg/kg cisplatin, 2 days post-cisplatin). The hippocampi from control and cisplatin treated rats were dissected out and homogenized in RIPA buffer (Thermo scientific). The homogenates were centrifuged at 12000 rpm for 20 minutes and total protein concentration of the supernatant was measured using the Bradford method. The extracted protein samples (10 µg) were separated on 4–12% gradient NuPage gels (Invitrogen, Carlsbad, CA), transferred to polyvinylidene difluoride membrane (Invitrogen, Carlsbad, CA), membrane and blocked with 5% dry non-fat milk for 1 hr. After blocking, the membrane was incubated with mouse anti-Bid (Chemicon, 1:5000, overnight) or rabbit anit-Bcl2a1 (Santa Cruz Biotechnology, Inc.; 1:500) followed by two hours incubation with appropriate hydrogen peroxidase conjugated secondary antibody (Pierce Chemical Co., Rockford, IL). Protein bands were visualized using the chemiluminescence detection method (Pierce Chemical Co., Rockford, IL) and samples evaluated using a Fuji model LAS 1000 imaging system (Stamford, CT). Afterwards, the membrane was stripped and re-probed with an antibody against actin to facilitate normalization. National Institute of Health (NIH) ImageJ software was used for densitometric analysis of the gels.
Immunohistochemistry
Three control rats and three cisplatin-treated rats (12 mg/kg, two days post-treatment) were deeply anesthetized (86 mg/kg, i.p, Fatal Plus, Vortech, Pharmaceutical Ltd.) and then perfused intracardially with 0.1 M phosphate buffered saline (PBS) followed by 10% formalin in PBS. The brain was removed, post-fixed in 10% formalin for 24 hr, and then cryoprotected in 15% sucrose in PBS for 6 hr followed by 30% sucrose in PBS for 12 hr. Coronal sections, 40 µm thick, were cut on a cryostat and stored in a cryoprotection solution of 30% ethylene glycol and 30% glycerol in PBS at −20 °C. Free-floating sections (20–25 sections from each rat) were blocked with 1% bovine serum albumin (BSA), 1% normal horse serum and 0.1% TritonX-100 (TX) in PBS for 30 min as previously reported (Manohar et al., 2012). The rabbit anti-Ki67 (Novocastra Laboratories Ltd, UK; 1:3000) was added to the solution and the sections were incubated overnight at 4 °C. Sections were then rinsed and incubated in a secondary antibody (biotinylated goat anti-rabbit IgG, BA 1000, Vector Laboratories) and processed with an avidin-biotin-peroxidase complex (ABC) kit (Vectastain, Vector Laboratories). Immunoreactivity was visualized using the glucose oxidase (Sigma-Aldrich)-diaminobenzidine (Sigma-Aldrich) method (Shu et al., 1988, Van Der Gucht et al., 2006). Sections were rinsed and mounted on Fisher “Superfrost” polarized slides (Fisher Scientific), allowed to dry, stained with cresyl-violet, dehydrated in ethanol, cleared in xylene and coverslipped with DePex mounting medium (Manohar et al., 2012). Microscopic images were photographed using a SPOT digital camera (SPOT Insight; Diagnostic Instruments, Inc). The images were assembled using Adobe Photoshop CS3 software. Quantification and statistical analysis were performed following a standard protocol (Kraus et al., 2010). Ki67 positive cells were counted in 25 sections from each normal and cisplatin-treated rat and Ki67 density was calculated by dividing the cell count by the length of sub granular zone. A two-tailed paired student’s t-test was performed to determine the statistical significance.
RESULTS
Cisplatin Induced Hippocampal Apoptotic Genes
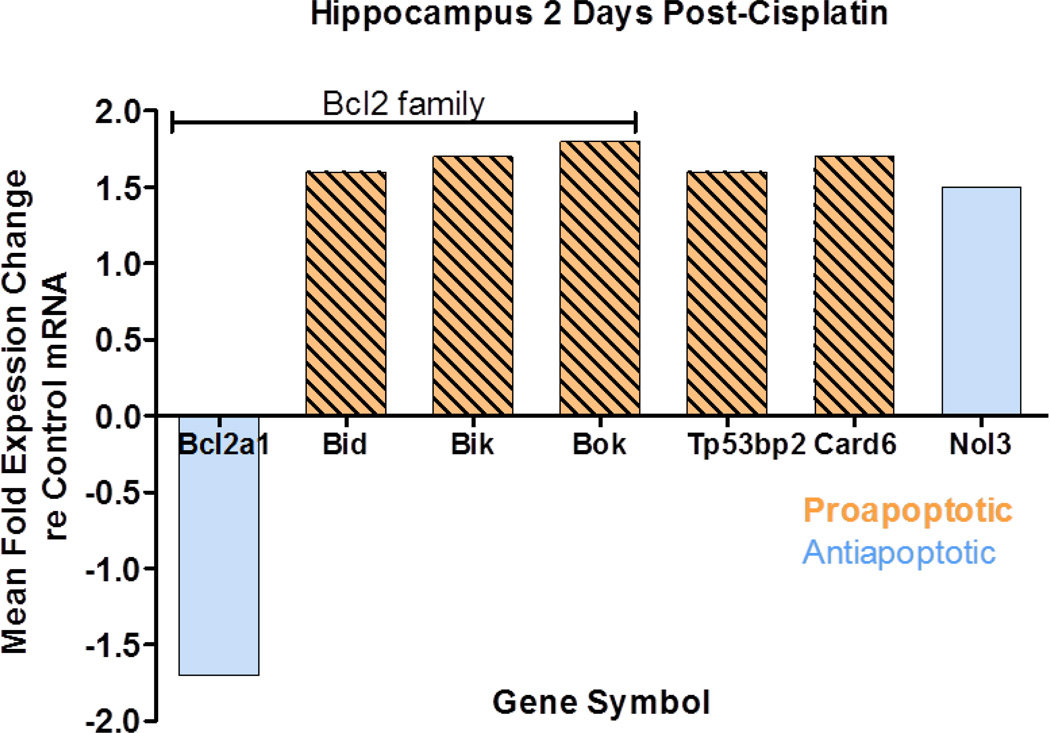
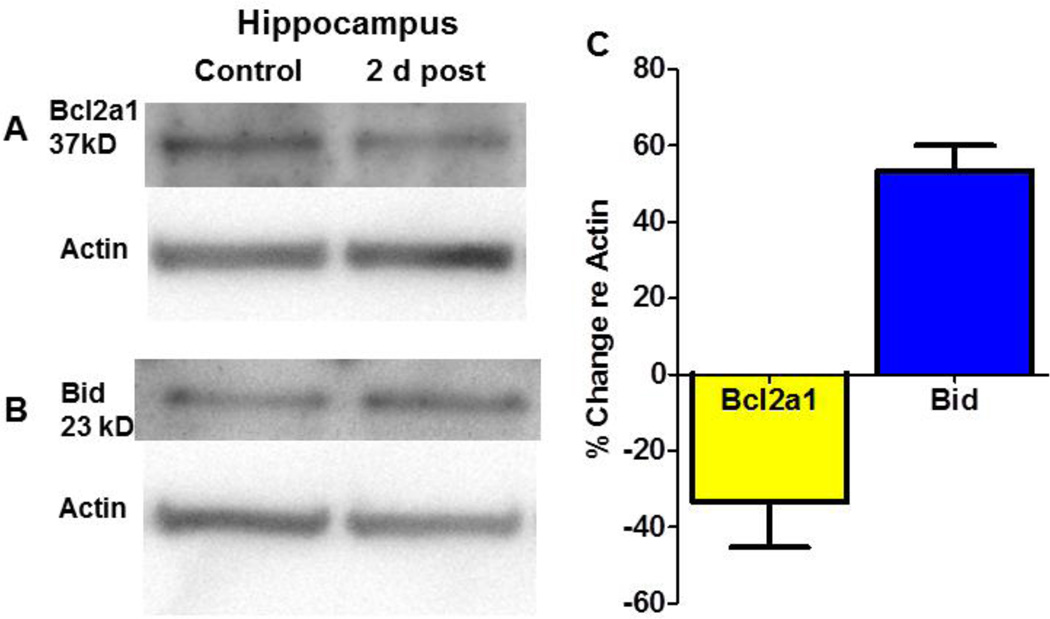
