Abstract
Astrocytes have been shown to protect neurons and increase their survival in many pathological settings. Manipulating astrocyte functions is thus an important strategy to enhance neuronal survival and improve outcome following cerebral ischemia. Increasing evidence supports the involvement of microRNAs (miRNA), some of them being astrocyte-enriched, in the regulation of cerebral ischemia. This mini review will focus on several recently reported astrocyte-enriched miRNAs (miR-181 and miR-29 families and miR-146a), their validated targets, regional expression and effects on outcome after cerebral ischemia.
Keywords: Astrocyte, microRNA, miR-181, miR-29, miR-146a, cerebral ischemia
1. Introduction
Cerebral ischemia is a key pathological event in several disease states; stroke, cardiac arrest and resuscitation, and head trauma being the most common. Stroke is one of the leading causes of death worldwide and the leading cause of long-term neurological disability. Despite many clinical stroke trials being conducted, the only efficacious treatment to date is thrombolysis [5]. Similarly, clinically effective treatment for the cerebral injury resulting from cardiac arrest and resuscitation is limited to hypothermia [4, 56]. Lack of consideration of the role of astrocytes, the importance of cell-cell interactions, the complex interplay among signaling pathways, and poorly defined treatment windows for specific targets are thought to be factors in the clinical failure of many potential neuroprotective strategies.
Astrocytes play many key roles both in normal and pathological central nervous system functioning. Astrocytes are central to K+ homeostasis, neurotransmitter uptake, synapse formation and regulation of the blood brain barrier. Astrocytes are the most abundant brain cell type, and in addition to multiple important homeostatic roles, they are essential to central nervous system development, helping to organize the structural architecture of the brain and modulate neuronal plasticity (for recent reviews see [3, 12, 54]).
1.1 Astrocytes as targets for protection
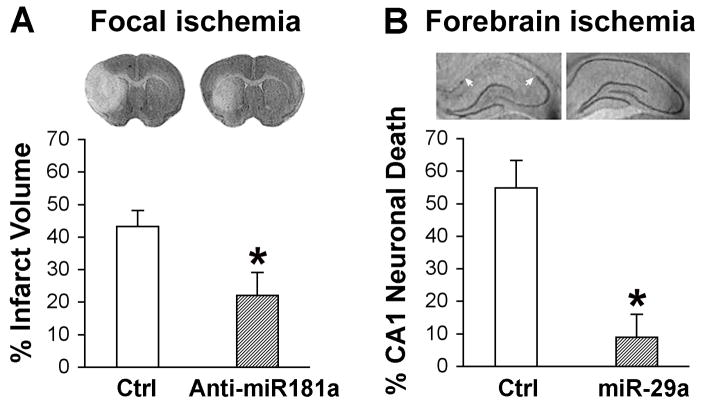
In focal cerebral ischemia, modeled commonly in rodents as transient middle cerebral artery occlusion (MCAO), the neurons die within hours in the center of the ischemic territory (ischemic core) forming the initial area of infarction (see image in Fig. 1 A) while the neurons in the adjacent peri-ischemic (penumbral) area may either survive via induction of pro-survival signaling pathways, or die at a later period of reperfusion (delayed neuronal death) via initiation of pro-apoptotic pathways [16]. Brief global cerebral ischemia, as seen with cardiac arrest and resuscitation, and modeled in rodents as forebrain ischemia or four vessel occlusion, causes delayed loss of neurons, with cornu ammonis 1 (CA1) pyramidal neurons being most vulnerable (image in Fig. 1B), whereas the nearby dentate gyrus (DG) neurons are relatively resistant.
Figure 1.
