Abstract
Background
Iron supplementation for hypoferremic anemia could potentiate bacterial growth in the cystic fibrosis (CF) lung, but clinical trials testing this hypothesis are lacking.
Methods
Twenty-two adults with CF and hypoferremic anemia participated in a randomized, double-blind, placebo-controlled, crossover trial of ferrous sulfate 325mg daily for 6 weeks. Iron-related hematologic parameters, anthopometric data, sputum iron, Akron Pulmonary Exacerbation Score (PES), and the sputum microbiome were serially assessed. Fixed-effect models were used to describe how ferrous sulfate affected these variables.
Results
Ferrous sulfate increased serum iron by 22.3% and transferrin saturation (TSAT) by 26.8% from baseline (p <0.05) but did not affect hemoglobin, sputum iron, Akron PES, and the sputum microbiome.
Conclusions
Low-dose ferrous sulfate improved hypoferremia without correcting anemia after 6 weeks. We did not observe significant effects on sputum iron, Akron PES, and the sputum microbiome. Although we did not identify untoward health effects of iron supplementation, a larger blinded randomized controlled trial would be needed to fully demonstrate safety.
BACKGROUND
Anemia affects an estimated 10–29% of adult cystic fibrosis (CF) patients (1–3). We (4) and others (2, 3) have observed low circulatory iron stores (hypoferremia) in 23–100% of anemic CF patients, suggesting that iron deficiency may restrict erythropoiesis (5). Nonetheless, accurate assessment of iron status is challenging in CF because serum ferritin (6) and transferrin saturation (TSAT) (7) are often increased and decreased, respectively, by inflammation, leading to overestimation or underestimation of total body iron reserves. In CF, an elevated serum soluble transferrin receptor (sTfR) level reflects hypoferremic anemia (7) and is not influenced by the acute phase response of infective exacerbation (8), but it cannot distinguish between ironlimited erythropoiesis and anemia of chronic disease (9) wherein iron is not mobilized for erythropoiesis (10). Therefore, no single blood test explains the finding of hypoferremia in CF.
However, iron supplementation is warranted for selected patients (11), but this practice is associated with several theoretical concerns. Bacteria in the CF lung require iron for growth and possess mechanisms to obtain this micronutrient from human tissues (12, 13). Iron enhances the formation of Pseudomonas aeruginosa (P.a.) biofilm communities (14) which can be visualized in the sputum of patients (15). In an epithelial co-culture model, ΔF508-CFTR increases iron in airway surface liquid and augments P.a. biofilm growth and antibiotic resistance (16, 17). Neovascular changes in bronchial arteries may lead to hemoptysis (18), also introducing iron into the airways. These observations prompted us to ask three related questions about oral iron supplementation: 1) does it increase sputum iron?; 2) does it alter bacterial communities (i.e., the microbiome of sputum from the CF lung)?; and 3) compared to placebo, does it increase the frequency of CF pulmonary exacerbation (CFPE)?
That iron supplementation might be harmful in CF is a concern of clinicians who reported the onset of CFPE symptoms in patients following infusion of intravenous iron (19). Ambiguity in the literature about the definition of CF-related anemia and its underlying mechanisms arguably contribute to the use of iron supplements in patients for whom additional iron is unlikely to be beneficial. We conducted this study to more fully understand the clinical ramifications of iron supplementation in CF.
METHODS
Subjects
Adults who were ≥18 years old with CF confirmed by genotype analysis were recruited from the programs at Dartmouth-Hitchcock Medical Center (DHMC) and Maine Medical Center (MMC). They provided written informed consent as part of identical protocols approved by institutional review boards (IRBs) at both sites. Participants were required to have serum transferrin saturation (TSAT) ≤21% and hemoglobin concentration <15.5 gm/dl (men) or <13.6 gm/dl (women) at screening. TSAT ≤21% is below the mean for 20–39 year old Caucasian women in the third National Health and Nutrition Examination Survey (NHANES III) (20). Cutoffs for hemoglobin are below the gender-specific means for 20–29 year old Caucasians in NHANES III (21). All subjects had a history of ≥1 P.a.-positive sputum culture. Exclusion criteria included use of iron-containing vitamins, history of an iron-overload condition or cirrhosis, pregnancy or breastfeeding, and recent visible hemoptysis.