Cisplatin-induced changes in the expression profile of apoptotic genes in the hippocampus two days post treatment. Significant changes were detected in the mRNA expression levels of seven of 84 genes on the array; those classified proapoptotic and antiapoptotic are indicated in legend of figure 1. Bcl2a1 (B-Cell leukemia/lymphoma 2 A1), a member of B-Cell leukemia/lymphoma 2 (Bcl2) gene family, was downregulated (1.7 fold). Bcl2a1, a pro-survival gene, encodes a protein that prevents the release of the cytochrome C from the mitochondria into the cytoplasm (Vogler, 2012). Release of cytochrome c into the cytoplasm initiates apoptosis through APAF-1 (apoptotic protease activating factor-1) and downstream caspases (Henshall et al., 2001). In contrast, three members of the Bcl2 gene family were significantly upregulated; these were Bid (BH3 interacting domain death agonist, 1.55 fold increase), Bik (Bcl2-interacting killer, 1.6 fold increase) and Bok (Bcl-2-related ovarian killer protein, 1.8 fold increase). Bid encodes a proapoptotic protein that interacts with the Bax protein; together they promote the opening of voltage dependent anion channels and the release of cytochrome c from mitochondria (Devarajan et al., 2002, Wang et al., 2001). Bik and Bok encodes proteins promote p53 dependent apoptosis (Li et al., 2008) (Hur et al., 2006, Yakovlev et al., 2004). The expression of three others genes increased significantly two days post-cisplatin. Card 6 (caspase recruitment domain gene family member 6), which was classified as proapoptotic increased 1.7 fold. Nol3 (apoptosis repressor with card domain protein Nuclear protein 3), considered antiapoptotic, increased 1.5 fold. Finally, Trp53bp2, also known as apoptosis-stimulating of p53 protein 2 (ASPP2)) and classified as proapoptotic increased 1.6 fold. Thus, the cisplatin-induced changes in mRNA expression the in the hippocampus were predominantly reflected by an increase in five proapoptotic genes plus and a decrease in one antiapoptotic genes, with the exception of an increase one antiapoptotic gene. Many of the gene expression changes occurred in the Bcl2 family. To extend and confirm these observations, the protein expression levels of Bcl2a1 and Bid were evaluated in the hippocampus, two days post treatment. Immunoblots detected Bcl2a1 and Bid protein bands at apparent molecular weights of 37 and 23 kD respectively. Cisplatin induced a 1.6 fold increase in Bid protein expression and a 0.7 fold decrease in Bcl2a1 protein expression (fig. 2), consistent with the cisplatin-induced changes in their mRNA expression. Collectively, these results highlight the activation of Bcl2 regulated apoptotic pathway in mediating the cytotoxic effects of cisplatin in the hippocampus.
Fig 1.
Seven genes showed a significant change (p<0.05) in mRNA expression in the hippocampus at 2-days post-cisplatin (12 mg/kg); results show mean fold changes in expression. Bcl2a1, Bid, Bik and Bok are members of the Bcl2 gene family. All five genes classified as pro-apoptotic showed an increase in expression. One of two genes classified as an antiapoptotic showed a decrease in expression; the other showed an increase. Gene expression levels normalized to five housekeeping genes (Hprt, Ldha, Rpl13a, Rplp1 and Actb). Mean data shown for four cisplatin and five control rats.
Fig 2.
Western blots showing changes in (A) Bcl2a1 (~37 kD) and (B) Bid protein (23 kD) expression in the hippocampus 2 days post-cisplatin and actin housekeeping protein shown below each protein. (C) Protein bands for Bcl2a1 and Bid proteins were normalized to actin housekeeping protein. Bcl2a1 was down-regulated approximately 30% while Bid was up-regulated nearly 60%.
