Abstract
Pure compounds extracted and purified from natural sources are crucial to lead discovery and drug screening. This study presents a novel two-dimensional hyphenation of expanded bed adsorption chromatography (EBAC) and high-speed countercurrent chromatography (HSCCC) for extraction and purification of target compounds from medicinal plants in a single step. The EBAC and HSCCC were hyphenated via a six-port injection valve as an interface. Fractionation of ingredients of Salvia miltiorrhiza and Rhizoma coptidis was performed on the hyphenated system to verify its efficacy. An amount each of 52.9 mg of salvianolic acid B and 2.1 mg of rosmarinic acid was obtained from Salvia miltiorrhiza by the two-dimensional system in a single step. The purities of the targets were over 96% of salvianolic acid B and 74% of rosmarinic acid. An amount each of 4.6 mg of coptisine and 4.1 mg of berberine was obtained from Rhizoma coptidis each with 98% and 82% purity, respectively. The processing time was nearly 50% that of the multi-step method. These results indicate that the present method is a rapid and green way to harvest targets from medicinal plants in a single step.
Keywords: High-speed countercurrent chromatography, Expanded bed chromatography, Medicinal plant, Hyphenation
INTRODUCTION
Pure compounds prepared from medicinal plants are the major sources of standards for drug analysis, drug screening, and lead for structure modification. As most target compounds are generally present in extremely complex matrixes at low levels, the conventional steps often include extraction, concentration and other following purification steps. Hence the multi-step process for extraction and purification of components is usually time-consuming and results in loss of other potentially interesting components. Furthermore, those conventional methods such as silica gel column chromatography, ion exchange resin chromatography, and prep-HPLC possibly lead to degradation of compounds under some circumstance.
During the early 1970s, a new separation technique, named high-speed countercurrent chromatography (HSCCC) had been developed. 1 HSCCC is a unique liquid-liquid partition chromatography using no solid support in the system. Consequently, this liquid-liquid partition method provides advantages over the conventional liquid chromatographic techniques in that it eliminates the irreversible adsorption from the support and the loaded samples can be fully recovered from the column. 2-7 It is now accepted as an efficient preparative technique, and widely used for separation and purification of various natural and synthetic compounds.2,4, 8-12
In recent years, various online chromatographic coupling techniques have been developed. In comprehensive two-dimensional chromatography, the sample is subjected to two different separation methods. Since the first paper of multidimensional CCC has been published in 1998, several researches have been reported on hyphenated CCC with other analytical techniques. 13 Yun Wei’s work14 on multidimensional countercurrent chromatography (MDCCC) has demonstrated that MDCCC is a useful method for separating pure compounds from medicinal plants. Pan and co-workers15, 16 also have improved 2DCCC in such a way that the samples are introduced in a trapping column as the interface where the mobile phase of 1st dimension is replaced by that of the 2nd dimension. Shihua Wu17,18 has separated highly pure compounds by an online hybrid 2D CCC×LC system. Yanbin Lu and his colleagues19 have developed a 2DCCC for preparative isolation and purification of three phenyl flavonoids. Yuchi Zhang et al.20 have hyphenated accelerated solvent extraction to CCC which was successfully used for extraction and online isolation of saponins from Panax notoginseng. From the above reports, we can see that 2DCCC can largely improve separation capacity and simplify the process of sample preparation.
The above researches on hyphenation of CCC either with CCC or other techniques suggest its great potential for separation of complex samples. However, samples still need a pretreatment process before injection to CCC, which definitely leads to a loss of target compounds. To address this issue, expanded bed adsorption chromatography (EBAC) is hyphenated to CCC to simplify the pretreatment of samples in the present work.
EBAC is an alternative bioseparation technique that greatly reduces the purification stepts required when target molecules are directly captured from particle-containing feed stocks. 21-23 It has been reported in many research works about how to efficiently obtain compounds by EBAC. 24-26 EBAC is an efficient technique which integrates extraction and separation in a single step. HSCCC is a favorable method for purification of compounds from crude extract because no solid-supported matrix is used and almost 100% of the sample is recovered. If combining these two techniques, there is a possibility to achieve highly pure target compounds from the original material in a single step. There are either online or offline mode to couple EBAC to HSCCC. In the offline mode, the sample for HSCCC is prepared in multi-step. Firstly fractions collected from EBAC are concentrated, dried, redissolved in the mobile phase of HSCCC, and then injected into HSCCC. Therefore, this offline mode will inevitably cause loss of sample to some extent. In the online mode, EBAC and HSCCC are hyphenated via an interface, which will take the advantages of both techniques to realize extraction and purification of target compounds from medicinal plants in a single step.
In this work, Salvia miltiorrhiza and Rhizoma coptidis were applied to the hyphenated EBAC-CCC system. The main water-soluble components of S. miltiorrhiza are salvianolic acid B and rosmarinic acid (Figure 1). Among those, salvianolic acid B is the most abundant bioactive compound. However, it is degradable in aqueous solution at high temperature or high-pH conditions, especially in the multi-step separation process.27,28 All this should be considered for developing an effective approach to prepare and separate natural compounds in a simplified process. R. coptidis is a main source of coptisine and berberine (Figure 1) which have been proved to possess antioxidant activity, inhibit the growth of prokaryotic organisms29, and suppress acetyl cholinesterase. 30
Figure 1.