Effect of miR-181a and miR-29a on focal and global cerebral ischemia. A. Representative cresyl violet-stained coronal sections demonstrate a decreased infarct size in miR-181a down-regulated brain compared with brain treated with miR-181 mismatch (Ctrl) subjected to 1 h MCAO and 48 h reperfusion. The bar graph shows quantitation of infarct size (modified from Fig. 5 in [42]). B. Representative cresyl violet-stained coronal hippocampal sections demonstrate selective loss of CA1 neurons (between white arrows) at 6 days reperfusion after 10 min forebrain ischemia in control ischemic animals and protection with miR-29a overexpression. Quantitation of CA1 neuronal loss is shown in the bar graph (modified from Fig. 6 in [46]).
It has been shown that loss of key astrocytic proteins and likely astrocyte cell death precedes delayed neuronal death in permanent focal [31] but not in global [45] cerebral ischemia. Astrocytic glial fibrillary acidic protein (GFAP) messenger RNA (mRNA) declined more quickly than neuronal glucose transporter 3 (GLUT3) mRNA in the ischemic core [31]. Our group showed that loss of glutamate transport activity and immunoreactivity for the astrocyte-specific glutamate transporter -1 (GLT-1) in astrocytes occurred at early reperfusion times, hours to days before the death of CA1 neurons [45].
Recently several strategies for protecting neurons by manipulating astrocytes have been shown to be effective, and the roles of astrocytes as both neuroprotective and neuron endangering in stroke were reviewed recently [66]. Astrocytes have been shown to protect neurons from oxidative stress by increasing neuronal glutathione levels, and by the interleukin 6 (IL6) pathway [19, 37, 50], and preconditioned astrocytes were shown to release protective factors, including erythropoietin, that protected neurons [60]. In vivo stimulation of the astrocyte specific purinergic P2Y1 receptor protected neurons from photothrombotic ischemia [67]. Glial supporting cells were recently shown to protect neighboring hair cell neurons by release of heat shock protein 70 (Hsp70) [33], and induction of brain-derived neurotrophic factor (BDNF) in astrocytes by galectin-1 reduced neuronal apoptosis in the ischemic boundary zone and improved functional recovery [49]. Ceftriaxone treatment, which induces GLT-1 expression, reduces CA1 delayed neuronal injury in hippocampal slice culture and in transient forebrain ischemia [45]. Finally, protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism [34]. Thus multiple strategies targeting astrocytes have been found effective in protecting neurons, encompassing several distinct mechanisms.
Enhancing astrocyte resistance to ischemic stress by overexpressing a heat shock protein or an antioxidant enzyme resulted in improved survival of CA1 neurons following forebrain ischemia [61]. These protective proteins, HSP72, or mitochondrial superoxide dismutase, were genetically targeted for expression in astrocytes using the astrocyte-specific human GFAP promoter. In both cases we found protection was accompanied by preservation of the astrocytic glutamate transporter GLT-1, and reduced oxidative stress in the CA1 region [61]. Similarly, selective overexpression of GLT-1 in astrocytes provided neuroprotection from moderate hypoxia-ischemia [59].
1.2 miRNAs in ischemia
MicroRNAs (miRNAs) are a novel and abundant class of 19- to 22-nucleotide (nt) noncoding RNAs that control gene expression at the post-transcriptional level. miRNAs bind target messenger RNAs (mRNA) and induce mRNA degradation or repression of translation. Because the targeting or seed sequence is relatively short, individual miRNAs have many targets, and single mRNAs can be targeted by multiple miRNAs. Increasing evidence supports a role for miRNAs in the response to cerebral ischemia, for review see [40, 44]. Changes in miRNAs with ischemic brain injury were first identified using miRNA profiling techniques in focal cerebral ischemia [14, 25, 32], in forebrain ischemia [64], and in stroke patients [55]. Recently studies have evaluated the significance of individual miRNAs and their regional expression in ischemic brain damage. miRNAs participate in synapse regulation and neuronal activity. The role of miRNAs in excitotoxicity and normal physiology was recently reviewed [15]. The faster post-transcriptional effect of miRNAs, and their ability to simultaneously regulate many target genes, suggests they may have greater therapeutic potential for stroke than therapies targeting a single gene by direct transcriptional control. Numerous miRNAs are expressed in a cell-specific or cell enriched manner, some specifically in astrocytes [46, 53].