Cisplatin Induces Site Specific Modulation of Bcl2 Family Genes in Brain
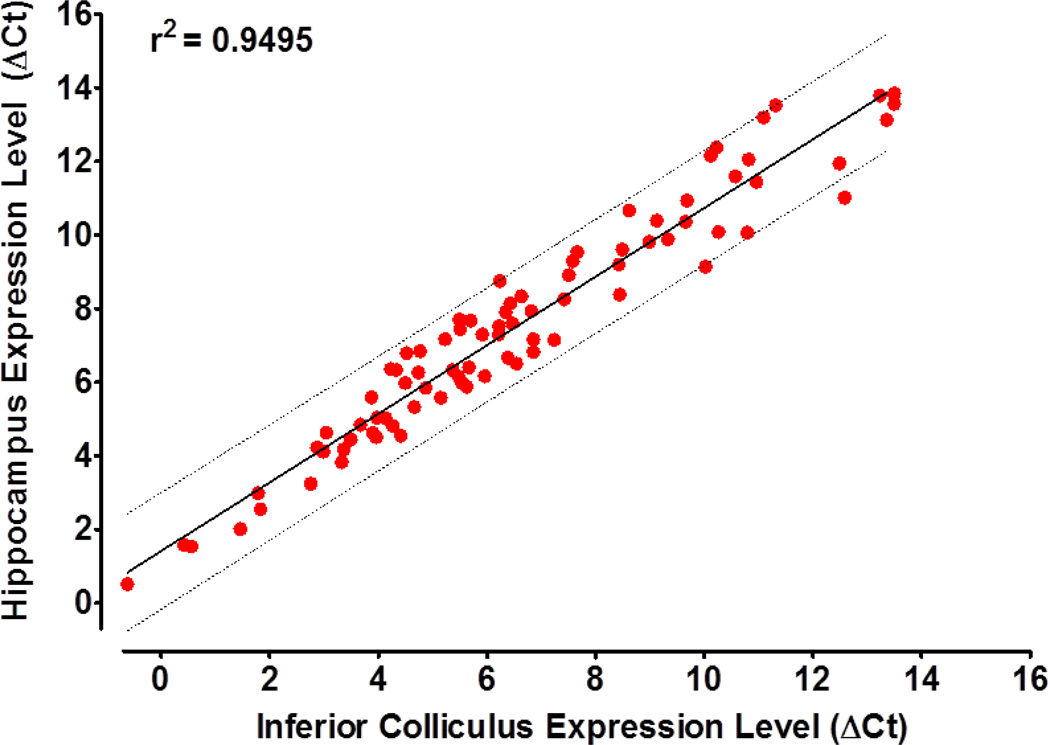
To determine if the effects of cisplatin on the hippocampus might be due to a unique apoptotic gene expression profile, we compared the relative abundance of the 84 apoptosis genes with those in the inferior colliculus, a region not known to be susceptible to apoptosis. Our results show that the baseline gene expression profiles of the hippocampus and the inferior colliculus were very similar to one another (Fig. 3). A linear regression analysis comparing the expression levels of the 84 apoptosis related genes in the hippocampus to those in the inferior colliculus revealed a high correlation (r2 = 0.9263). Genes that were expressed at high or low levels in the hippocampus were also expressed at high or low levels in the inferior colliculus under basal conditions.
Fig 3.
Correlation of apoptotic gene expression levels (re housekeeping genes) in the hippocampus versus the inferior colliculus in normal controls. Linear regression showed a strong correlation (r2=0.9495) in gene expression levels in these two regions. Thick solid line shows linear regression; dotted lines show the 95% confidence interval.
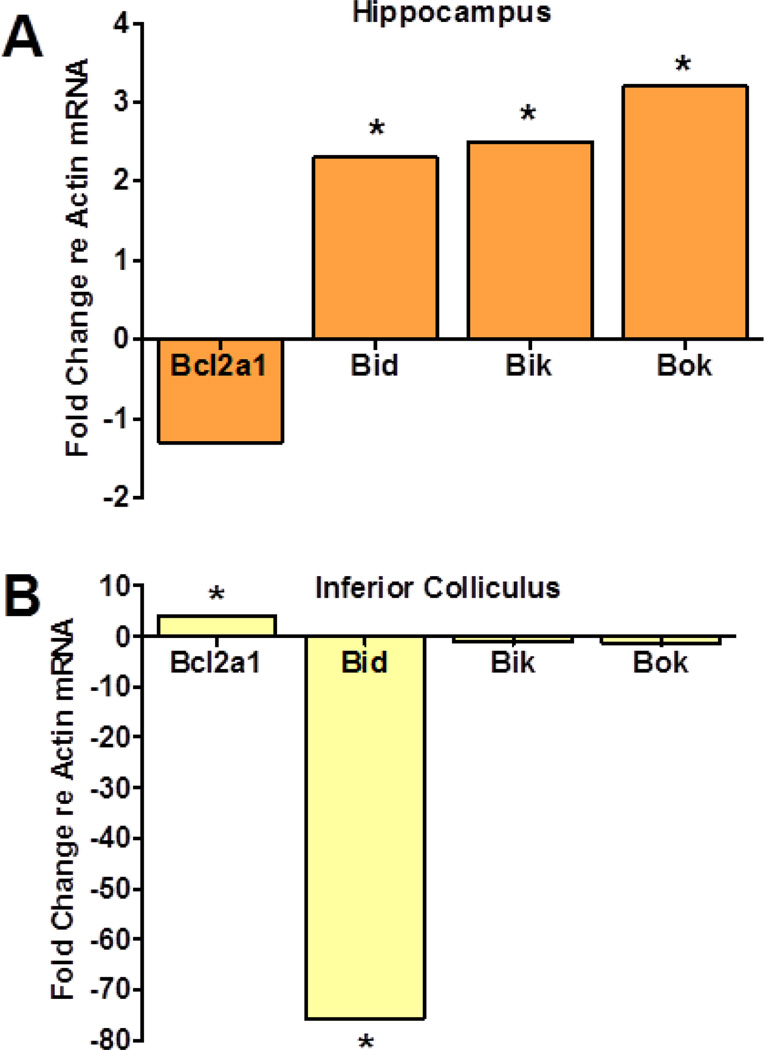
To validate the hippocampus gene array data and to determine if cisplatin would induce an apoptotic response in the inferior colliculus similar to that seen in the hippocampus, we measured the cisplatin induced changes in expression of Bcl2a1, Bid, Bik and Bok in these two brain regions two days post-cisplatin. The effects of cisplatin on the expression of Bcl2a1, Bid, Bik and Bok genes were strikingly different in the inferior colliculus than the hippocampus (Fig. 4). In the hippocampus, mRNA levels of the antiapoptotic gene, Bcl2a1, was down-regulated whereas it increased slightly in the inferior colliculus. In contrast, the three proapoptotic genes, Bid, Bik and Bok, were significantly up-regulated in the hippocampus whereas all three proapoptotic genes decreased in the inferior colliculus. Bid expression in the inferior colliculus decreased more than 70 fold while Bik and Bok decreased only slightly. These results indicate that the hippocampus responds in an apoptotic manner to cisplatin whereas the inferior colliculus mounts an antiapoptotic response.
Fig 4.
Fold change in expression of four Bcl2 genes, Bcl2a1, Bid, Bik and Bok in hippocampus (A) and inferior colliculus (B) two days post-cisplatin. For each group, the mRNA expression was normalized to the beta-actin housekeeping gene. In the hippocampus (A), apoptotic genes Bid (p = 0.001), Bik (p = 0.025) and Bok (p = 0.004) were up-regulated significantly (p < 0.05) while antiapoptotic gene Bcl2a1 (p = 0.22) was down-regulated moderately. In contrast, in the inferior colliculus, apoptotic gene Bid was down-regulated significantly (p = 0.0004) while the antiapoptotic gene Bcl2a1 was significantly up-regulated (p = 0.02).