Study Design
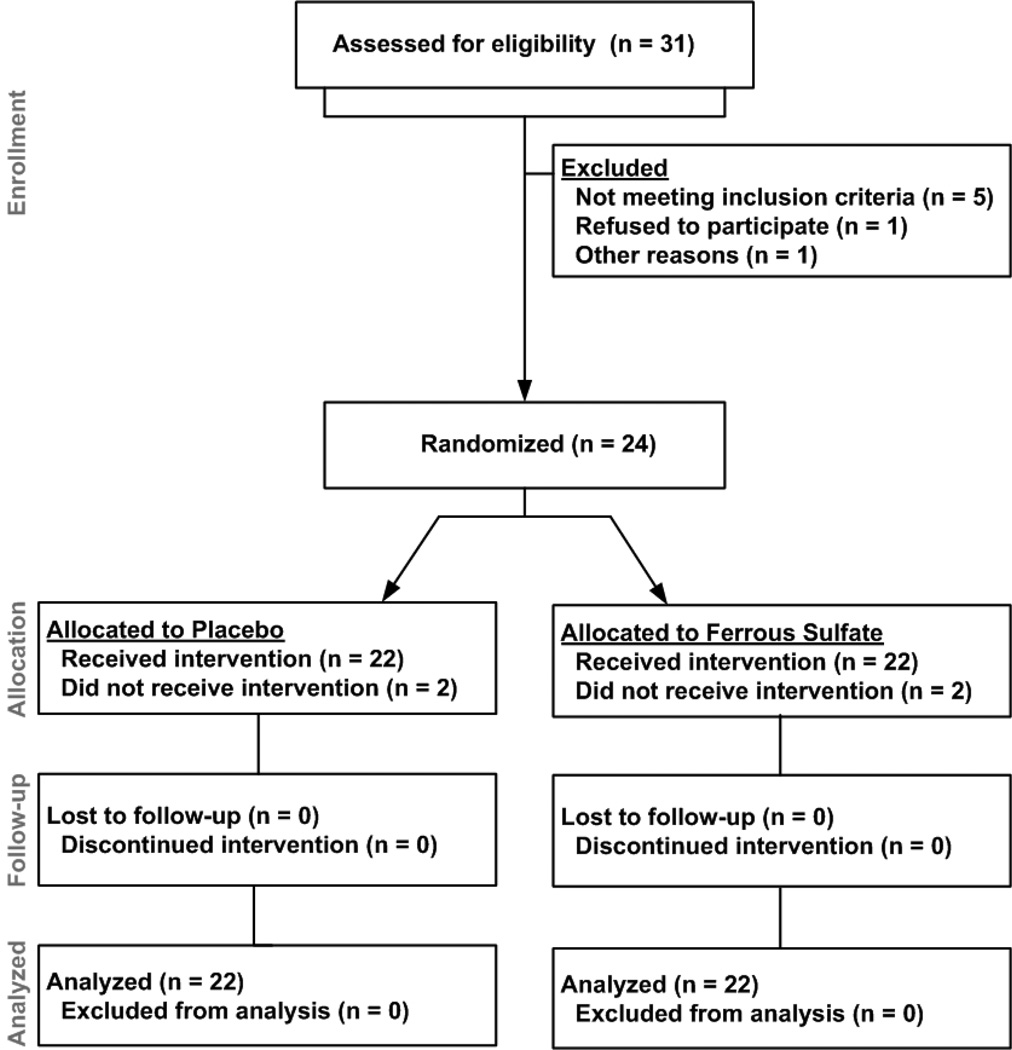
This investigation was a randomized, double-blind, placebo-controlled, crossover trial of ferrous sulfate 325mg taken orally once a day for 6 weeks. Subjects were randomized in a 1:1 allocation. A 30-day washout period occurred between arms. Subjects attended follow-up visits at weeks 0, 3, and 6 of each arm. The CONSORT flow chart (Figure 1) further describes subject participation. Treatment adherence was determined by pill counts and asking subjects about remaining pills at each visit. Because constipation is a common problem in CF (22) that could be worsened by iron, study personnel tracked this symptom. Subjects were advised to avoid taking the study drug at the same time as fluoroquinolones, as iron limits their absorption (23). Systemic antibiotic use was documented at each visit because of its effect on iron homeostasis (24). CFPE was defined by an Akron Pulmonary Exacerbation Score (PES) ≥5 (25).