The importance of astrocytes for neuroprotection after cerebral ischemia has been reviewed by our group and others recently [3, 54, 66]. This mini review focuses on the novel regulation of astrocytes by astrocyte-enriched miRNAs in this setting.
2. Astrocyte-enriched miRNAs and their targets
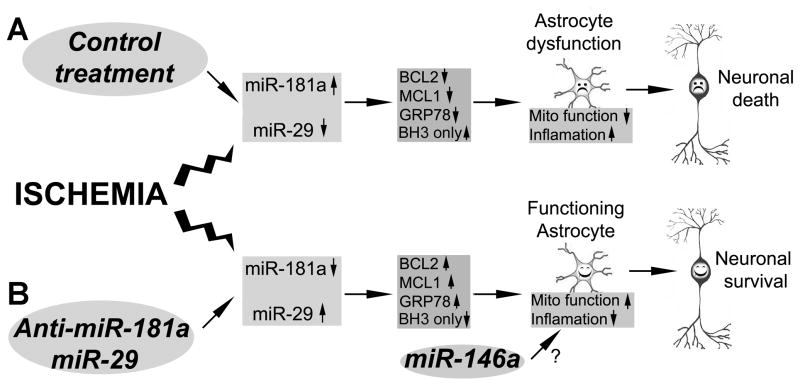
We demonstrated recently that two brain-enriched miRNAs, miR-181a [42] and miR-29a [46], are involved in the regulation of outcome following cerebral ischemia (Fig. 1) (see sections 3.1 and 3.2 below). Interestingly, the literature and our experiments suggest that both the miR-181 and miR-29 families are astrocyte-enriched [22, 46].
To define the role of a miRNA, determining its molecular targets and cellular expression are the first critical steps. Targets are often determined by testing the ability of the miRNA to suppress expression when the 3′ untranslated region (UTR) of the putative target mRNA is placed downstream of the luciferase reporter gene. Several bioinformatically predicted miRNA targets in cerebral ischemia have been validated. miR-497 was shown to be induced in mouse brain after MCAO, and knockdown of miR-497 is protective against MCAO-induced neuronal death [63]. miR-497 directly targets two anti-apoptotic genes, B-cell lymphoma (BCL) 2 and BCL-w at the same time to trigger ischemic brain cell death [63] and knockdown of miR-497 enhances BCL2 and BCL-w protein levels in the brain.
A recent microarray analyses of miRNA expression in the four principal cell types of the CNS (neurons, astrocytes, oligodendrocytes, and microglia) using primary cultures revealed that neural miRNA expression is highly cell-type specific, with 116 of the 351 miRNAs examined being differentially expressed fivefold or more across the four cell types [27]. Certain miRNAs are found preferentially expressed in neurons, e.g., miR-124 and miR-128 [27, 30, 53], whereas others seem restricted to astrocytes. The next section focuses on a few broadly conserved astrocyte enriched miRNA families and their validated targets.
2.1 miR-181 family
The miR-181 family consists of four mature members (miR-181a, miR-181b, miR-181c, and miR-181d) from three polycistronic miRNA genes – miR-181a-1/b-1, miR-181a-2/b-2, and miR-181c/d. The miR-181 family was first reported as an important immune cell development regulator [9]. These studies found that miR-181a and miR-181b, are enriched in brain [9, 35] and aberrant expression is associated with brain diseases. miR-181a and miR-181b are reduced in human gliomas and expression is negatively correlated with tumor grade [52]. miR-181a sensitizes malignant glioma cells to radiation by targeting anti-apoptotic BCL2 [10]. Expression of the miR-181 family is strongly enriched in cultured astrocytes compared to neurons derived from neural stem cells [22], and expression is significantly higher in adult cortex relative to embryonic telencephalon, an early developmental stage prior to astrogenesis.