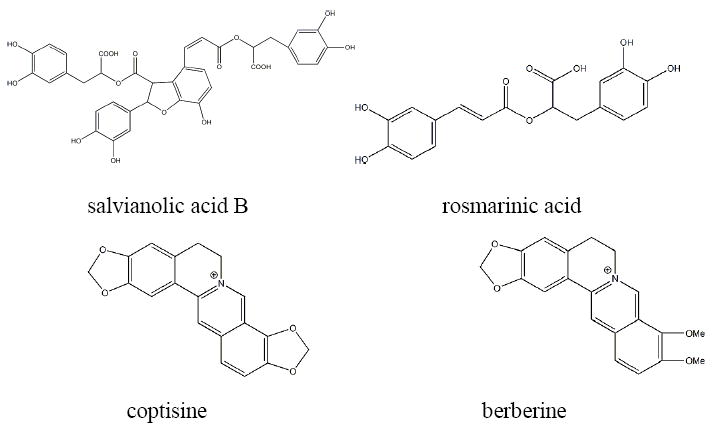
Chemical structures of the main compounds of Salvia miltiorrhiza and Rhizoma coptidis
The aim of this work is to establish an online system to obtain pure target compounds from medicinal plants in a single step. In the present research, an online two dimensional chromatographic system was developed based on the hyphenation of an EBAC column and a HSCCC system using a six-port injection valve as an interface.
EXPERIMENTAL SECTION
Chemicals
Chemicals for EBAC and HSCCC were of analytical grade and purchased from Titan chemical (Titan Tech, Shanghai). Acetonitrile used for HPLC analysis was of chromatographic grade (Burdick & Jackson, Ulsan, Korea). The macroporous resins of HZ-816 were purchased from the Huazhen Science and Technology Co., Ltd. (Shanghai, China).
Plant materials
S. miltiorrhiza and R. coptidis were both purchased from Shanghai Hongqiao Pharmaceutical Co., Ltd. (Shanghai, China). The content of salvianolic acid B in S. miltiorrhiza is about 2.0%, while those of coptisine and berberine in R. coptidis are about 2.1% and 6.5%.
Apparatus
The EBAC (30×2.6 cm, I.D.) was assembled with a UV detector (STI501, Surwit Technology Inc., Hangzhou, China) and a BT00-300M peristaltic pump (Longer Precision Pump Co., Ltd. Baoding, China). Extraction was conducted in an SCQ-5201E ultrasonic bath (Shengyan Ultrasonics Equipment Co., Ltd, Shanghai, China). Separations were performed on a TBE-300B HSCCC (Tauto Biotech, Shanghai, China) with three polytetrafluoroethylene coils (tubing I.D.=1.6 mm, column capacity=300 mL). The interface was a V-541 six-port switching valve (Tauto Biotech, Shanghai, China). The detector for HSCCC apparatus was a full wavelength of ultraviolet detector (Tauto Biotech, Shanghai, China). HPLC used for analysis was Agilent 1200 system (Agilent, US).
EBAC-HSCCC instrumentation
A schematic diagram of the EBAC-HSCCC setup is shown in Figure 2. The core part of the system is the interface unit consisting of a six-port switching valve with a sample loop, allowing an aliquot of effluent to directly transfer from the EBAC to HSCCC systems.
Figure 2.
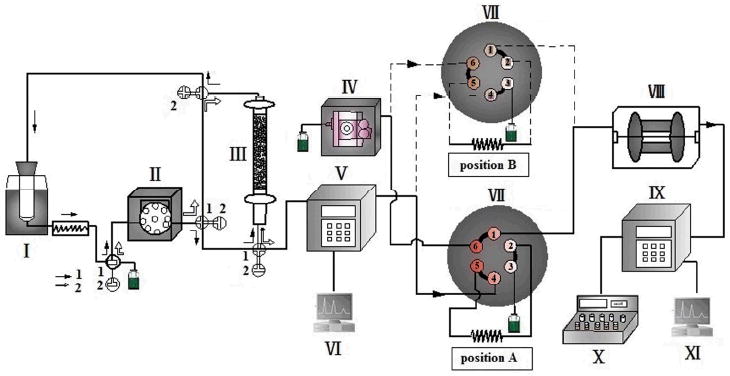
Schematic diagram of the online EBAC-HSCCC system. EBAC coupled to the HSCCC using a six-port injection valve equipped with sample loops of different volumes. (I ultrasonic bath; II peristaltic pump; III EBAC; IV constant flow pump; V UV detector; VI computer; VII six-port injection valve; VIII CCC; IX UV detector; X fraction collector; XI computer)
For extraction step, an expanded bed (I.D.=26 mm) is integrated with a dynamic extraction column (I.D.=50 mm) equipped with an ultrasonic bath (I) outside, as shown in Figure 2. An amount of the plant material is put into the dynamic extraction column along with water. Then, the expanded bed column (III) packed with macroporous resins is expanded to a certain expansion ratio. The target compounds extracted from the dynamic extraction column are continuously adsorbed onto the expanded bed column by circulating the solvent through the loop with pump II as shown by a set of solid arrows. When the extraction and adsorption processes are completed, the expanded bed is eluted by water and ethanol aqueous solution as shown by the open arrows with pump (II). At this moment, the switching valve (VII) is at the “load” position (A) allowing effluent pass through the monitor V, the injection loop, and then to the fraction collector (not shown), as indicated by the solid line. Meanwhile the HSCCC column is filled with stationary phase and equilibrated. When the target peak starts to emerge in the UV chromatogram (monitor V), the “load” position (A) of sliding valve VII is held for a few minutes to ensure that the loop is filled with the sample and then switched to the “injection” position (B). Afterwards the target compounds eluted from the 1st dimension (EBAC) are directly transferred to the second dimension system (HSCCC) by pump IV through the dashed line. In this way the effluents from the expanded bed column (III) are subjected to further separation and purification in the HSCCC system and eluted through the UV monitor (IX) into the fraction collector (X).