Cisplatin Decreases Ki67 in the SGZ
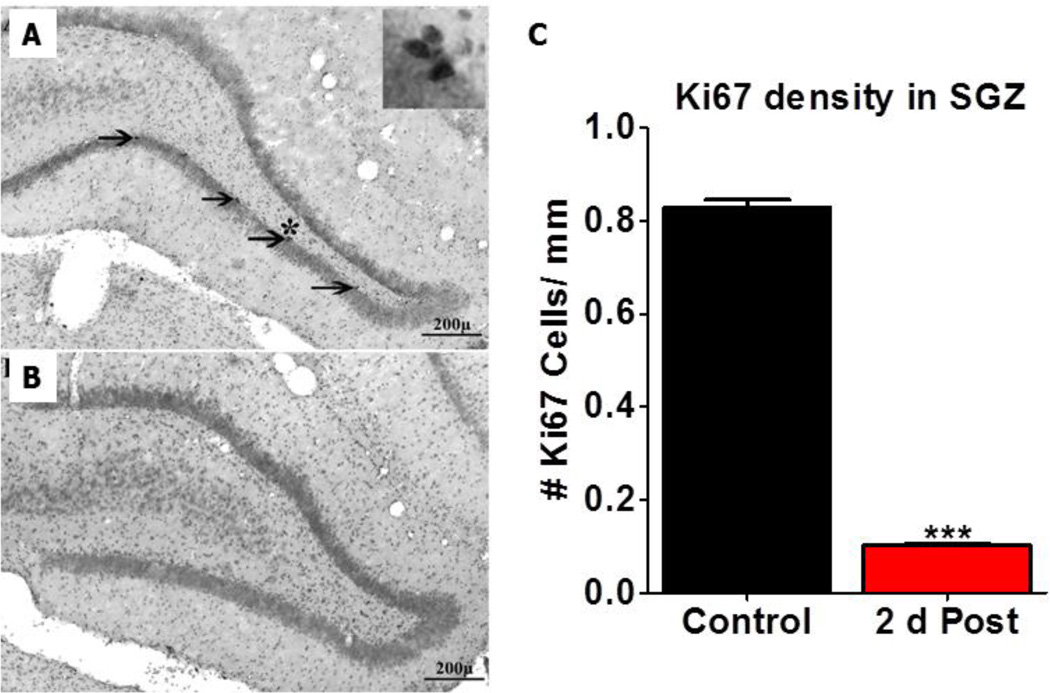
In addition to inducing apoptosis, many anticancer drugs suppress cell proliferation and neurogenesis in the hippocampus (Dietrich et al., 2006). To determine if our cisplatin treatment affected cell proliferation, an antibody against Ki67 was used to quantify cell proliferation in the SGZ of the hippocampus of control rats and cisplatin treated rats sacrificed two days post-treatment (Bullwinkel et al., 2006, Kraus et al., 2010). Figure 5A–B show representative coronal sections from a control rat and a cisplatin treated rat respectively; sections were immunolabeled with Ki67 and counterstained with cresyl violet. Consistent with our previous results, Ki67 positive cells were present in the SGZ of the hippocampus of control rats (arrows, Fig. 5A) (Kraus et al., 2010). The high magnification inset located near the asterisk in figure 5A shows a cluster of Ki67 positive nuclei. In contrast, few Ki67 immunolabeled cells were seen in the hippocampus two days post-cisplatin. Ki67 positive cells were counted along the length of the SGZ of the hippocampus of three control and three cisplatin treated rats. The mean numbers of Ki67 cells per mm length of SGZ decreased from approximately 0.82 to 0.1, a decline of nearly 90% (fig. 5).
Fig 5.
Typical coronal sections from a control (A) and a cisplatin treated rat (B) immunolabeled with an antibody against Ki67 and counterstained with cresyl violet. In controls (A) Ki67 immunopositive nuclei (arrows) were present in the SGZ of the dentate gyrus. Insert in upper, right of panel A shows a cluster of Ki67 positive cells from the region with the asterisk (p<0.001, ***). Ki67 labeling was almost completely absent from the hippocampus 2 days after cisplatin treatment. (C) Histogram showing the mean (n=3/group, SEM) numbers of Ki67 positive cells per mm length of the SGZ. Ki67 immunolabeling was significantly (t=35.67 & df =2) reduced 2 days post-cisplatin relative to controls.
DISCUSSION
Although cisplatin does not readily cross the blood-brain barrier (Jacobs et al., 2010, Minami et al., 1998), it nevertheless has been found to exert neurotoxic effects. Clinically, the neurological side effects of cisplatin are manifested as psychological, memory, perceptual and cognitive deficits (Kaasa et al., 1988, Troy et al., 2000, Verstappen et al., 2003, Whitney et al., 2008). Cisplatin potently inhibits cell proliferation, differentiation and neurogenesis in the hippocampus, alters neurotransmitter concentrations and damages neurons and oligodendrocytes (Dietrich et al., 2006, James et al., 2008, Liu et al., 2003). Since the molecular mechanisms underlying the neurotoxic effects of cisplatin on the in hippocampus are poorly understood, we utilized an apoptosis array consisting of 84 genes to identify biologically and clinically relevant targets significantly upregulated or downregulated two days after cisplatin treatment. Our results suggest that cisplatin initially promotes apoptosis in the hippocampus by increasing the expression of five proapoptotic genes, Bid, Bik, Bok, Tp53bp2 and Card6 while reducing the expression of one ant-apoptotic gene Bcl2a1 (Fig. 1). In contrast, expression of one antiapoptotic gene, Nol3, was significantly increased. Moreover, cisplatin almost completely inhibited cell proliferation in the SGZ of hippocampus consistent with previous results (Dietrich et al., 2006). These changes may provide insights into the biological mechanisms that give rise to the psychological, emotional and cognitive impairments that develop after cisplatin treatment (Shabani et al., 2012, Troy et al., 2000).
Hippocampal Cell Death Genes
The Bcl2 gene family, which codes for antiapoptotic, proapoptotic and BH3 (Bcl-2 homology 3) proteins, regulates essential cellular functions such as programmed cell death, immune responses and tissue turnover (Martinou and Youle, 2011, Youle and Strasser, 2008). Activation of the Bcl2 cell death pathway results in numerous changes such as loss of mitochondrial membrane integrity, release of cytochrome c and upregulation of executioner caspase 3 (Zhang et al., 2005). Cisplatin upregulated the proapoptotic genes Bid, Bik and Bok while down regulating the antiapoptotic gene Bcl2a1. These results are consistent with studies showing that upregulation of Bik significantly enhances cisplatin induced apoptosis (Li et al., 2008). Likewise, cisplatin promotes the calpain-mediated cleavage of Bid which enhances apoptosis (Mandic et al., 2002). Conversely, cells over expressing Bcl2a1 are resistant to cisplatin and other chemotherapeutic compounds (Cheng et al., 2000, Kim et al., 2004b, Vogler, 2012). Since, Bcl2a1 interacts with Bid (Chen et al., 2005b, Mandic et al., 2002, Werner et al., 2002), Bok (Hsu et al., 1997) and Bik (Chen et al., 2005b, Holmgreen et al., 1999, Li et al., 2008) to prevent the loss of mitochondrial outer membrane integrity (Zhang et al., 2005), the cisplatin-induced changes in these four genes strongly implicate Bcl2 genes as major contributors to cisplatin mediated cell death in the hippocampus.