Recently miR-181a was shown to directly target chaperone glucose regulated protein 78/BIP [42], anti-apoptotic members of the BCL2 family BCL2 and myeloid cell leukemia (MCL) 1 [41] and X-linked inhibitor of apoptosis (XIAP) [22] as well as some targets involved in controlling mitochondrial function, redox state, and inflammatory pathways (for recent reviews see [40, 43]). Although miR-181a antagomir reduces miR-181a levels and significantly inhibits the decrease of GLT-1 after forebrain ischemia, miR-181a does not directly target GLT-1 [36]. One interesting observation is the difference in effect of anti-miR-181a treatment in different cell types. Reducing miR-181a increased BCL2 as one of the targets and increased survival of primary astrocytes [41] while it failed to significantly change levels of BCL2 and did not change outcome in primary neurons after ischemia-like injury in vivo [36].
Some evidence supports the concept that not only mature miRNAs but also pri-/pre-miRNAs function in target recognition and repression [8]. Although miR-181a-2/b-2 and miR-181a-1/b-1 produce identical mature miR-181a and miR-181b, deletion of miR-181a-1/b-1, but not miR-181a-2/b-2, selectively inhibits tumor transformation by Notch oncogenes [17]. Because of the differential effects in neurons vs. astrocytes, future studies should assess which miR-181a gene(s) (miR-181a-1/or a-2) are expressed and the possible role of pri/pre-miR-181a in cell type specific responses.
2.2 miR-29 family
The miR-29 family consists of three members (a, b, and c) that map to two genomic loci in clusters [46]: miR-29 a/b-1 on chromosome 6 in mouse, 7 in human, and miR-29c/b-2 on chromosome 1 in both species. miR-29a/b-1 is developmentally regulated in mouse brain with highest expression in adults [21, 29]. During brain development miR-29 is more strongly expressed in astrocytes than neurons [53]. Recently we compared postnatal brain, primary neuron and astrocyte cultures, and found miR-29a increased in brain, astrocytes, and neurons with development, but levels in cultured astrocytes were always 20–40X higher than in cultured neurons with brain levels about 1/2 that in astrocytes [46]. This suggests that the developmental increase of brain miR-29a largely reflects increases in astrocyte miR-29a.
The study of miR-29 began in cancer research and focused on its role in regulation of apoptotic pathways. However, a question that has stirred controversy is whether miR-29 is pro-survival or pro-apoptotic [47]. Elevated miR-29 expression in some cancers suggested it may be an oncogene [18, 20], while other reports suggested tumor suppressor functions for miR-29 [48, 57]. This question is just as relevant in ischemia research as in cancer. While downregulation of miR-29 protected hearts against ischemia-reperfusion injury [62], upregulation of miR-29 protected neurons from apoptosis during neuronal maturation [29] forebrain [46] and focal ischemia [28].
The BCL2 protein family, central regulator of life/death decisions [1], largely influences cell death through regulating mitochondrial membrane integrity and function. The BCL2 protein family consists of 3 subgroups: the pro-survival proteins (BCL2, BCL-xL, BCL-w, MCL1 and A1), the multi-domain pro-apoptotic proteins (BAX and BAK) and the BH3-only pro-apoptotic proteins (BIM, PUMA, BID, BAD, BIK, BMF, HRK, NOXA). In response to diverse intracellular and extracellular signals the decision to undergo apoptosis is determined by interactions between these three subgroups. Luciferase assays indicate that the miR-29 family targets both pro- and anti-apoptotic BCL2 family members including BCL-w, MCL1, BAK, BMF and PUMA (Fig. 2). Our results demonstrating targeting of several BCL2 family members strongly suggests that the reported pro-apoptotic and anti-apoptotic effects of miR-29 likely reflect inhibition of different targets in different cells (similar to miR-146a in astrocytes and neurons mentioned below) and under different physiological or pathological settings. Kole et al., reported that miR-29b is activated during neuronal maturation and targets pro-apoptotic BIM, BMF, HRK, PUMA, and BAK in the BCL2 family [29]. We found that miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia [46]. Loss of miR-29b assessed in the infarct, contributed to injury in focal ischemia [28]. Another study reported miR-29b increased with MCAO, but these authors measured miR-29b in whole brain after stroke, so it is unclear if the increase was primarily on the contralateral side [51].
