Abstract
An infant born with hypospadias and no palpable gonads was diagnosed with persistent mullerian duct syndrome (PMDS) based on history, physical examination, laboratory testing, and radiologic imaging. A robot-assisted laparoscopic hysterectomy, right gonadal biopsy, and bilateral orchiopexies were performed without incident. Final pathology confirmed the diagnosis of PMDS. To our knowledge, this is only the second report of PMDS managed through a robot-assisted laparoscopic approach.
Keywords: persistent mullerian duct syndrome, robotic surgery, infant, hysterectomy, orchiopexy
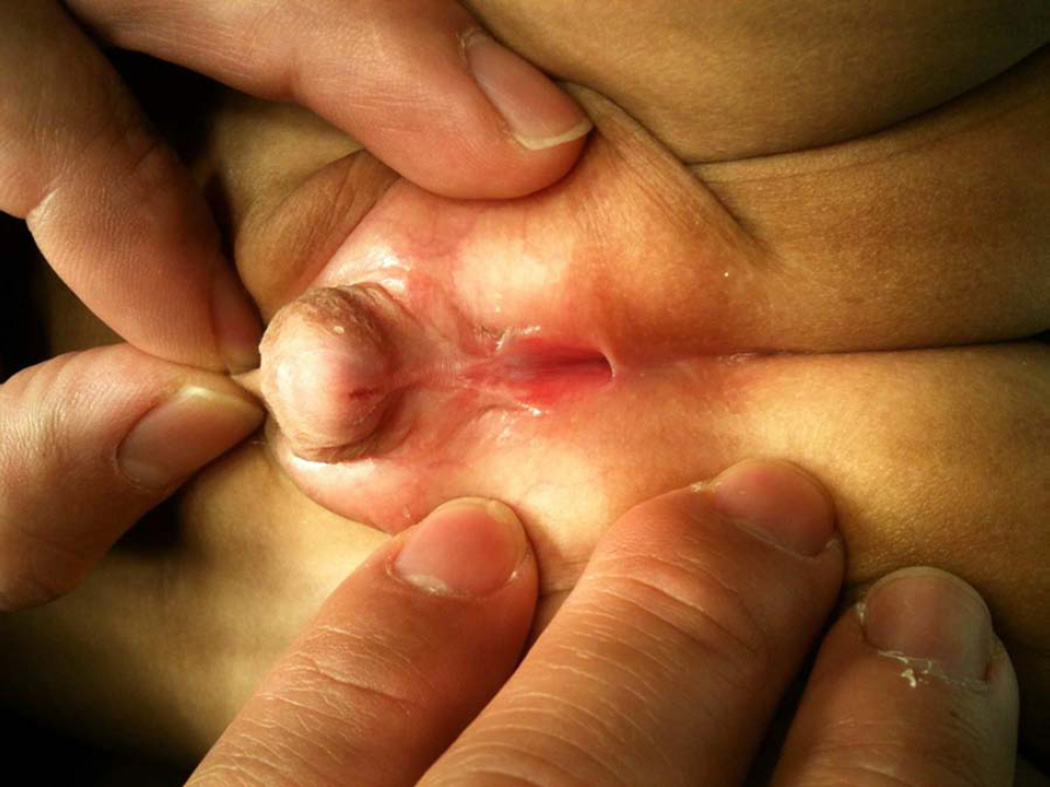
A newborn with ambiguous genitalia featured a phallus of ~2.5 cm length and 1.2 cm girth, scrotal hypospadias with significant ventral curvature, a dorsally hooded prepuce, penoscrotal transposition, a bifid scrotum, and no palpable gonads in the labioscrotal folds (Figure 1).
Figure 1.
Photograph of patient’s genitals demonstrating scrotal hypospadias with significant ventral curvature, a dorsally hooded prepuce, penoscrotal transposition, and a bifid scrotum.
The newborn’s karyotype was 46XY. An inhibin B level was somewhat low at 50.6 pg/mL, indicating the presence of testicles. The neonate’s 17-hydroxyprogesterone level was 591 ng/dL, typical for a newborn. His testosterone level was high (651 ng/dL; normal 12–21), indicating a virilizing signal. Anti-mullerian hormone level was low at 5.2 ng/mL (normal 15.5 to 48.7 ng/mL). Serum electrolytes were with normal limits.