Cisplatin also significantly increased the expression of Tp53bp2, which codes for the apoptosis stimulating protein of p53-2 (ASPP2). ASPP2 promotes apoptosis and regulates cell growth (Chen et al., 2005a). Card6 expression was also significantly elevated two days postcisplatin. The Card6 gene encodes a microtubule-associated protein containing a caspase recruitment domain that interacts with signaling pathways involved in inflammation and apoptosis (Dufner et al., 2006). In contrast to the preceding findings, cisplatin exerted antiapoptotic effects by significantly increasing the expression of Nol3 (also known as ARC, apoptosis repressor with CARD domain). Nol3 codes for an antiapoptotic protein that reduces enzyme activities linked to caspase 2, caspase 8 and tumor protein 53 (Medina-Ramirez et al., 2011, Mercier et al., 2005, Wang et al., 2012, Wei et al., 2010). Upregulation of Nol3 could contribute to cisplatin drug resistance.
In addition to direct effects, cisplatin also increases the expression of a number of genes coding for inflammatory proteins such us TNF , RANTES (CCL5), chemokine receptors CCR1/CCR5, IL-1, and IL-6 (Ju et al., 2008, Li et al., 2005, So et al., 2008). Inflammatory molecule IL-1 and IL-1 increase the expression of proapoptotic protein Bid and Bik (Mezosi et al., 2004, Wakahara et al., 2005) while cytokines INF-β and INF-γ upregulate Card6 (Dufner et al., 2008). Thus, the inflammation may promote the increase in Bid, Bik and Card6 mRNA and provide an indirect pathway by which cisplatin may promote apoptosis in the hippocampus.
Hippocampus and Inferior Colliculus
The hippocampus is vulnerable to many different forms of traumatic brain injury and neurotoxic compounds including many anticancer drugs (Chang, 1990, Geddes et al., 2003, Lowenstein et al., 1992). While high doses of cisplatin can lead to apoptosis in the hippocampus (Dietrich et al., 2006), we are unaware of any studies showing that cisplatin induces cell death in the inferior colliculus. Why the hippocampus, but not the inferior colliculus, is susceptible to cisplatin damage is unclear, but could be related to differences in the genes or proteins expressed in these regions. To partially address this issue, we compared the apoptotic gene expression profiles of the hippocampus and inferior colliculus and found them to be remarkably similar in normal animals (Fig. 3). However, the effects of cisplatin on Bcl2a1, Bid, Bik and Box gene expression were distinctly different in these two regions. Cisplatin significantly increased the expression of the pro-apoptotic genes Bid, Bik and Bok in the hippocampus, but had the opposite or no effect on these genes in the inferior colliculus. Moreover, cisplatin decreased the expression of Bcl2a1 in the hippocampus (Fig. 1, 3), but significantly increased the expression this prosurvival gene in the inferior colliculus. Thus, in response to cisplatin, the hippocampus appears to mount a largely proapoptotic response whereas the response of the inferior colliculus is largely antiapoptotic, which could contribute to drug resistance.
Cisplatin Inhibits Hippocampal Cell Proliferation
The stem cell niche in the rat hippocampus gives rise to approximately 9000 newborn cells each day many of which differentiate into neurons or glial cells (Aimone et al., 2009, Cameron and McKay, 2001, Deng et al., 2010, Jessberger et al., 2009). Using Ki67 immunolabeling, we found that cell proliferation in the SGZ of the hippocampus was suppressed by nearly 90% 2-days postcisplatin. Many different factors can modulate cell proliferation in the hippocampus (Couillard-Despres et al., 2009, Hattiangady et al., 2004, Kim et al., 2004a, Mustafa et al., 2008, Veena et al., 2009). Since cisplatin forms intra- and interstrand crosslinks with DNA, this could be a factor that directly suppresses cell proliferation in the hippocampus (Dietrich et al., 2006). However, the uptake of cisplatin into the central nervous system is somewhat limited (Gregg et al., 1992, Jacobs et al., 2010); therefore other mechanisms need to be considered. Cisplatin is known to induce oxidative stress and strong inflammatory responses (Jung et al., 2009, Kang et al., 2009, Lee et al., 2006, Lim et al., 2005, Ramesh and Reeves, 2004, Tsuji et al., 2009) which may act to suppress neurogenesis (Bachstetter et al., 2010, Barha et al., 2011, Graciarena et al., 2010, Iosif et al., 2006, Shih et al., 2006). In addition, cisplatin increased the expression of Trp53bp2 mRNA is known to contribute cell cycle arrest (Chen et al., 2005a, Lane, 1992, Naumovski and Cleary, 1996).