Figure 1.
Diagram of enrollment, allocation, follow-up, and analysis of subjects.
Sample Size and Statistical Analyses
The primary endpoint of this study was the absolute change from baseline in hemoglobin concentration attributed to ferrous sulfate. Ater et al. (26) found that 8 out of 22 CF patients (36%) treated with ferrous sulfate (6 mg/kg/day) experienced a ≥1.0 gm/dl increase in hemoglobin after 4–5 weeks. We calculated that 28 subjects would be needed to observe this endpoint with power of 80% and α = 0.05. Secondary endpoints were absolute changes from baseline attributed to ferrous sulfate and antibiotic use for the following parameters: serum iron, hepcidin-25, TSAT, sputum iron, and incremental and dichotomized PES (< or ≥5 points). Paired Student’s t-tests were used to compare sputum microbiome parameters.
All data were checked for normality (Kolmogorov-Smirnov test) and were otherwise log-transformed. Heterogeneity of the carry-over effects between the two treatment sequences was refuted by permutation test. Fixed-effect models accounted for repeated measurements within subjects and treatment sequence (27). For log-transformed predictor variables, the estimated effect is expressed as percent change relative to baseline. Otherwise, the estimated effect signifies the absolute change from baseline and (standard error) that is explained by each variable. Multicollinearity disallowed simultaneous inclusion of serum iron and TSAT in the models. Fisher’s exact test was used to compare side effect frequency and adherence between arms. SAS 9.3® (SAS Institute, Inc., Cary, NC) and GraphPad Prism® 5.04 (GraphPad Software, Inc., La Jolla, CA) were used for all analyses. A two-tailed p-value <0.05 was significant.
Diagnostic Testing
Phlebotomy was performed at weeks 0, 3, and 6 of each arm. Complete blood count (CBC), serum iron, TSAT, and reticulocyte count were measured by automated hematology analyzers in the clinical laboratories of DHMC and MMC. FEV1% reflected percentages of predicted normal values (28). Serum hepcidin-25 and erythropoietin (EPO) were determined at Intrinsic LifeSciences (La Jolla, CA) using a competitive enzyme-linked immunosorbent assay (ELISA) (29) and a commercial ELISA (Quantikine® IVD®, R&D Systems, Inc., Minneapolis, MN), respectively. Total sputum iron was measured by inductively coupled plasma-mass spectrometry (ICP-MS) (24, 30) and is expressed as nanograms of iron per mg of sample (ng/mg). The Akron PES was calculated by study personnel at weeks 0, 3, and 6 of each arm using the version published by Kraynack et al. (25)
Sputum Microbiome Analysis
Relative abundance of P.a. was determined by sequencing, and total bacterial diversity was measured by 454 pyrosequencing of the V4–V6 regions of the 16S rRNA gene from genomic DNA isolated from patient sputum samples, as previously described (31). Deep sequencing, bioinformatic quality filtering, and operational taxonomic unit (OTU) assignments were performed, as previously described (31). Bacterial diversity was calculated using Simpson Diversity Index (SDI). Individual reads, taxon assignments, and descriptions of individual clusters and diversity calculations are accessible on the website Visualization and Analysis of Microbial Population Structures (http://vamps.mbl.edu).