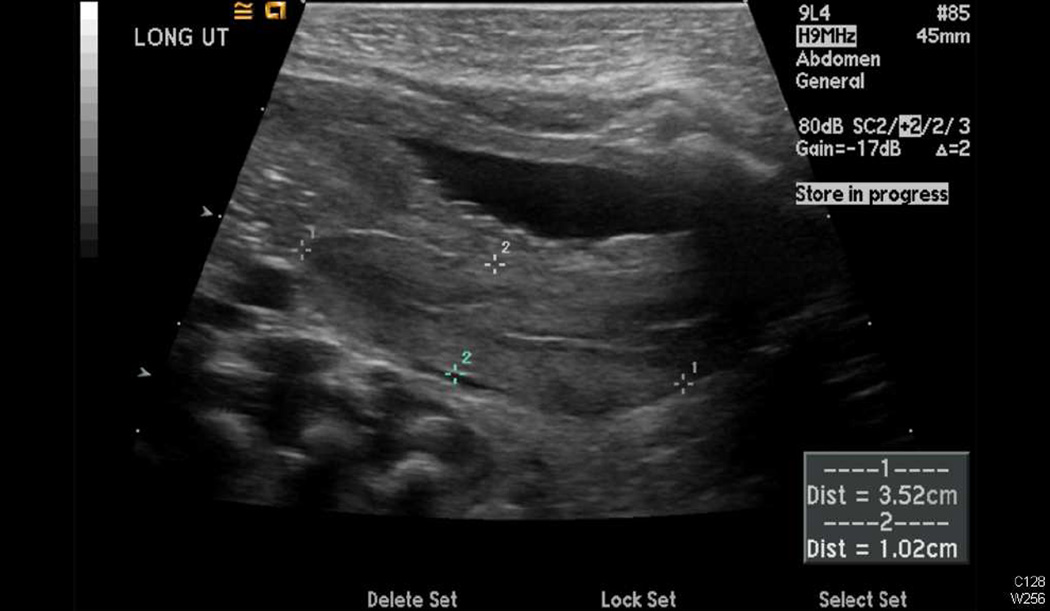
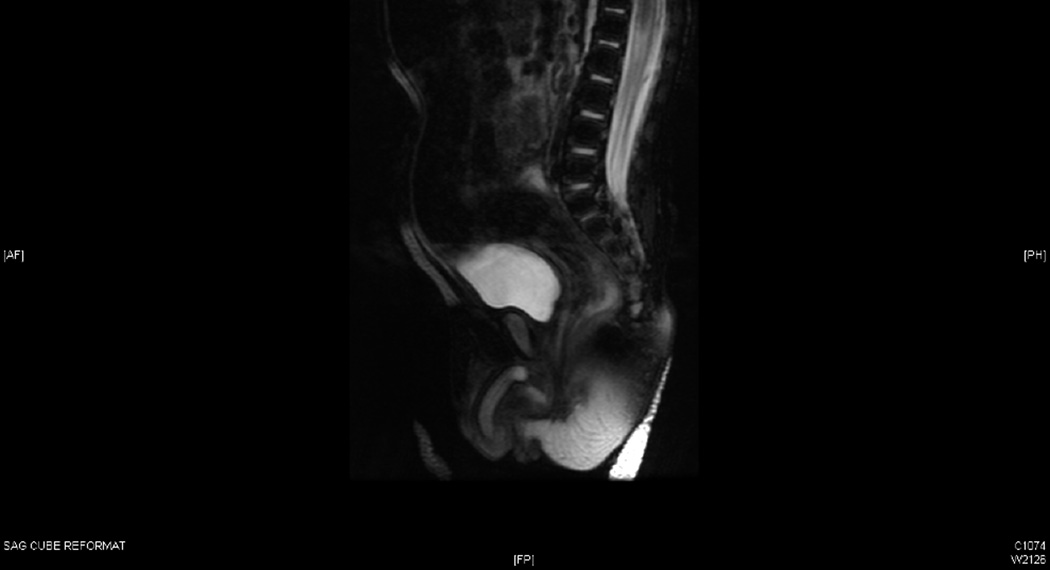
A pelvic ultrasound performed on day of life 3 revealed perineal gonads (6×5 mm and 8×4 mm) and a uterus measuring 3.5×1.4×1 cm (Figure 2A). The kidneys and bladder appeared normal. An MRI was performed, which confirmed the presence of a phallus, gonads, and a uterus (Figure 2B). Another ultrasound was performed four months later, at which point the gonads (left: 7×6×8 mm; right: 8×5×6 mm) were seen in the proximal portion of the labioscrotal folds. The uterus measured 20×7×8 mm.