CONCULSION
Although cisplatin does not readily enter the central nervous system, it nevertheless damages the hippocampus and blocks hippocampal cell proliferation. Cisplatin promotes cell death in the hippocampus by increasing the expression of several proapoptotic genes while reducing the expression of antiapoptotic gene; these gene expression changes were not seen in the inferior colliculus. Future studies are needed to determine the effects of long term treatment with cisplatin and its long term effects on the hippocampus
References
- Abe K, Wakatsuki T, Katsushima F, Monoe K, Kanno Y, Takahashi A, et al. A case of advanced intrahepatic cholangiocarcinoma successfully treated with chemosensitivity test-guided systemic chemotherapy. World J Gastroenterol. 2009;15:5228–5231. doi: 10.3748/wjg.15.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby PA, Kempermann G, Wiskott L. The role of additive neurogenesis and synaptic plasticity in a hippocampal memory model with grid-cell like input. PLoS Comput Biol. 2011;7:e1001063. doi: 10.1371/journal.pcbi.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Jernberg J, Schlunk A, Vila JL, Hudson C, Cole MJ, et al. Spirulina promotes stem cell genesis and protects against LPS induced declines in neural stem cell proliferation. PLoS One. 2010;5:e10496. doi: 10.1371/journal.pone.0010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol. 2011;231:72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri MR, Small GA. Neuroimaging of hypoxia and cocaine-induced hippocampal stroke. J Neuroimaging. 2004;14:290–291. doi: 10.1177/1051228404265751. [DOI] [PubMed] [Google Scholar]
- Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chang LW. The neurotoxicology and pathology of organomercury, organolead, and organotin. J Toxicol Sci. 1990;15(Suppl 4):125–151. doi: 10.2131/jts.15.supplementiv_125. [DOI] [PubMed] [Google Scholar]
- Chen D, Padiernos E, Ding F, Lossos IS, Lopez CD. Apoptosis-stimulating protein of p53-2 (ASPP2/53BP2L) is an E2F target gene. Cell Death Differ. 2005a;12:358–368. doi: 10.1038/sj.cdd.4401536. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005b;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Lee HH, Li Y, Parks TP, Cheng G. Upregulation of Bcl-x and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-kappaB inhibition. Oncogene. 2000;19:4936–4940. doi: 10.1038/sj.onc.1203861. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Wuertinger C, Kandasamy M, Caioni M, Stadler K, Aigner R, et al. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol Psychiatry. 2009;14:856–864. doi: 10.1038/mp.2008.147. [DOI] [PubMed] [Google Scholar]
- Dawe RJ, Bennett DA, Schneider JA, Arfanakis K. Neuropathologic correlates of hippocampal atrophy in the elderly: a clinical, pathologic, postmortem MRI study. PLoS One. 2011;6:e26286. doi: 10.1371/journal.pone.0026286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, et al. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner A, Duncan GS, Wakeham A, Elford AR, Hall HT, Ohashi PS, et al. CARD6 is interferon inducible but not involved in nucleotide-binding oligomerization domain protein signaling leading to NF-kappaB activation. Mol Cell Biol. 2008;28:1541–1552. doi: 10.1128/MCB.01359-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner A, Pownall S, Mak TW. Caspase recruitment domain protein 6 is a microtubule-interacting protein that positively modulates NF-kappaB activation. Proc Natl Acad Sci U S A. 2006;103:988–993. doi: 10.1073/pnas.0510380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femenia T, Gomez-Galan M, Lindskog M, Magara S. Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res. 2012;1476:58–70. doi: 10.1016/j.brainres.2012.03.053. [DOI] [PubMed] [Google Scholar]
- Fuse-Nagase Y, Suwa K, Nagao Y. Partial seizures associated with cisplatin administration: a case report. Clin Electroencephalogr. 1997;28:55–56. doi: 10.1177/155005949702800110. [DOI] [PubMed] [Google Scholar]
- Geddes DM, LaPlaca MC, Cargill RS., 2nd Susceptibility of hippocampal neurons to mechanically induced injury. Exp Neurol. 2003;184:420–427. doi: 10.1016/s0014-4886(03)00254-1. [DOI] [PubMed] [Google Scholar]
- Graciarena M, Depino AM, Pitossi FJ. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain Behav Immun. 2010;24:1301–1309. doi: 10.1016/j.bbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Gregg RW, Molepo JM, Monpetit VJ, Mikael NZ, Redmond D, Gadia M, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol. 1992;10:795–803. doi: 10.1200/JCO.1992.10.5.795. [DOI] [PubMed] [Google Scholar]
- Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21:289–295. doi: 10.1097/IGC.0b013e31820aaafd. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Bonislawski DP, Skradski SL, Araki T, Lan JQ, Schindler CK, et al. Formation of the Apaf-1/cytochrome c complex precedes activation of caspase-9 during seizure-induced neuronal death. Cell Death Differ. 2001;8:1169–1181. doi: 10.1038/sj.cdd.4400921. [DOI] [PubMed] [Google Scholar]
- Highley M, Meller ST, Pinkerton CR. Seizures and cortical dysfunction following high-dose cisplatin administration in children. Med Pediatr Oncol. 1992;20:143–148. doi: 10.1002/mpo.2950200210. [DOI] [PubMed] [Google Scholar]
- Holmgreen SP, Huang DC, Adams JM, Cory S. Survival activity of Bcl-2 homologs Bclw and A1 only partially correlates with their ability to bind pro-apoptotic family members. Cell Death Differ. 1999;6:525–532. doi: 10.1038/sj.cdd.4400519. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kaipia A, McGee E, Lomeli M, Hsueh AJ. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective antiapoptotic Bcl-2 family members. Proc Natl Acad Sci U S A. 1997;94:12401–12406. doi: 10.1073/pnas.94.23.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Bell DW, Dean KL, Coser KR, Hilario PC, Okimoto RA, et al. Regulation of expression of BIK proapoptotic protein in human breast cancer cells: p53-dependent induction of BIK mRNA by fulvestrant and proteasomal degradation of BIK protein. Cancer Res. 2006;66:10153–10161. doi: 10.1158/0008-5472.CAN-05-3696. [DOI] [PubMed] [Google Scholar]
- Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs MS, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat. 2006;98:343–348. doi: 10.1007/s10549-006-9171-6. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida N, Akaike M, Tsutsumi S, Kanai H, Masui A, Sadamatsu M, et al. Trimethyltin syndrome as a hippocampal degeneration model: temporal changes and neurochemical features of seizure susceptibility and learning impairment. Neuroscience. 1997;81:1183–1191. doi: 10.1016/s0306-4522(97)00220-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Kubo T, Shibanoki S, Matsumoto A, Hata H, Asai S. Hippocampal degeneration inducing impairment of learning in rats: model of dementia? Behav Brain Res. 1997;83:39–44. doi: 10.1016/s0166-4328(97)86043-3. [DOI] [PubMed] [Google Scholar]