EBAC extraction process
The EBAC operation process was based on a previous report. 23 For S. miltiorrhiza, 37.0 g of the material was put into the dynamic extraction column along with 250 mL of water, while 70 mL of HZ-816 macroporous resins were packed into the expanded bed with an expansion ratio of 1.4. The ultrasonic extraction was carried out for 3 h, while the target compounds extracted were continuously adsorbed onto the reins in the expanded bed column. When the adsorption process was completed, the expanded bed was eluted with 5 bed volumes (BVs) of water and 5 BVs of 30% (v/v) ethanol aqueous solution at a flow rate of 2.4 BV/h.
For R. coptidis, 10.0 g of the material was put into the dynamic extraction column along with 150 mL of water, while 60 mL of HZ-816 macroporous resins were packed into the expanded bed with an expansion ratio of 1.6. The ultrasonic extraction was carried out for 2 h and the target compounds were directly adsorbed onto the resins packed in the expanded bed column. When the adsorption process was completed, the expanded bed was eluted with 2 BVs of water and 5 BVs of 50% ethanol aqueous solution at 2.0 BV/h.
Solvent system selection for HSCCC separation
The first step of HSCCC separation is selection of the biphasic solvent system and determination of the partition coefficients (K) of the targets. For S. miltiorrhiza, various solvent systems composed of n-hexane-ethyl acetate-methanol-acetic acid-water and n-hexane-ethyl acetate-ethanol-acetic acid-water with different volume ratios were prepared for test. An equal volume of the upper and lower phases of a solvent system were put into a test tube. Then, 3 mg of the crude sample was added to the test tube and thoroughly mixed. After the sample was dissolved, both phases were each transferred to separate vials and then dried by nitrogen, and then 500 μl of 50% methanol was added to dissolve the residues. Afterwards, both upper and lower phases were analyzed by HPLC. The partition coefficient is defined as K=Aupper/Alower, where Aupper is the HPLC peak area of the target in the upper phase and Alower is that in the lower phase.
For R. coptidis various solvent systems with different ratios of methyl tert.-butyl ether (MTBE)-acetonitrile (ACN)-H2O, MTBE-BuOH-H2O and MTBE-H2O were tested. The partition coefficients (K) for each sample in these two-phase solvent systems were determined using the conventional test tube method in acidic (Kacid) and basic (Kbase) conditions using the same method stated above.
HSCCC separation procedure
At the beginning of the separation of salvinolic acids from S. miltiorrhiza, the column was filled with the stationary phase (the upper phase of n-hexane-ethyl acetate-methanol-acetic acid-water). Then, the mobile phase (the lower phase of the system) was pumped into the coil at 2 mL/min from the head toward the tail with a rotational speed of 850 rpm at 25°C. When hydrodynamic equilibrium was established, the crude sample of S. miltiorrhiza was injected into the column via a sample loop. The effluent was monitored by an online UV detector.
When the separation of alkaloids from R. coptidis was performed on CCC, the column was first filled with the stationary phase (the upper phase of MTBE-n-butanol- ethyl acetate-water), followed by injection of the crude sample extract. The mobile phase (the lower phase of MTBE-n-butanol- ethyl acetate-water) was pumped into the column at 2.0 mL/min in the head to tail mode at 850 rpm. The fractions were monitored by a UV detector at 345 nm. The pH value of each fraction was measured with a pH meter. All the fractions were analyzed by HPLC after dried by a rotary vaporization under reduced pressure.
HPLC analysis
The HPLC analysis for salvianolic acids was carried out on a C18 column (Alltech, 250 mm×4.6 mm, 5 μm) at 25°C with an injection volume of 20 μl. The mobile phase was composed of water-acetonitrile-formic acid (A, 90: 10: 0.4, v/v/v) and acetonitrile (B). The gradient elution was started at 0% B, rising to 30% B after 40 min, at a flow rate of 1.0 mL/min. The chromatogram was recorded at the wavelength of 280 nm.31
For analysis of alkaloids, the separation was performed on a C18 column (Agilent, 150 mm×4.6 mm, 5 μm). The mobile phase was acetonitrile-0.05 mol/L KH2PO4 (aq) (60:50), adding 0.4 g of sodium dodecyl sulfate per 100 mL of mobile phase, then the pH was adjusted to 4.0 with H3PO4. The analysis was carried out under isocratic conditions with a flow rate of 0.8 mL/min at the detection wavelength of 345 nm.
Comparison with offline and online modes of EBAC-HSCCC
In the offline mode, the medicinal plant was extracted with H2O. Then the extraction solution was centrifuged, filtrated, and concentrated under reduced pressure to remove the solvent to prepare the sample for macroporous resin column separation. The effluentwas concentrated and dried. Then the sample was redissolved in the stationary phase of the CCC solvent system.
RESULTS AND DISCUSSION
Optimization of extraction and separation conditions on EBAC
Expansion ratio is a parameter used to measure the bed expansion. It is defined as R=Hexp/H0, where R is the expansion ratio, Hexp is expanded bed height, H0 is initial bed height. Expansion ratio optimization for the expanded bed operation focuses on the expansion effect of resins on extraction and separation. Different expansion ratios were tested within the range of flow rates from 0 to 16.8 mL/min. The maximum target recovery was achieved at the expansion ratio of 1.4. Extraction time optimization was undertaken to investigate the end point of extraction. After 180 min of extraction, 95% of the target had been extracted. Therefore, the extraction was considered to be terminated at 180 min.