Figure 2.
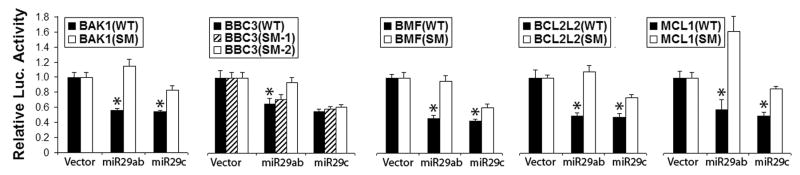
Luciferase assay validates miR-29 family targets. Dual luciferase activity assays using the wild type 3′UTRs or their seed mutants (SM) of five BCL2 family members shows that miR-29ab reduces luciferase activity. miR-29ab targets all and miR-29c targets four. Assays were performed 3 times in triplicate. *P<0.01 compared to the 3′UTRs-SM group.
2.3 miR-146a
Of the miR-146 family (miR-146a, miR-146b and miR-146c) only miR-146a is found enriched in human brain in astrocytes [2, 13]. miR-146a is brain abundant and widely investigated as a key regulator of the astrocyte-mediated inflammatory response [23] in neurological diseases such as epilepsy [2] and Alzheimer’s Disease [13]. In situ hybridization confirmed the increased expression of miR-146a in reactive astrocytes [23] in disease. Using primary cultures from newborn rat cortex, miR-146a was strongly expressed in astrocytes but reduced in neurons [27].
The miR-146a promoter contains two consensus nuclear factor (NF)-κB sites that are essential for the transcriptional activation of miR-146a in response to inflammatory stimuli (for recent review [6]). By repressing tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) and IL-1 receptor associated kinase 1 (IRAK1), miR-146a acts as a negative regulator of the NF-κB pathway and an important regulator of toll-like receptor signaling [6]. In the astrocyte-mediated inflammatory pathway miR-146a has many targets including IRAK-1, IRAK-2, TRAF-6, IL-6 etc. [23]. Recently miR-146a was reported to target superoxide dismutase (SOD) 2, an endogenous mitochondrial antioxidant enzyme, and regulate cell viability in H2O2 treated PC12 cells [26]. Astrocyte-enriched miRNAs such as miR-146a target some astrocyte-specific mRNAs and promote astrocytic differentiation in neural stem cells [27]. Interestingly miR-146a has the opposite effect on proneuronal differentiation by targeting neuroligin 1 (Nlgn1) [27]. Thus expression of miR-146a regulates redox and immune response in astrocytes prevents astrocytes from adopting neuron-specific phenotypes.
3. Targeting protection of astrocytes to protect neurons: the potential of miRNAs
Because of the importance of astrocytes in the brain and their early changes after cerebral ischemia, they are now recognized as important targets for manipulation of delayed neuronal injury after both focal and global cerebral ischemia. Indeed our studies and those of other laboratories have demonstrated that this can be a successful strategy. Here we examine possible uses of miRNAs in this context.
3.1 Astrocyte-enriched miRNA-181a
Recently a few studies have evaluated the significance of some of the astrocyte-enriched miRNAs mentioned above and their regional expression in ischemic brain damage. As described above, miR-181a increased in vulnerable regions [36, 42], and decreased in ischemia-resistant areas. Antagomir to miR-181a reduced infarct size in focal ischemia (Fig. 1A) [42], CA1 neuronal loss in global cerebral ischemia [36], and inhibitor protected primary astrocyte but not neuronal cultures from ischemia-like stresses [36, 41].