- Jacobs S, McCully CL, Murphy RF, Bacher J, Balis FM, Fox E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother Pharmacol. 2010;65:817–824. doi: 10.1007/s00280-009-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SE, Burden H, Burgess R, Xie Y, Yang T, Massa SM, et al. Anti-cancer drug induced neurotoxicity and identification of Rho pathway signaling modulators as potential neuroprotectants. Neurotoxicology. 2008;29:605–612. doi: 10.1016/j.neuro.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamesdaniel S, Ding D, Kermany MH, Davidson BA, Knight PR, 3rd, Salvi R, et al. Proteomic analysis of the balance between survival and cell death responses in cisplatinmediated ototoxicity. J Proteome Res. 2008;7:3516–3524. doi: 10.1021/pr8002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, et al. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Ju C, Hamaue N, Machida T, Liu Y, Iizuka K, Wang Y, et al. Anti-inflammatory drugs ameliorate opposite enzymatic changes in ileal 5-hydroxytryptamine metabolism in the delayed phase after cisplatin administration to rats. Eur J Pharmacol. 2008;589:281–287. doi: 10.1016/j.ejphar.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Jung M, Hotter G, Vinas JL, Sola A. Cisplatin upregulates mitochondrial nitric oxide synthase and peroxynitrite formation to promote renal injury. Toxicol Appl Pharmacol. 2009;234:236–246. doi: 10.1016/j.taap.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Kaasa S, Olsnes BT, Thorud E, Host H. Reduced short-term neuropsychological performance in patients with nonsmall-cell lung cancer treated with cisplatin and etoposide. Antibiot Chemother. 1988;41:226–231. doi: 10.1159/000416209. [DOI] [PubMed] [Google Scholar]
- Kang KP, Kim DH, Jung YJ, Lee AS, Lee S, Lee SY, et al. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24:3012–3020. doi: 10.1093/ndt/gfp242. [DOI] [PubMed] [Google Scholar]
- Kannarkat G, Lasher EE, Schiff D. Neurologic complications of chemotherapy agents. Curr Opin Neurol. 2007;20:719–725. doi: 10.1097/WCO.0b013e3282f1a06e. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Williams JM. Memory for magnitude of reinforcement: dissociation between the amygdala and hippocampus. Neurobiol Learn Mem. 1995;64:237–244. doi: 10.1006/nlme.1995.0006. [DOI] [PubMed] [Google Scholar]
- Kim JB, Ju JY, Kim JH, Kim TY, Yang BH, Lee YS, et al. Dexamethasone inhibits proliferation of adult hippocampal neurogenesis in vivo and in vitro. Brain Res. 2004a;1027:1–10. doi: 10.1016/j.brainres.2004.07.093. [DOI] [PubMed] [Google Scholar]
- Kim JK, Kim KD, Lee E, Lim JS, Cho HJ, Yoon HK, et al. Up-regulation of Bfl-1/A1 via NF-kappaB activation in cisplatin-resistant human bladder cancer cell line. Cancer Lett. 2004b;212:61–70. doi: 10.1016/j.canlet.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, et al. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyd RJ, Bilkey DK. Hippocampal place cells show increased sensitivity to changes in the local environment following prefrontal cortex lesions. Cereb Cortex. 2005;15:720–731. doi: 10.1093/cercor/bhh173. [DOI] [PubMed] [Google Scholar]
- Lane DP. Worrying about p53. Curr Biol. 1992;2:581–583. doi: 10.1016/0960-9822(92)90154-3. [DOI] [PubMed] [Google Scholar]
- Lee S, Moon SO, Kim W, Sung MJ, Kim DH, Kang KP, et al. Protective role of L-2-oxothiazolidine-4-carboxylic acid in cisplatin-induced renal injury. Nephrol Dial Transplant. 2006;21:2085–2095. doi: 10.1093/ndt/gfl209. [DOI] [PubMed] [Google Scholar]
- Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7:1647–1655. doi: 10.1158/1535-7163.MCT-07-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289:F469–F480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, et al. Antioxidant and antiinflammatory activities of xanthorrhizol in hippocampal neurons and primary cultured microglia. J Neurosci Res. 2005;82:831–838. doi: 10.1002/jnr.20692. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hamaue N, Endo T, Hirafuji M, Minami M. 5-hydroxytryptamine (5-HT) concentrations in the hippocampus, the hypothalamus and the medulla oblongata related to cisplatin-induced pica of rats. Res Commun Mol Pathol Pharmacol. 2003;113–114:97–113. [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft T, Amaral OB, Schwartsmann G, Roesler R. Transient disruption of fear-related memory by post-retrieval inactivation of gastrin-releasing peptide or N-methyl-D-aspartate receptors in the hippocampus. Curr Neurovasc Res. 2008;5:21–27. doi: 10.2174/156720208783565672. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Paolone NA, Bleichfeld M, Hayes SH, Salvi RJ, Baizer JS. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2012;202:169–183. doi: 10.1016/j.neuroscience.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Takayama T, Tashiro M, Nakamura Y, Ohashi Y, Shimozuma K. Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients--evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer. 2005;12:279–287. doi: 10.2325/jbcs.12.279. [DOI] [PubMed] [Google Scholar]
- Medina-Ramirez CM, Goswami S, Smirnova T, Bamira D, Benson B, Ferrick N, et al. Apoptosis inhibitor ARC promotes breast tumorigenesis, metastasis, and chemoresistance. Cancer Res. 2011;71:7705–7715. doi: 10.1158/0008-5472.CAN-11-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier I, Vuolo M, Madan R, Xue X, Levalley AJ, Ashton AW, et al. ARC, an apoptosis suppressor limited to terminally differentiated cells, is induced in human breast cancer and confers chemo-and radiation-resistance. Cell Death Differ. 2005;12:682–686. doi: 10.1038/sj.cdd.4401631. [DOI] [PubMed] [Google Scholar]
- Mezosi E, Wang SH, Utsugi S, Bajnok L, Bretz JD, Gauger PG, et al. Interleukin-1beta and tumor necrosis factor (TNF)-alpha sensitize human thyroid epithelial cells to TNF-related apoptosis-inducing ligand-induced apoptosis through increases in procaspase-7 and bid, and the down-regulation of p44/42 mitogen-activated protein kinase activity. J Clin Endocrinol Metab. 2004;89:250–257. doi: 10.1210/jc.2003-030697. [DOI] [PubMed] [Google Scholar]
- Minami T, Okazaki J, Kawabata A, Kuroda R, Okazaki Y. Penetration of cisplatin into mouse brain by lipopolysaccharide. Toxicology. 1998;130:107–113. doi: 10.1016/s0300-483x(98)00103-6. [DOI] [PubMed] [Google Scholar]
- Moita MA, Rosis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37:485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morgan RJ, Jr, Leong L, Chow W, Gandara D, Frankel P, Garcia A, et al. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: a California cancer consortium study. Invest New Drugs. 2012;30:723–728. doi: 10.1007/s10637-010-9557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S, Walker A, Bennett G, Wigmore PM. 5-Fluorouracil chemotherapy affects spatial working memory and newborn neurons in the adult rat hippocampus. Eur J Neurosci. 2008;28:323–330. doi: 10.1111/j.1460-9568.2008.06325.x. [DOI] [PubMed] [Google Scholar]