Eluent, flow rate of eluting, and elution volume have great influence on the separation on EBAC. The flow rate of the eluent and elution volume optimization for EBAC was conducted on a packed column. Considering solvent consumption, an extracting solution volume of 5 BVs was chosen. The flow rate of 2.4 BV/h gave a relatively high desorption ratio. Considering the compatibility of effluent of EBAC and the solvent system of HSCCC, the influence of relative concentrations of ethanol to the solvent system of HSCCC was investigated. Eventually 30% ethanol was found to be optimal for the separation of S. miltiorrhiza on EBAC. To avoid peak tailing, 50% ethanol was selected for R. coptidis. A relatively high recovery was achieved at the expansion ratio of 1.6 for R. coptidis. The flow rate of 2 BV/h and elution volume of 5 BVs gave a relatively high desorption ratio.
Figure 3 shows the elution curves of salvianolic acids and alkaloids on EBAC. We collected heart-cutting fractions shaded in Figure 3 as the sample for the 2nd dimensional HSCCC.
Figure 3.
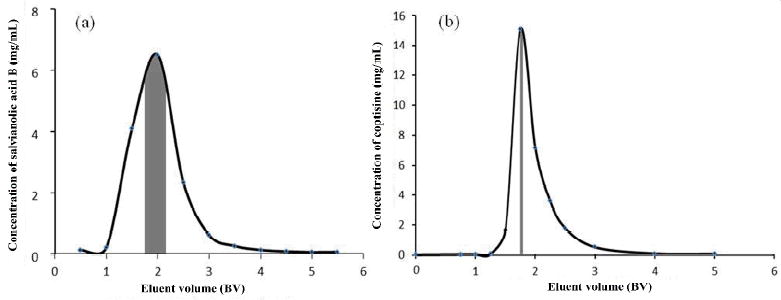
The elution curves of salvianolc acid B (a) and coptisine (b) on EBAC
Solvent system selection for HSCCC separation
The systems of n-hexane-ethyl acetate-methanol-acetic acid-water and n-hexane-ethyl acetate-ethanol-acetic acid-water at various volume ratios were investigated for separation of salvianolic acids from S. Miltiorrhiza. The results of the determinations of K values are listed in Table 1. The settling time of the systems No. 1-3 was shortened by increasing the proportion of water, meantime the KB values were increased. The shorter the settling time of solvent system was, the higher the stationary phase would be retained. So from the view of stationary phase retention, the solvent system with a large proportion of the water should be selected. Acetic acid was chosen for adjusting the pH of the lower phase to prevent the targets from degradation. In the solvent systems of No. 3-5 in Table 1, as the ratio of acetic acid is increased, K value is gradually reduced, which may lead to a poor peak resolution. So the ratio of acetic acid should not be too high. By raising the proportion of ethanol, the settling time will become longer, and the K value will be decreased. Therefore, we chose n-hexane-ethyl acetate-ethanol-acetic acid-water (1:5:1.75:1:7, v/v/v/v/v) as the solvent system for separation of salvianolic acids.
Table 1.
Partition coefficient of the targets in S. miltiorrhiza in different solvent systems
| No. | Solvent system | Ratio (v/v) | K1 | K2 | K3 | K4 |
|---|---|---|---|---|---|---|
| 1 | n-hexane-ethyl acetate-methanol-acetic acid-water | 1:6:1.5:1.5:6 | 0.51 | 0.97 | 1.67 | 1.72 |
| 2 | 1:6:1.5:1.5:4 | 0.46 | 0.52 | 1.03 | 1.19 | |
| 3 | 1:6:1.5:1.5:8 | 0.70 | 1.51 | 2.24 | 2.61 | |
| 4 | 1:6:1.5:2.5:8 | 0.30 | 0.39 | 0.77 | 0.84 | |
| 5 | 1:6:1.5:1:8 | 1.49 | 1.81 | 2.42 | 3.36 | |
| 6 | 1:5.5:1.5:1:7 | 0.95 | 1.77 | 2.21 | 2.87 | |
| 7 | 1:5:1.5:1:7 | 0.89 | 1.35 | 1.97 | 2.79 | |
|
| ||||||
| 8 | n-hexane-ethyl acetate-ethanol-acetic acid-water | 1:5:1.5:1:7 | 1.24 | 1.98 | 2.09 | 3.51 |
| 9 | 1:5:1.75 :1:7 | 1.15 | 1.66 | 1.90 | 2.89 | |
| 10 | 1:5:2:1:7 | 1.00 | 1.33 | 1.71 | 2.45 | |
Note: K was calculated as HPLC peak area percentage in the upper phase divided by that in the lower phase; K1 is for salvianolic acid G, K2 is for salvianolic acid B, K3 is for protocatechualdehyde, K4 is for rosmarinic acid.
For the separation of alkaloids from R. coptidis, different ratios of retainer and eluter were tested. The K values of the different solvent systems were measured. First, different polar solvent systems such as methyl tert.-butyl ether (MTBE)-ACN-H2O, MTBE-BuOH-H2O, MTBE-H2O were tested, but the K values of the compounds were not suitable for the separation on HSCCC. By adding triethylamine (TEA, 25 mM) to the upper organic stationary phase as a retainer and hydrochloric acid (HCl, 10 mM) to the aqueous mobile phase as an eluter, a two-phase solvent system composed of MTBE-n-BuOH-EtOAc-water (2:2:1:8, v/v/v/v) was explored. The result is listed in Table 2. As the concentration of retainer was increased, Kbase became higher. Reasonable K values (K1=0.80, k2=0.15) were achieved when the concentration of retainer was at 25 mM. Therefore, we chose MTBE-BuOH-EtOAc-H2O (2:2:1:8) as the solvent system, 25 mM of TEA as the retainer, and 10 mM of HCl as the eluter.