Figure 2.
Radiographic confirmation of Mullerian structures. A, Sagittal view of a pelvic ultrasound showing the presence of a uterus behind the bladder. B, Sagittal view of an MRI demonstrating a phallus, gonads, and a uterus.
Based on the data, the patient was diagnosed with persistent mullerian duct syndrome (PMDS), penoscrotal hypospadias, and bilateral undescended testes. Sertoli cell failure was suspected based on the low levels of anti-mullerian hormone and inhibin B. The attending pediatric urologist recommended hysterectomy and bilateral orchiopexies followed by hypospadias repair in a separate procedure. The family and attending discussed surgical options, including open, pure laparoscopic, and robot-assisted laparoscopic approaches. Informed consent was obtained for a robot-assisted laparoscopic surgery.
At six months of age the patient was brought to the operating room. After induction of general anesthesia, a Veress needle was used to obtain peritoneal access through an umbilical incision. A 12 mm Patton Surgical robotic trocar was placed.
Under direct vision using bladeless obturators, two 8 mm robotic ports were placed on both sides of the abdomen at the level of the umbilicus. A da Vinci Standard Surgical System bedside cart was docked to the ports.
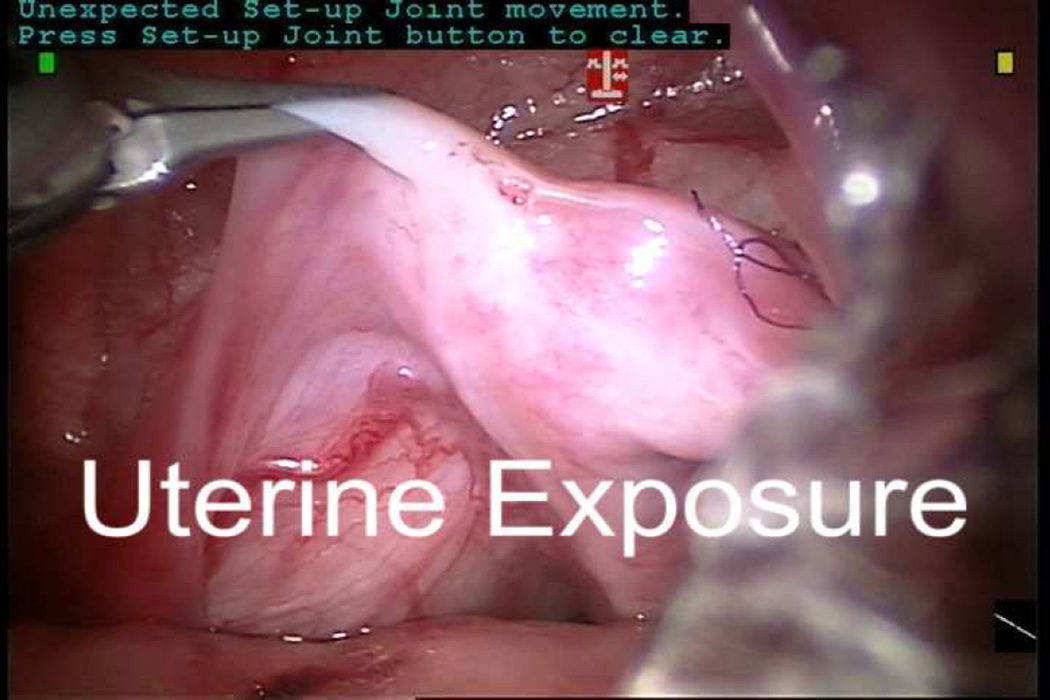
“Peeping” gonads just proximal to the internal ring were identified bilaterally. Since the right gonad was very intimately attached to the uterus, a small biopsy of this gonad was performed and sent to pathology (Video 1). The frozen section confirmed the presence of immature testicular tissue so the testis was preserved. A hitch stich was placed transabdominally through the peritoneum overlying the mesorchium to immobilize the right testis. A 6–0 PDS suture was used to close the biopsy site in a running fashion. A separate hitch stitch was placed transabdominally through the fundus of the uterus to immobilize it. Bipolar cautery and a Harmonic Scalpel were used to perform the hysterectomy. The distal end of the mullerian remnant was transected close to its urethral insertion. Urethral catheter manipulation was performed under laparoscopic vision to ensure a urethrotomy had not occurred.