- Naumovski L, Cleary ML. The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M. Mol Cell Biol. 1996;16:3884–3892. doi: 10.1128/mcb.16.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Quiroga I, Hernandez-Valdes M, Lin SL, Naegele JR. Postnatal cellular contributions of the hippocampus subventricular zone to the dentate gyrus, corpus callosum, fimbria, and cerebral cortex. J Comp Neurol. 2006;497:833–845. doi: 10.1002/cne.21037. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E, Ghigo JO. Alzheimer dementia: an overview and a promising new concept. Am J Electroneurodiagnostic Technol. 2011;51:82–91. [PubMed] [Google Scholar]
- Ortin I, Gonzalez JF, Cuesta Ede L, Manguan-Garcia C, Perona R, Avendano C. Cytotoxicity mechanisms of pyrazino[1,2-b]isoquinoline-4-ones and SAR studies. Bioorg Med Chem. 2009;17:8040–8047. doi: 10.1016/j.bmc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Piccolini VM, Cerri S, Romanelli E, Bernocchi G. Interactions of neurotransmitter systems during postnatal development of the rat hippocampal formation: Effects of cisplatin. Exp Neurol. 2012;234:239–252. doi: 10.1016/j.expneurol.2011.12.044. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Paterna JC, Bueler H, Feldon J, Yee BK. Bidirectional changes in water-maze learning following recombinant adenovirus-associated viral vector (rAAV)-mediated brain-derived neurotrophic factor expression in the rat hippocampus. Behav Pharmacol. 2007;18:533–547. doi: 10.1097/FBP.0b013e3282da0bf6. [DOI] [PubMed] [Google Scholar]
- Prestia A, Boccardi M, Galluzzi S, Cavedo E, Adorni A, Soricelli A, et al. Hippocampal and amygdalar volume changes in elderly patients with Alzheimer's disease and schizophrenia. Psychiatry Res. 2011;192:77–83. doi: 10.1016/j.pscychresns.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int. 2004;(Suppl):S56–S61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- Rzeski W, Pruskil S, Macke A, Felderhoff-Mueser U, Reiher AK, Hoerster F, et al. Anticancer agents are potent neurotoxins in vitro and in vivo. Ann Neurol. 2004;56:351–360. doi: 10.1002/ana.20185. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. 1957. J Neuropsychiatry Clin Neurosci. 2000;12:103–113. doi: 10.1176/jnp.12.1.103. [DOI] [PubMed] [Google Scholar]
- Sehouli J, Runnebaum IB, Fotopoulou C, Blohmer U, Belau A, Leber H, et al. A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC-R): a NOGGO-AGO Intergroup Study. Ann Oncol. 2012;23:2259–2264. doi: 10.1093/annonc/mdr628. [DOI] [PubMed] [Google Scholar]
- Shabani M, Larizadeh MH, Parsania S, Hajali V, Shojaei A. Evaluation of destructive effects of exposure to cisplatin during developmental stage: no profound evidence for sex differences in impaired motor and memory performance. Int J Neurosci. 2012;122:439–448. doi: 10.3109/00207454.2012.673515. [DOI] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- So H, Kim H, Kim Y, Kim E, Pae HO, Chung HT, et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Watanabe Y, Kato H, Araki T. Immunohistochemical changes related to ageing in the mouse hippocampus and subventricular zone. Mech Ageing Dev. 2007;128:303–310. doi: 10.1016/j.mad.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Troy L, McFarland K, Littman-Power S, Kelly BJ, Walpole ET, Wyld D, et al. Cisplatin-based therapy: a neurological and neuropsychological review. Psychooncology. 2000;9:29–39. doi: 10.1002/(sici)1099-1611(200001/02)9:1<29::aid-pon428>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Kato A, Yasuda H, Miyaji T, Luo J, Sakao Y, et al. The dimethylthioureainduced attenuation of cisplatin nephrotoxicity is associated with the augmented induction of heat shock proteins. Toxicol Appl Pharmacol. 2009;234:202–208. doi: 10.1016/j.taap.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Valente T, Hidalgo J, Bolea I, Ramirez B, Angles N, Reguant J, et al. A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neurogenesis in the subventricular zone and hippocampus of adult mouse brain. J Alzheimers Dis. 2009;18:849–865. doi: 10.3233/JAD-2009-1188. [DOI] [PubMed] [Google Scholar]
- Van Der Gucht E, Youakim M, Arckens L, Hof PR, Baizer JS. Variations in the structure of the prelunate gyrus in Old World monkeys. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:753–775. doi: 10.1002/ar.a.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. The hippocampus as an olfacto-motor mechanism: were the classical anatomists right after all? Behav Brain Res. 2001;127:25–47. doi: 10.1016/s0166-4328(01)00354-0. [DOI] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87:831–843. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63:1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- Vogler M. BCL2A1: the underdog in the BCL2 family. Cell Death Differ. 2012;19:67–74. doi: 10.1038/cdd.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, et al. Bikunin suppresses lipopolysaccharide-induced lethality through down-regulation of tumor necrosis factor-alpha and interleukin-1 beta in macrophages. J Infect Dis. 2005;191:930–938. doi: 10.1086/428134. [DOI] [PubMed] [Google Scholar]
- Wang GQ, Gastman BR, Wieckowski E, Goldstein LA, Gambotto A, Kim TH, et al. A role for mitochondrial Bak in apoptotic response to anticancer drugs. J Biol Chem. 2001;276:34307–34317. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li A, Wang H, Wang J. Knockdown of apoptosis repressor with caspase recruitment domain (ARC) increases the sensitivity of human glioma cell line U251MG to VM-26. Int J Clin Exp Pathol. 2012;5:555–561. [PMC free article] [PubMed] [Google Scholar]
- Wei L, Ding D, Salvi R. Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience. 2010;168:288–299. doi: 10.1016/j.neuroscience.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B. Chemobrain: a translational challenge for neurotoxicology. Neurotoxicology. 2008;29:891–898. doi: 10.1016/j.neuro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AB, de Vries E, Tait SW, Bontjer I, Borst J. Bcl-2 family member Bfl-1/A1 sequesters truncated bid to inhibit is collaboration with pro-apoptotic Bak or Bax. J Biol Chem. 2002;277:22781–22788. doi: 10.1074/jbc.M201469200. [DOI] [PubMed] [Google Scholar]
- Whitney KA, Lysaker PH, Steiner AR, Hook JN, Estes DD, Hanna NH. Is "chemobrain" a transient state? A prospective pilot study among persons with non-small cell lung cancer. J Support Oncol. 2008;6:313–321. [PubMed] [Google Scholar]
- Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J, et al. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 2012;314:232–243. doi: 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Di Giovanni S, Wang G, Liu W, Stoica B, Faden AI. BOK and NOXA are essential mediators of p53-dependent apoptosis. J Biol Chem. 2004;279:28367–28374. doi: 10.1074/jbc.M313526200. [DOI] [PubMed] [Google Scholar]
- Yang FY, Lee PY. Efficiency of drug delivery enhanced by acoustic pressure during blood-brain barrier disruption induced by focused ultrasound. Int J Nanomedicine. 2012;7:2573–2582. doi: 10.2147/IJN.S31675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhang N, Hartig H, Dzhagalov I, Draper D, He YW. The role of apoptosis in the development and function of T lymphocytes. Cell Res. 2005;15:749–769. doi: 10.1038/sj.cr.7290345. [DOI] [PubMed] [Google Scholar]