Table 2.
Partition coefficient of the targets in R. Coptidis in different solvent systems
| No. | solvent system(v/v) | retainer/eluter | K1 | K2 | |
|---|---|---|---|---|---|
| 1 | MTBE/BuOH/EtOAc/H2O(2:2:1:8) | 10mM (retainer) | Kbase | 0.47 | 0.14 |
| 2 | 15mM (retainer) | Kbase | 0.78 | 0.17 | |
| 3 | 20mM (retainer) | Kbase | 0.78 | 0.17 | |
| 4 | 25mM (retainer) | Kbase | 0.80 | 0.15 | |
| 5 | 30mM (retainer) | Kbase | 0.92 | 0.17 | |
| 6 | 10mM (eluter) | Kacid | 0.04 | 0.06 |
Note: K was calculated as HPLC peak area percentage in the upper phase divided by that in the lower phase; K1 is for coptisine, K2 is for berberine.
Application of the online EBAC-HSCCC system
In the online mode of EBAC-HSCCC, since the eluant containing ethanol from EBAC as a sample was charged to the HSCCC column, the sample will affect the equilibrium of the solvent system on HSCCC. In order to achieve the compatibility between the solvents used in EBAC and HSCCC and to minimize the impact of the solvent in sample on the hydrodynamic equilibrium of the solvent system of HSCCC, the sample volume to the HSCCC column was optimized.
When the separation of salvianolic acids from S. Miltiorrhiza was conducted on HSCCC using the above optimized solvent system, the mobile phase was mostly composed of water and ethanol, while the stationary phase mainly contained n-hexane and ethyl acetate. When the sample solution containing ethanol was injected to HSCCC, the hydrodynamic equilibrium of the column would be affected. To evaluate the possibility of direct injection of effluent from EBAC into the HSCCC column, 0.1 g of crude extract obtained from EBAC was dissolved in 3 mL of 30% ethanol aqueous solution and injected into the HSCCC column in an offline mode. The separation yielded 12.5 mg of salvianolic acid B at 91.0% purity, which indicated that sample in ethanol aqueous solution can be directly introduced into the CCC system with a minimum effect on separation. The similar investigation was undertaken to the separation of alkaloids from R. coptidis. The result showed that ethanol in sample solution again did not affect the separation of alkaloids.
Then, the effect of injection volume on the separation in an online mode was examined. The effluent of 8 mL to 26 mL from EBAC was directly injected into the HSCCC column. Figure 4 (a) shows the HSCCC chromatograms of separation of salvianolic acids with different injection volumes. When 8 mL of sample was injected, 30.8 mg of salvianolic acid B was harvested at purity of 96% and 1.6 mg of rosmarinic acid with 83% pure. Injection of 13 mL of sample solution yielded 52.9 mg of salvianolic acid B with 96% of purity and 2.1 mg rosmarinic acid with 74% purity. And 108.5 mg of salvianolic acid B with 92% of purity and 2.8 mg of rosmarinic acid with 63% were obtained from 26 mL effluent injection. Based on the above results, 13 mL injection volume was selected as the injection volume.
Figure 4.
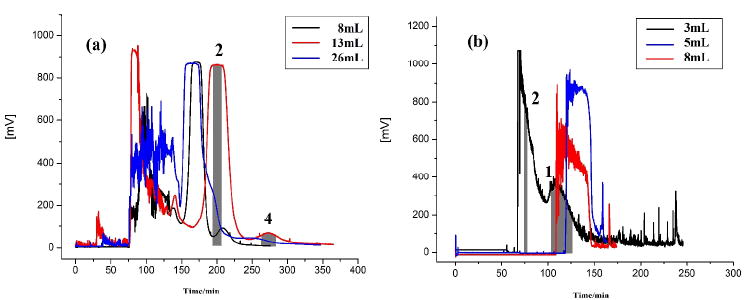
HSCCC chromatograms of different injection volumes. Solvent system (a): n-hexane-EtOAc-ethanol-acetic acid-water (1:5:1.75:1:7, v/v/v/v/v); flow rate: 2.0 mL/min; rotationary speed: 850 rpm; detection wavelength: 280 nm; temperature: 25°C; stationary phase retention: 45%. solvent system b: MTBE-n-butyl alcohol-ethyl acetate-water (2:2:1:8, v/v/v/v), 25 mM triethylamine in upper organic stationary-phase and 10 mM hydrochloric acid in lower aqueous phase; flow rate: 2.0 mL/min; revolution speed: 850 rpm; detection wavelength: 345 nm; column temperature: 25°C; stationary phase retention: 50%.
Figure 4 (b) shows the HSCCC chromatogram of the extract from R. coptidis with different injection volumes. Different injection volumes were tested for the separation of alkaloids from R. coptidis. It was found that 3 mL was a suitable injection volume where 4.6 mg of coptisine was yielded with 98% of purity. When the sample volume was increased to 5 mL, the stationary phase retention was decreased to 20% and the purity of the target was less than 50%. When the sample volume increased to 8 mL, the sample was carried over with the solvent front and there was nearly no retention of the stationary phase.