3.2 miR-29a and miR-29b
Interestingly, another astrocyte-enriched miRNA, miR-29a, changed in the opposite direction compared to miR-181a after transient forebrain ischemia, decreasing in CA1 and increasing in DG [46]. Overexpressing miR-29a protected CA1 neurons from forebrain ischemia (Fig. 1B) [46]. A recent report demonstrated that delivery of miR-29b mimic decreased MCAO infarct [28] and reported that miR-29b levels were regulated by 12-lipoxygenase activity.
Knowing that the targets of miR-181a and miR-29a validated to date (GRP78, BCL2 family members) are ER-mitochondria related [39, 40, 44], a common mechanism for these two miRNAs in regulating cerebral ischemia may involve influencing mitochondrial function. Indeed using primary cultured astrocytes we have found that miR-181a inhibitor and miR-29a mimic both preserve mitochondrial function and protect astrocytes from ischemia-like stress [42, 46].
3.3 miR-146a
Recently miR-146a was found to be involved in ischemia-reperfusion injury in liver [11], heart [58] and small intestine [7]. A study of 678 patients with ischemic stroke reported that miR-146a is associated with risk of silent brain infarction (evident by brain imaging but without clinical prsentation) [24]. Interestingly the only efficacious treatment of stroke to date using tissue plasminogen activator (tPA) for thrombolysis correlates with increased miR146a [65]. Because inflammation is one of the widely accepted mechanisms of cell death after cerebral ischemia (for recent reviews, see [38, 43]) and miR-146a has been found to target several proteins involved in astrocyte-mediated inflammation, it will be interesting to explore the regional and cellular expression as well as the effect of this astrocyte-enriched miRNA in different models of ischemic brain damage in future research.
4. Future directions
Strategies to improve the neuronal supportive functions of astrocytes have been used successfully in animal and in vitro studies. We speculate that miRNAs may have greater therapeutic potential as candidates for the treatment of stroke than therapies targeting induction of a single gene because of their faster post-transcriptional effect and their ability to simultaneously regulate many target genes. Several astrocyte-enriched miRNAs such as miR-181a and miR-29a have been demonstrated to regulate focal and global cerebral ischemia in animal models by targeting several important cell death/survival pathways (Fig. 3). While pretreatment has been tested in these early studies, investigating post-treatment effects of manipulating these astrocyte-enriched miRNAs is an essential next step. Recent work suggests that in some settings individual miRNAs have modest effects, while coordinate changes in several miRNAs targeting the same mRNA may produce large effects. Thus combination therapy with astrocyte-enriched miRNAs may be even more neuroprotective than a single one. Several miRNAs are already in clinical trials in other diseases, suggesting that formulation and administration will be possible in a new disease setting or for a new miRNA target.
Figure 3.
Targeting protection of astrocytes using astrocyte-enriched miRNAs or their antagomirs improves neuronal survival after cerebral ischemia. A. Cerebral ischemia induces changes in astrocyte-specific miRNAs (increase in miR-181a and decrease in miR-29a and miR-29b) in vulnerable regions of the brain. By targeting specific proteins astrocyte mitochondrial function is inhibited and inflammatory pathways activated in astrocytes, producing “unhappy” astrocytes that endanger neurons. B. Treatment with miR-181a antagomir, miR-29 and possibly miR-146a protects astrocyte function and supports neuronal survival.
Highlights.
Astrocytes and miRNAs are key players in cerebral ischemia
Some miRNAs are astrocyte-enriched
Targeting astrocyte-enriched miRNAs protects neurons from ischemia
Acknowledgments
Grant sponsor: NIH; Grant numbers: GM049831, NS053898, NS080177. The authors thank William Magruder for help preparing the manuscript.