The peritoneum overlying the testicles was mobilized. The spermatic cords remained quite tight despite this mobilization due to a wide arcade of short blood vessels stretching from the midline and across the broad ligament to each testis. Selective division of small branches of the vascular arcade was performed to mobilize the testes while preserving as much blood supply as possible. Sutures were placed through the mesorchium of each testis and brought through the skin to facilitate subsequent identification. The robot was undocked and the excised mullerian remnant was laparoscopically placed in an entrapment sac and removed. The ports were removed and the fascia closed with 4-0 PDS. Inguinal incisions were made bilaterally and the stay sutures were followed down to the internal ring on each side. The testes were delivered into the surgical field. The peritoneum overlying each spermatic cord was further mobilized, but because the left testis became dusky with minimal manipulation and the right testicular cord was very short, both testes were tacked in place in the distal inguinal canals. The external oblique fascia was closed with 4-0 Vicryl. The skin incisions were closed with 5-0 Monocryl and dermal adhesive. Total operative time was 334 minutes. The estimated blood loss was 10 cc.
The patient was discharged the following day. The permanent sections of the gonadal biopsy and mullerian remnant confirmed the presence of testicular and uterine tissue, respectively.
A testicular ultrasound was performed three months after surgery and showed left and right inguinal testes measuring 1.1×0.4×0.7 cm (volume 0.2 ml) and 1×0.3×0.7 cm (volume 0.1 ml), respectively. Both testes exhibited microlithiasis and intact blood flow.
Nine and 16 months after hysterectomy/orchiopexy, the patient underwent a single stage hypospadias repair and scrotoplasty, respectively, without complications. He was last seen 21 months after hysterectomy/orchiopexy and is clinically doing well. The patient’s PMDS-related mutations are being evaluated at the UCLA disorders of sexual development clinic. The patient continues to be followed by our institution’s pediatric urology, pediatric endocrinology, and andrology services.
PMDS is a disorder of sexual development which features a failure of involution of mullerian structures 1,2. Typically this occurs due to functional mutations in either anti-mullerian hormone or anti-mullerian hormone receptor type II (reviewed by Salehi et al.3). PMDS is primarily inherited in an autosomal recessive fashion, although sex-linked inheritance has been reported. Cryptorchidism and other forms of testicular ectopia are commonly associated with PMDS, likely due to intimate association of the testis and spermatic cord with mullerian structures. Resection of mullerian remnants and orchiopexies, if necessary, are recommended to reduce the risk of malignant degeneration of these tissues. The close anatomic association of wolffian and mullerian structures can make surgical management of PMDS challenging, since care must be taken to avoid damaging the spermatic cord during hysterectomy and resection of the fallopian tubes. This has led to recommendations to consider partial hysterectomy when the mullerian remnant cannot readily separated from the spermatic cord(s).
Numerous surgeons have reported varied use of laparoscopy in the diagnosis and treatment of PMDS 4–13. Some surgeons have only used laparoscopy for diagnostic purposes, while others have utilized it to perform orchiopexies, orchiectomies, and/or hysterectomies. To our knowledge, only one prior publication has reported using robot-assisted laparoscopy to perform orchiopexies and hysterectomy in a child with PMDS 14. In this report, Najmaldin and Antao reported an operative time of 193 minutes associated with a 5 day hospitalization. Although it is tempting to compare this to our operative time of 334 minutes and overnight hospitalization, there is insufficient patient detail in Najmaldin’s report to make such appraisals. Regardless, we attribute our long operative time to multiple factors: the complex anatomy of the patient (especially the vascular supply of both testes running across the broad ligament); small patient size, which made instrument clash and tissue dissection more challenging; use of the now-outdated da Vinci Standard System, which features less range-of-motion and inferior optics compared to the newer systems; and our surgical team’s relative inexperience with infant PMDS surgery.
Despite these issues, we found the robot to be particularly useful for suture closure of the gonadal biopsy site. We believe that as technology improves and is adopted by pediatric surgeons, the utilization of robot-assisted surgery for PMDS will increase and facilitate evaluation of its benefits, if any, over other forms of surgery.
Supplementary Material
Video. Intraoperative footage of robot-assisted laparoscopic hysterectomy, gonadal biopsy, and orchiopexies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant financial disclosures.