Figure 5 shows the HPLC chromatograms of the decoction, effluent of EBAC, rosmarinic acid, and salvianolic acid. From these chromatograms, we can see that there are more peaks in the early stage of the chromatogram of the decotion than that of the eluent of EBAC. This was due to the degradation of salvianolic acids at high temperature when the decoction was prepared. 27 By comparison with HPLC fingerprint chromatogram of S. miltiorrhiza, they should be the decomposition products from salvianolic acid B. Due to the closed online EBAC-HSCCC system, it needed less manual operation, less amount of time, and solvent consumption, which resulted in higher recovery and less degradation.
Figure 5.
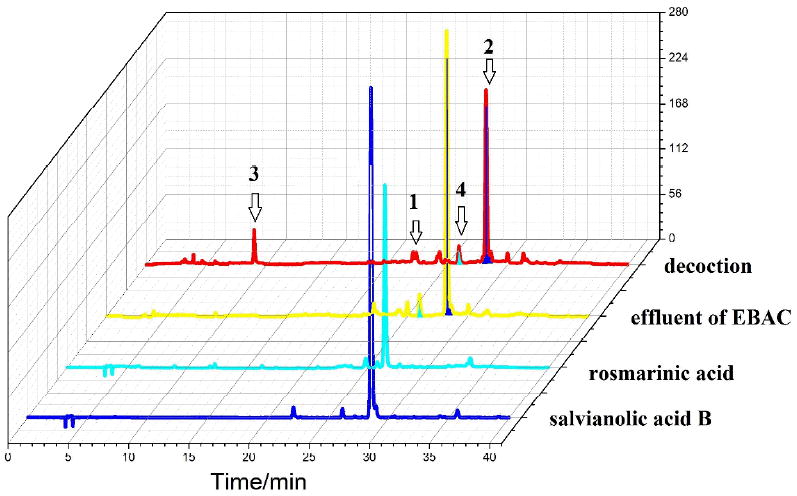
HPLC chromatograms of the decoction of Salvia miltiorrhiza, effluent of EBAC, purified rosmarinic acid, and salvianolic acid B.
Comparison between online EBAC-HSCCC method and the conventional method
The comparison of online EBAC-HSCCC method and the conventional multi-step method in terms of processing time, solvent consumption, and the purity of targets is listed in Table 3. The consumptions of time and solvents were estimated by summing those in each step. In the offline mode, the consumed time and solvent in decoction, filtration, concentration, separation on resin column, freeze-drying, and separation on HSCCC were covered. 32, 33 In the online mode, the time and solvent used on EBAC and HSCCC were counted. From Table 3, the time of online separation of salvianolic acid is reduced from 28 h to 12 h and that for R. coptidis is 5 h shorter. Thus, the hyphenation system can save almost 50% of separation time. Furthermore, the consumption of solvent is also reduced.
Table 3.
The comparison between online EBAC-HSCCC and the traditional method
| salvianolic acid B | rosmarinic acid | coptisine | berberine | ||
|---|---|---|---|---|---|
| Online | Time (h) | 12 | 7 | ||
| Solvent amount (L) | 1.9 | 1.3 | |||
| Purity (%) | 96 | 74 | 98 | 82 | |
| Offline | Time (h) | 28 | 12 | ||
| Solvent amount (L) | 2.1 | 1.6 | |||
| Purity (%) | 96 | 88 | 98 | 80 | |
The interface and compatibility of solvent systems
To build an online hyphenated chromatography system, the issues of interface selection and solvents compatibility should be addressed. There are usually various sets can play the role of interface. These are trapping column, parallel second dimension columns, stop-flow interface, and vacuum-assisted evaporation interface, etc. Among them, the injection sample loop is the simplest interface which can be easily operated. In this study, a six-port injection valve acted as the interface in which the effluent from EBAC was transferred from the 1st dimension to the 2nd dimension.
For HSCCC, the sample is usually dissolved in both mobile and stationary phases. However, in this study the effluent of the EBAC containing ethanol aqueous was directly used as the sample for HSCCC separation. Since the compositions of sample are same, we only focused on the sample volume effect on separation. Figure 4 shows the HSCCC chromatograms of different inject volumes performed on EBAC-HSCCC system. The results showed that the ethanol effluent made very different impacts on the two solvent systems for the separation of S. miltiorrhiza and R. coptidis.
The separation results of different injection volumes are listed in Table 4. We have probed the effect of different injection volumes on purity, yield, and stationary phase retention (Sf). The less sample was injected, the purer target was harvested. The more sample was injected, the less stationary phase was retained. Large volume injection led to loss of extra stationary phase especially in the separation of alkaloids from R. coptidis. Pure coptisine could not be obtained when 5 mL or 8 mL of sample was injected. We concluded that the solvent in the effluent affected the Sf. and purity of targets. Then we have investigated the effect of other solvents on stationary phase retention and the result is listed in Table 5.
Table 4.
Separation results of different injection volumes
| Injection volumes (mL) | Purity (%) | Yield (mg) | Sf (%) | |||
|---|---|---|---|---|---|---|
| salvia miltiorrhiza | salvianolic acid B | rosmarinic acid | salvianolic acid B | rosmarinic acid | ||
| 8 | 96 | 83 | 30.8 | 1.6 | 35 | |
| 13 | 96 | 74 | 52.9 | 2.1 | 30 | |
| 26 | 92 | 63 | 108.5 | 2.8 | 10 | |
| Rhizoma coptidis | coptisine | berberine | coptisine | berberine | ||
| 3 | 98 | 82 | 4.6 | 4.1 | 50 | |
| 5 | - | - | - | - | 20 | |
| 8 | - | - | - | - | 0 | |
Table 5.