Footnotes
The authors have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010;31:1100–1107. doi: 10.1111/j.1460-9568.2010.07122.x. [DOI] [PubMed] [Google Scholar]
- 3.Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11:164–173. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 5.Blakeley JO, Llinas RH. Thrombolytic therapy for acute ischemic stroke. J Neurol Sci. 2007;261:55–62. doi: 10.1016/j.jns.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-kappaB. Immunol Rev. 2012;246:205–220. doi: 10.1111/j.1600-065X.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 7.Chassin C, Hempel C, Stockinger S, Dupont A, Kubler JF, Wedemeyer J, Vandewalle A, Hornef MW. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CZ. An unsolved mystery: The target-recognizing RNA species of microRNA genes. Biochimie. 2013;95:1663–1676. doi: 10.1016/j.biochi.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P, Hu W. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Kong L, Xu X, Geng Q, Tang W, Jiang W. Down-regulation of microRNA-146a in the early stage of liver ischemia-reperfusion injury. Transplant Proc. 2013;45:492–496. doi: 10.1016/j.transproceed.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ. Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem. 2010;285:38951–38960. doi: 10.1074/jbc.M110.178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eacker SM, Dawson TM, Dawson VL. The interplay of microRNA and neuronal activity in health and disease. Frontiers in Cellular Neuroscience. 2013;7 doi: 10.3389/fncel.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 17.Fragoso R, Mao T, Wang S, Schaffert S, Gong X, Yue S, Luong R, Min H, Yashiro-Ohtani Y, Davis M, Pear W, Chen CZ. Modulating the strength and threshold of NOTCH oncogenic signals by mir-181a-1/b-1. PLoS Genet. 2012;8:e1002855. doi: 10.1371/journal.pgen.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano G, Kavanagh TJ, Costa LG. Mouse cerebellar astrocytes protect cerebellar granule neurons against toxicity of the polybrominated diphenyl ether (PBDE) mixture DE-71. NeuroToxicology. 2009;30:326–329. doi: 10.1016/j.neuro.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YC, Park CY, Bhagat G, Zhang J, Wang Y, Fan JB, Liu M, Zou Y, Weissman IL, Gu H. microRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, Wood WH, 3rd, Lehrmann E, Camandola S, Becker KG, Gorospe M, Mattson MP. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61:1018–1028. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet WG, van Rijen PC, Gorter JA, Aronica E. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One. 2012;7:e44789. doi: 10.1371/journal.pone.0044789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeon YJ, Kim OJ, Kim SY, Oh SH, Oh D, Kim OJ, Shin BS, Kim NK. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler Thromb Vasc Biol. 2013;33:420–430. doi: 10.1161/ATVBAHA.112.300251. [DOI] [PubMed] [Google Scholar]
- 25.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 26.Ji G, Lv K, Chen H, Wang T, Wang Y, Zhao D, Qu L, Li Y. MiR-146a Regulates SOD2 Expression in H2O2 Stimulated PC12 Cells. PLoS One. 2013;8:e69351. doi: 10.1371/journal.pone.0069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jovicic A, Roshan R, Moisoi N, Pradervand S, Moser R, Pillai B, Luthi-Carter R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. J Neurosci. 2013;33:5127–5137. doi: 10.1523/JNEUROSCI.0600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna S, Rink C, Ghoorkhanian R, Gnyawali S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang Y, Roy S, Sen CK. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab. 2013;33:1197–1206. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125–130. doi: 10.1101/gad.1975411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 32.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May LA, Kramarenko II, Brandon CS, Voelkel-Johnson C, Roy S, Truong K, Francis SP, Monzack EL, Lee FS, Cunningham LL. Inner ear supporting cells protect hair cells by secreting HSP70. The Journal of Clinical Investigation. 2013;123:3577–3587. doi: 10.1172/JCI68480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao Y, Qiu Y, Lin Y, Miao Z, Zhang J, Lu X. Protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism. Mol Biol Rep. 2011;38:3235–3242. doi: 10.1007/s11033-010-9998-0. [DOI] [PubMed] [Google Scholar]