RESULTS
Enrollment and Subject Characteristics
A total of 31 subjects were screened (Figure 1). Of the 26 subjects who met screening criteria, 24 were randomized to receive ferrous sulfate or placebo. During the initial treatment arm, one subject was lost to follow-up, and one subject was excluded due to lung transplant. Data from these two subjects were not analyzed. All 22 remaining subjects (18 at DHMC, 4 at MMC) finished both arms.
Baseline characteristics of the study population are presented in Table 1. Participants were predominantly males in their third decade of life with moderately-severe lung function impairment. Three-quarters of them were homozygous for ΔF508-CFTR. Two-thirds of the cohort had CF-related diabetes (CFRD).
Table 1.
Subject Baseline Characteristics
| Parameter | Values |
|---|---|
| Number (n) | 22 |
| Age (years) | 32.1 (13.6) |
| Gender (M / F) | 14 / 8 |
| FEV1 (% predicted) | 56 (21) |
| Body weight (kg) | 63.9 (11.9) |
| dF508 heterozygote (%) | 100 |
| dF508 homozygote (%) | 77 |
| CF-related diabetes (%) | 68 |
| Hemoglobin (gm/dl)* | 13.6 (0.9) (men) 12.6 (0.7) (women) |
| Serum iron (µg/dl)** | 62 (38) (men) 51 (22) (women) |
| TSAT (%)* | 13 (5) (men) 10 (4) (women) |
| Serum hepcidin-25 (ng/ml)** | 48.6 (41.9) |
| Sputum iron (ng/mg)**, † | 1.44 (1.0) |
Data are presented as mean (standard deviation) unless otherwise noted.
Measured at screening
Measured at randomization
Based on sputum samples collected from 21 of 22 subjects.
Treatment Adherence and Side Effects
A total of 3 subjects experienced 3 constipation events during ferrous sulfate use. For comparison, 5 such events were observed in 4 subjects during placebo treatment. The relative risk for constipation on ferrous sulfate was no greater than placebo (RR 1.3, 95% CI 0.7–2.3, p = 0.71). Subjects took 914 of 924 ferrous sulfate doses (99%) and 898 of 924 placebo doses (97%). Risk of missing a dose was higher for placebo (RR 1.4, 95% 1.2–1.8, p = 0.02).
Effect of Ferrous Sulfate on Hemoglobin, Reticulocyte Count, and Serum EPO
In this and subsequent statistical models, data are presented as estimated effects followed by standard error in parentheses. Ferrous sulfate insignificantly increased hemoglobin by 0.04 (0.18) gm/dl (p = 0.81). However, the model showed that every kilogram of body weight lost was associated with a drop in hemoglobin of 0.17 gm/dl (p = 0.01). No other parameter predicted hemoglobin variation. Reticulocyte count and serum EPO were also unaffected by ferrous sulfate.