Effect of different sample solvent for HSCCC solvent system on Sf (%)
| 30% ethanol | H2O | 30% methanol | 30% acetonitrile | |
|---|---|---|---|---|
| Salvia miltiorrhiza | 31 | 45 | 11 | 10 |
| n-butyl alcohol | H2O | 30% acetone | 30% methanol | |
| Rhizoma coptidis | 70 | 73 | 57 | 23 |
Higher injection volumes lead to more loss of stationary phase especially for the separation of Rhizoma coptidis. It failed to produce a pure coptisine from the separation by 5 and 8 mL of injection volumes. However, the impact for Salvia miltiorrhiza is less. We considered that this phenomenon may be related to the solvent system for Salvia miltiorrhiza containing ethanol. Then, we tested some other 3 mL solvents (BuOH, H2O, 30% acetone, 30% methanol) for Rhizoma coptidis and some 5 mL solvents (30% ethanol, H2O, 30% methanol, 30% acetonitrile) for Salvia miltiorrhiza, where Sf was used as the index to evaluate the effect of different solvents.
The results are shown in Table 5 which indicates that the stationary phase retention rate of the solvent system (Sf) which contains the same solvent with the sample solution is higher than that of the solvent system which does not contain the sample solvent. Therefore, the1st dimensional elution solvent system that shares the solvents with the 2nd dimensional solvent system is suitable for the interface for the hyphenation. It can be a tip for future researches using EBAC-HSCCC to separate various other compounds.
CONCLUSIONS
The present study presents the successful development of online hyphenation of EBAC to HSCCC for direct extraction and purification of ingredients from medicinal plants. EBAC and HSCCC were interfaced via an injection valve which was capable of transferring effluents from EBAC to HSCCC. This setup was effectively used for the separation of salvianolic acids from S. miltiorrhiza and alkaloids from R. coptidis. Salvianolic acid B, rosmarinic acid, coptisine, and berberine could be recovered at high purity. In a typical run, 52.9 mg of 96% pure salvianolic acid B and 2.1 mg of 74% pure rosmarinic acid were harvested. While in another run, 4.6 mg of 98% pure coptisine and 4.1 mg of 82% pure berberine were recovered. These results clearly indicate that this EBAC-HSCCC system is an efficient andhigh-yieldalternative technique which can be applied for extraction and purification of bioactive components from various medicinal plants.
Figure 6.
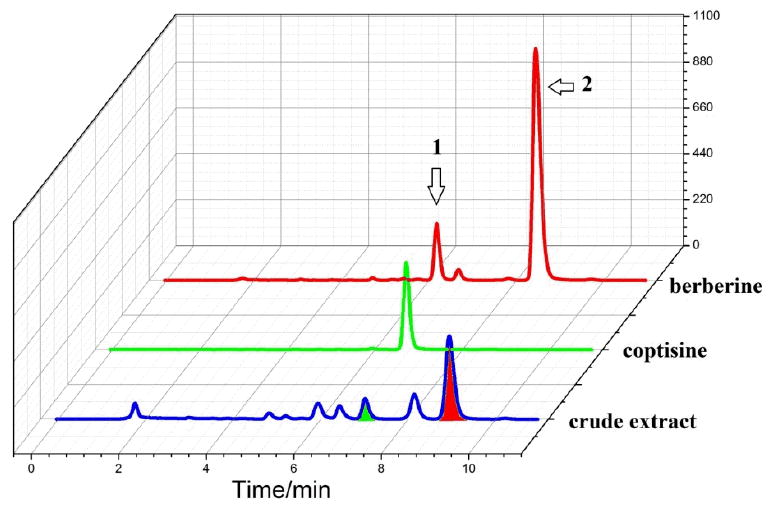
HPLC chromatograms of the crude extract, purified coptisine and berberine.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 81273481 and 21006023), the Fundamental Research Funds from the Central Universities, and National Science and Technology Major Project for Significant New Drugs Development (No. 2013ZX09507005).