- 35.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5:R68. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon JM, Xu L, Giffard RG. Inhibition of microRNA-181 reduces forebrain ischemia induced neuronal loss. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.157. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noguchi Y, Shinozaki Y, Fujishita K, Shibata K, Imura Y, Morizawa Y, Gachet C, Koizumi S. Astrocytes Protect Neurons against Methylmercury via ATP/P2Y1 Receptor-Mediated Pathways in Astrocytes. PLoS One. 2013;8:e57898. doi: 10.1371/journal.pone.0057898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouyang YB. Inflammation and stroke. Neurosci Lett. 2013;548:1–3. doi: 10.1016/j.neulet.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Ouyang YB, Giffard RG. ER-Mitochondria Crosstalk during Cerebral Ischemia: Molecular Chaperones and ER-Mitochondrial Calcium Transfer. Int J Cell Biol. 2012;2012:493934. doi: 10.1155/2012/493934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang YB, Giffard RG. MicroRNAs Regulate the Chaperone Network in Cerebral Ischemia. Transl Stroke Res. 2013:1–11. doi: 10.1007/s12975-013-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG. miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiol Dis. 2012;45:555–563. doi: 10.1016/j.nbd.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang YB, Stary CM, White RE, Voloboueva LA, Giffard RG. The use of microRNAs to modulate redox and immune response to stroke. Antiox Redox Signaling. 2013 doi: 10.1089/ars.2013.5757. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouyang YB, Stary CM, Yang GY, Giffard R. microRNAs: innovative targets for cerebral ischemia and stroke. Curr Drug Targets. 2013;14:90–101. doi: 10.2174/138945013804806424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-Enriched miR-29a Targets PUMA and Reduces Neuronal Vulnerability to Forebrain Ischemia. Glia. 2013 doi: 10.1002/glia.22556. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1:224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 49.Qu WS, Wang YH, Wang JP, Tang YX, Zhang Q, Tian DS, Yu ZY, Xie MJ, Wang W. Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochem Res. 2010;35:1716–1724. doi: 10.1007/s11064-010-0234-z. [DOI] [PubMed] [Google Scholar]
- 50.Rathinam ML, Watts LT, Narasimhan M, Riar AK, Mahimainathan L, Henderson GI. Astrocyte mediated protection of fetal cerebral cortical neurons from rotenone and paraquat. Environmental Toxicology and Pharmacology. 2012;33:353–360. doi: 10.1016/j.etap.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp Brain Res. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 52.Shi L, Cheng Z, Zhang J, Li R, Zhao P, Fu Z, You Y. hsa-mir-181a and hsa-mir-181b function as tumor suppressors in human glioma cells. Brain Res. 2008;1236:185–193. doi: 10.1016/j.brainres.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 53.Smirnova L, Grafe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 54.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.THACAS. Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14:369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res. 2013;97:432–442. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ. Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia-ischemia. Neuroscience. 2008;155:1204–1211. doi: 10.1016/j.neuroscience.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Zhou C, Du F, Lu Y, Peng B, Chen L, Zhu L. Ginkgolide B preconditioning on astrocytes promotes neuronal survival in ischemic injury via up-regulating erythropoietin secretion. Neurochemistry International. 2013;62:157–164. doi: 10.1016/j.neuint.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–542. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 63.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY. MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. J Clin Neurosci. 2010;17:774–778. doi: 10.1016/j.jocn.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32:1856–1864. doi: 10.1161/ATVBAHA.112.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Rempe D. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7:439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng W, Talley Watts L, Holstein DM, Wewer J, Lechleiter JD. P2Y1R-initiated, IP3R-dependent stimulation of astrocyte mitochondrial metabolism reduces and partially reverses ischemic neuronal damage in mouse. J Cereb Blood Flow Metab. 2013;33:600–611. doi: 10.1038/jcbfm.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]