Effect of Ferrous Sulfate on TSAT and Serum Iron Concentration
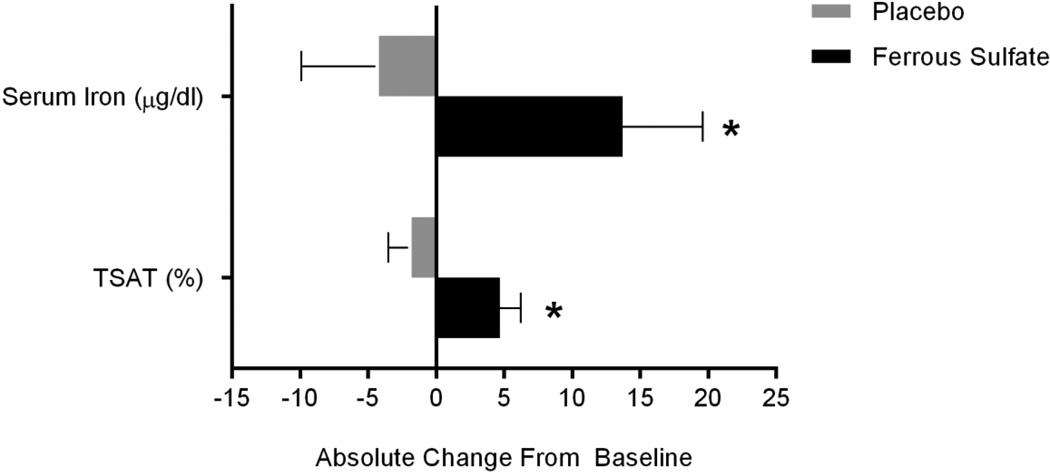
Data for TSAT and serum iron required log-transformation. For TSAT, the estimated treatment effect was an increase of 26.8 (10.6)% from baseline (p = 0.02). For serum iron, the estimated treatment effect was an increase of 22.3 (9.9)% from baseline (p = 0.03). Absolute changes in TSAT and serum iron for ferrous sulfate and placebo are shown in Figure 2. Antibiotic use, body weight, Akron PES, and sputum iron did not predict TSAT or serum iron variation. In a responder analysis of data from the ferrous sulfate arm, we found no significant difference in hemoglobin between subjects who did and did not achieve a 13.7 µg/dl increase in mean serum iron (Figure 2).
Figure 2.
Treatment Related Differences in Serum Iron and TSAT. Bars and whiskers denote mean differences from baseline and (standard error), respectively. After 6 weeks, serum iron increased by 13.7 (5.9) µg/dl for ferrous sulfate but fell by 4.2 (5.7) µg/dl for placebo. TSAT increased 4.7 (1.5) % for ferrous sulfate and fell by 1.8 (1.7) % for placebo. * p <0.05 for comparison of ferrous sulfate to placebo.
Effect of Ferrous Sulfate on Sputum Iron Content
Use of ferrous sulfate and antibiotics did not significantly affect sputum iron variation from baseline (Table 2). The number of sputum samples permitted a power of 68% to detect the 0.281 ng/mg difference in sputum iron. Thus, the study was underpowered for this endpoint. Each ng/ml increase in serum hepcidin-25 from baseline was associated with a 1.1 (0.4)% increase in sputum iron (p = 0.02). Each mU/ml increase in serum EPO from baseline accounted for a 5.2 (1.9)% elevation in sputum iron (p = 0.01). No other factor in Table 2 predicted sputum iron changes.
Table 2.
Fixed-effect Model for Predictors of Change in Sputum Iron from Baseline
| Effect* | Estimate | S.E. | t-value | p-value |
|---|---|---|---|---|
| Intercept | −3.418 | 1.854 | −1.84 | 0.08 |
| Drug sequence | 0.236 | 0.248 | 0.95 | 0.35 |
| Period | −0.126 | 0.181 | −0.70 | 0.49 |
| Ferrous sulfate | −0.281 | 0.196 | −1.43 | 0.16 |
| Antibiotic use | 0.054 | 0.228 | 0.24 | 0.81 |
| FEV1 (% predicted) | −0.017 | 0.013 | −1.30 | 0.20 |
| Serum iron (µg/dl)* | 0.410 | 0.251 | 1.64 | 0.11 |
| Serum hepcidin-25 (ng/ml) | 0.011 | 0.004 | 2.51 | 0.02 |
| Akron PES | −0.032 | 0.041 | −0.77 | 0.45 |
| Serum EPO (mU/ml) | 0.052 | 0.020 | 2.66 | 0.01 |
| Hemoglobin (gm/dl) | 0.082 | 0.142 | 0.58 | 0.57 |
| Body weight (kg) | −0.014 | 0.063 | −0.22 | 0.83 |
S.E. = standard error
Log-transformed prior to introduction into model.