References
- 1.Sloan WR, Walsh PC. Familial persistent Müllerian duct syndrome. [Accessed August 4, 2013];The Journal of urology. 1976 115(4):459–461. doi: 10.1016/s0022-5347(17)59242-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4634. [DOI] [PubMed] [Google Scholar]
- 2.Gallucci C, Abrao A. [Seminoma of the testicle with bilateral cryptorchidism and persistence of müllerian formations (uterus and tubes)] [Accessed August 4, 2013];Anais paulistas de medicina e cirurgia. 1950 59(4):359–372. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15425834. [PubMed] [Google Scholar]
- 3.Salehi P, Koh CJ, Pitukcheewanont P, Trinh L, Daniels M, Geffner M. Persistent Müllerian duct syndrome: 8 new cases in Southern California and a review of the literature. [Accessed August 4, 2013];Pediatric endocrinology reviews: PER. 10(2):227–233. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23539834. [PubMed] [Google Scholar]
- 4.Colacurci N, Cardone A, De Franciscis P, Landolfi E, Venditto T, Sinisi AA. Laparoscopic hysterectomy in a case of male pseudohermaphroditism with persistent Müllerian duct derivatives. [Accessed August 4, 2013];Human reproduction (Oxford, England) 1997 12(2):272–274. doi: 10.1093/humrep/12.2.272. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9070709. [DOI] [PubMed] [Google Scholar]
- 5.Farikullah J, Ehtisham S, Nappo S, Patel L, Hennayake S. Persistent Müllerian duct syndrome: lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Müllerian remnants. [Accessed August 4, 2013];BJU international. 2012 110(11 Pt C):E1084–E1089. doi: 10.1111/j.1464-410X.2012.11184.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22540537. [DOI] [PubMed] [Google Scholar]
- 6.Parelkar SV, Gupta RK, Oak S, et al. Laparoscopic management of persistent mullerian duct syndrome. [Accessed August 1, 2013];Journal of pediatric surgery. 2009 44(9):e1–e3. doi: 10.1016/j.jpedsurg.2009.05.033. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19735801. [DOI] [PubMed] [Google Scholar]
- 7.Chertin B, Koulikov D, Alberton J, Hadas-Halpern I, Reissman P, Farkas A. The use of laparoscopy in intersex patients. [Accessed August 4, 2013];Pediatric surgery international. 2006 22(5):405–408. doi: 10.1007/s00383-006-1662-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16521001. [DOI] [PubMed] [Google Scholar]
- 8.Shirasaki Y, Nagai A, Nasu Y, Iguchi H, Kumon H. Laparoscopic removal of Müllerian structures and orchiopexy for persistent Müllerian duct syndrome. [Accessed August 4, 2013];Urology. 2003 62(6):1121. doi: 10.1016/j.urology.2003.07.005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14665371. [DOI] [PubMed] [Google Scholar]
- 9.El-Gohary MA. Laparoscopic management of persistent müllerian duct syndrome. [Accessed August 4, 2013];Pediatric surgery international. 2003 19(7):533–536. doi: 10.1007/s00383-003-0984-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/13680290. [DOI] [PubMed] [Google Scholar]
- 10.Nishio R, Fuse H, Akashi T, Furuya Y. Persistent Müllerian duct syndrome: a surgical approach. [August 4, 2013];Archives of andrology. 49(6):479–482. doi: 10.1080/01485010390236440. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14555334. [DOI] [PubMed] [Google Scholar]
- 11.Lima M, Morabito A, Libri M, et al. Laparoscopic removal of a persistent mullerian duct in a male: case report. [Accessed August 4, 2013];European journal of pediatric surgery: official journal of Austrian Association of Pediatric Surgery …[et al] = Zeitschrift für Kinderchirurgie. 2001 11(2):142. doi: 10.1055/s-2001-15471. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11371038. [DOI] [PubMed] [Google Scholar]
- 12.Wiener JS, Jordan GH, Gonzales ET. Laparoscopic management of persistent Müllerian duct remnants associated with an abdominal testis. [Accessed August 4, 2013];Journal of endourology / Endourological Society. 1997 11(5):357–359. doi: 10.1089/end.1997.11.357. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9355954. [DOI] [PubMed] [Google Scholar]
- 13.Ng JW, Koh GH. Laparoscopic orchidopexy for persistent müllerian duct syndrome. [August 4, 2013];Pediatric surgery international. 1997 12(7):522–525. doi: 10.1007/BF01258717. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9238122. [DOI] [PubMed] [Google Scholar]
- 14.Najmaldin A, Antao B. Early experience of tele-robotic sugery in children †. The International journal of medical robotics and computer assisted surgery. 2007 Aug;3:199–202. doi: 10.1002/rcs.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. Intraoperative footage of robot-assisted laparoscopic hysterectomy, gonadal biopsy, and orchiopexies.