References
- 1.Ito Yoichiro, Bowman Robert L. Anal Chem. 1971;43:69A–75A. [Google Scholar]
- 2.Sutherland Ian A, Fisher Derek. J Chromatogr A. 2009;1216:740–753. doi: 10.1016/j.chroma.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Angel MJ, Carda-Broch S, Levet A, Berthod A. J Chromatogr A. 2011;1218:6044–6052. doi: 10.1016/j.chroma.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Ito Yoichiro. J Chromatogr A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Michel Thomas, Destandau Emilie, Elfakir Claire. J Chromatogr A. 2011;1218:6173–6178. doi: 10.1016/j.chroma.2011.01.070. [DOI] [PubMed] [Google Scholar]
- 6.Ito Yoichiro, Bowman Robert L. Science. 1970;16:281–283. doi: 10.1126/science.167.3916.281. [DOI] [PubMed] [Google Scholar]
- 7.Ito Yoichiro. Nature. 1987;326:419–420. doi: 10.1038/326419a0. [DOI] [PubMed] [Google Scholar]
- 8.Franco Pilar, Blanc Javier, Oberleitner Wolfgang R, Maier Norbert M, Lindner Wolfgang, Minguillon Cristina. Anal Chem. 2002;74:4175–4183. doi: 10.1021/ac020209q. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Kohei, Kuribayashi Hiroaki, Watanabe Haruna, Shimasaki Tomomi, Azuma Kenzaburo, Horie Yohei, Saitoh Kazunori, Saito Shingo, Shibukawa Masami. Anal Chem. 2013;85:978–984. doi: 10.1021/ac302546s. [DOI] [PubMed] [Google Scholar]
- 10.Weisz Adrian, Mazzola Eugene P, Ito Yoichiro. J Chromatogr A. 2011;1218:8249–8254. doi: 10.1016/j.chroma.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisz Adrian, Witten Jacob J, Zeng Yun, Mazzola Eugene P, Ito Yoichiro. J Chromatogr A. 2012;1237:106–114. doi: 10.1016/j.chroma.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisz Adrian, Scher Alan L, Shinomiya Kazufusa, Fales Henry M, Ito Yoichiro. J Am Chem Soc. 1994;116:704–708. [Google Scholar]
- 13.Yang Fuquan, Quan Jiang, Zhang Tian You, Ito Yoichiro. J Chromatogr A. 1998;803:298–301. doi: 10.1016/s0021-9673(97)01273-9. [DOI] [PubMed] [Google Scholar]
- 14.Wei Yun, Ito Yoichiro. J Chromatogr A. 2006;1115:112–117. doi: 10.1016/j.chroma.2006.02.081. [DOI] [PubMed] [Google Scholar]
- 15.Lu Yanbin, Hu Ruilin, Pan Yuanjiang. Anal Chem. 2010;82:3081–3085. doi: 10.1021/ac100121j. [DOI] [PubMed] [Google Scholar]
- 16.Hu Ruilin, Dai Xiaojing, Xu Xia, Sun Cuirong, Pan Yuanjiang. J Chromatogr A. 2011;1218:6085–6091. doi: 10.1016/j.chroma.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 17.Guo Mengzhe, Liang Junling, Wu Shihua. J Chromatogr A. 2010;1217:5398–5406. doi: 10.1016/j.chroma.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Liang Junling, Yang Zhi, Cao Xiaoji, Wu Bing, Wu Shihua. J Chromatogr A. 2011;1218:6191–6199. doi: 10.1016/j.chroma.2010.10.092. [DOI] [PubMed] [Google Scholar]
- 19.Lu Yanbin, Sun Cuirong, Wang Yu, Pan Yuanjiang. J Chromatogr A. 2007;1151:31–36. doi: 10.1016/j.chroma.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Yuchi, Liu Chunming, Qi Yanjuan, Li Sainan, Wang Jing. Sep Purif Technol. 2013;106:82–89. [Google Scholar]
- 21.Li Jing, Chase Howard A. J Chromatogr A. 2009;1216:8730–8740. doi: 10.1016/j.chroma.2009.02.092. [DOI] [PubMed] [Google Scholar]
- 22.Tolner Berend, Smith Lisa, Begent Richard HJ, Chester KA. Nat Protoc. 2006;1:1213–1222. doi: 10.1038/nprot.2006.127. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Min, Yang Huihua, Chen Xian, Zhou Yongchuan, Zhang Hongyang, Wang Yuerong, Hu Ping. Sep Purif Technol. 2011;80:677–682. [Google Scholar]
- 24.Bermejo Ruperto, Ruiz Esmer alda, Ramos Amparo, Acien FGabriel. Sep Sci Technol. 2013;48:1913–1922. [Google Scholar]
- 25.Ramos Amparo, Acien F Gabriel, Fernandez-Sevilla Jose M, Gonzalez Cynthia V, Bermejo Ruperto. J Chem Technol Biotechnol. 2010;85:783–792. [Google Scholar]
- 26.Yap Wei Boon, Tey Beng Ti, Mohamed Alitheen Noorjahan Banu, Tan Wen Siang. J Chromatogr A. 2010;1217:3473–3480. doi: 10.1016/j.chroma.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Guo Yongxue, Zhang Daijia, Wang Hui, Xiu Zhilong, Wang Longxing, Xiao Hongbin. J Pharm Biomed Anal. 2007;43:435–439. doi: 10.1016/j.jpba.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Guo Yongxue, Xiu Zhilong, Zhang Daijia, Wang Hui, Wang Longxing, Xiao Hongbin. J Pharm Biomed Anal. 2007;43:1249–1255. doi: 10.1016/j.jpba.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Yan Dan, Jin Cheng, Xiao Xiaohe, Dong Xiaoping. J Biochem Bioph Meth. 2008;70:845–849. doi: 10.1016/j.jbbm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Xiao Haitao, Peng Jiao, Liang Yan, Yang Jie, Bai Xue, Hao Xiaoyan, Yang Fumei, Sun Qianyun. Nat Prod Res. 2011;25:1418–1422. doi: 10.1080/14786410802496911. [DOI] [PubMed] [Google Scholar]
- 31.Hu Ping, Luo Guoan, Zhao Zhongzhen, Jiang Zhihong. Chem Pharm Bull. 2005;53:481–486. doi: 10.1248/cpb.53.481. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Min, Ignatova Svetlana, Liang Qionglin, Jun FrankWu, Sutherland Ian, Wang Yiming, Luo Guoan. J Chromatogr A. 2009;1216:3869–3873. doi: 10.1016/j.chroma.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Min, Ignatova Svetlana, Hu Ping, Liang Qionglin, Wang Yiming, Sutherland Ian, Jun Frank Wu, Luo Guoan. J Chromatogr A. 2011;1217:6031–6037. doi: 10.1016/j.chroma.2010.12.118. [DOI] [PubMed] [Google Scholar]