Correlation Between Ferrous Sulfate and Akron PES
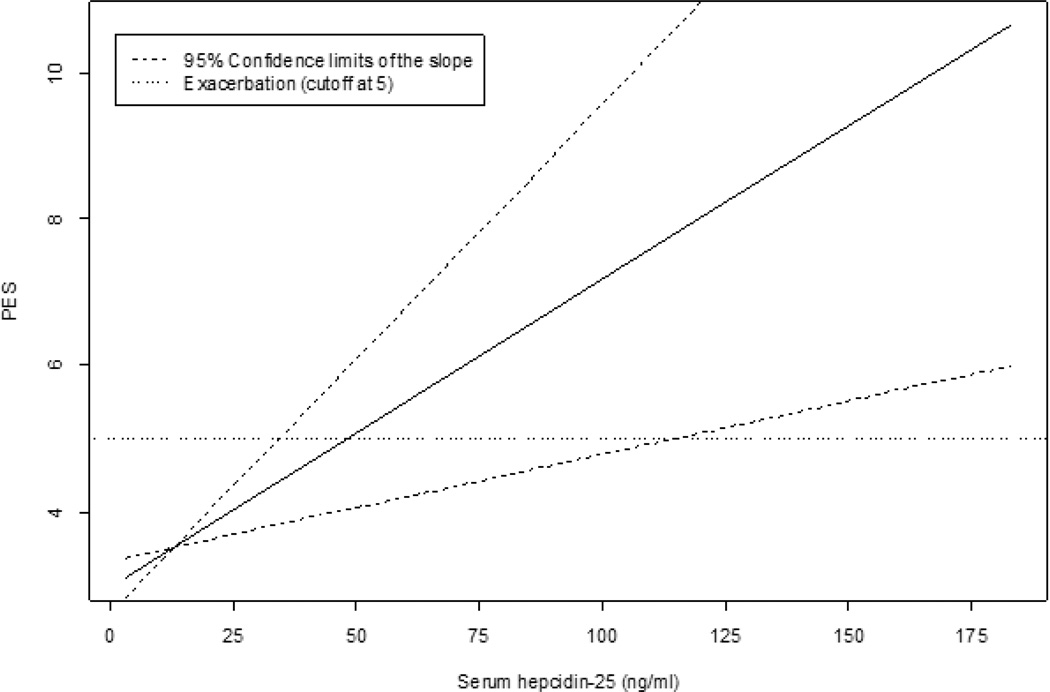
Ferrous sulfate was not associated with incremental (Table 3) or dichotomized (PES ≥5) scores on the Akron instrument. Therefore, short term treatment did not trigger CFPE as defined by this inventory. The power to detect the observed difference in Akron PES between ferrous sulfate and placebo arms was 42%. Controlling for other variables, antibiotic use was associated with a 1.6 (0.7) point increase from baseline PES (p = 0.04). Each ng/ml increase in serum hepcidin-25 was associated with a PES increase of 0.04 (0.01) points (p = 0.006) (Figure 3). In cases where PES was 5 or higher (i.e., CFPE), each ng/ml increase in serum hepcidin-25 was associated with a 0.07 (0.03) point increase in PES (p = 0.03). The fixed-effect model for PES (Table 3) showed that a gain of 1 kg in body weight predicted a decrease of 0.4 ± 0.2 points for PES (p = 0.047).
Table 3.
Fixed-effect Model for Predictors of Change in Akron PES from Baseline
| Effect | Estimate | S.E. | t-value | p-value |
|---|---|---|---|---|
| Intercept | −1.773 | 6.554 | −0.27 | 0.79 |
| Drug sequence | 1.459 | 0.839 | 1.74 | 0.10 |
| Period | −0.365 | 0.624 | −0.58 | 0.56 |
| Ferrous sulfate | −0.738 | 0.684 | −1.08 | 0.29 |
| Antibiotic use | 1.585 | 0.731 | 2.17 | 0.04 |
| FEV1 (% predicted) | −0.021 | 0.046 | −0.46 | 0.65 |
| Serum iron (µg/dl)* | 0.995 | 0.901 | 1.10 | 0.28 |
| Serum hepcidin-25 (ng/ml) | 0.042 | 0.014 | 2.98 | 0.006 |
| Reticulocyte count (%) | 0.781 | 0.975 | 0.80 | 0.43 |
| Body weight (kg) | −0.424 | 0.206 | −2.06 | 0.047 |
| Hemoglobin (gm/dl) | −0.929 | 0.469 | −1.98 | 0.06 |
| Sputum iron (ng/mg)* | −0.244 | 0.499 | −0.49 | 0.63 |
S.E. = standard error
Log-transformed prior to introduction into model.
Figure 3.
Relationship Between Akron PES and Serum Hepcidin-25 in CF. The fixed-effect model for serum hepcidin-25 revealed that every ng/ml increase in serum hepcidin-25 above baseline was associated with a 0.04 (0.01) point increase in Akron PES above baseline (p <0.05). 95% confidence intervals for the slope of this relationship intersect PES = 5 at 35 ng/ml and 115 ng/ml for hepcidin-25.
Correlation Between Ferrous Sulfate and the Sputum Microbiome
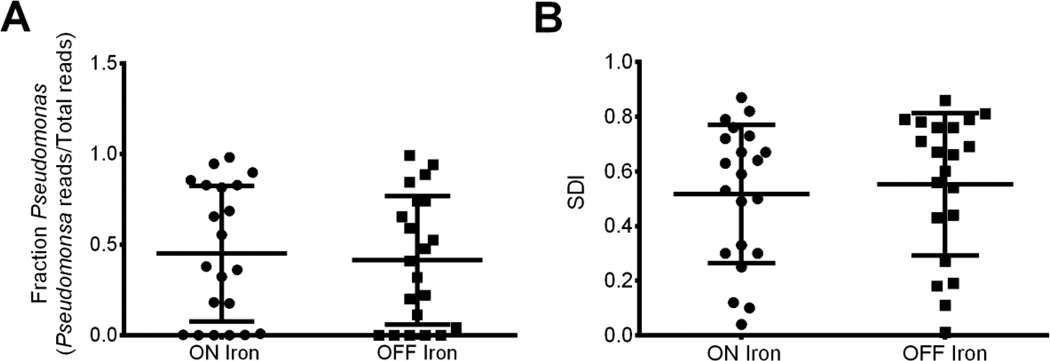
Ferrous sulfate did not impact relative abundance of P.a. or community diversity within subjects (Figure 4). Given that the standard deviation of P.a. fractions (Figure 4A) was 0.37, we would expect to resolve a difference of 0.30 with 80% power and p <0.01 with 21 paired samples. Relative abundance of CF pathogens, including Stenotrophomonas, Staphylococcus, Haemophilus, Rothia, Achromobacter, and all other microbes analyzed was similarly unaffected by ferrous sulfate (data not shown). Hierarchical clustering and principal coordinate analyses revealed no dichotomy between subjects based on treatment status (data not shown). These observations are consistent with the finding that iron supplementation does not increase the availability of iron in CF sputum (Table 2).
Figure 4.
Sputum Microbiome is Unaltered by Iron Supplementation. The CF sputum microbiome is unaltered by iron supplementation. A) Relative abundance of P.a. as calculated by deep sequencing (P.a. reads/Total reads) for sputum samples obtained from 21 subjects while on or off iron supplementation. Sputum from week 6 of Arm 1 and Arm 2 were compared and analyzed for difference by paired Student’s t-test. Each symbol indicates an individual sputum sample. Horizontal line indicates the mean and error bars indicate the standard deviation. There is no significant difference in P.a. relative abundance in subjects on or off iron supplementation (p >0.05). B) Simpson Diversity Index (SDI) for sputum samples obtained while on or off iron supplementation for the same samples and by the same method described in panel A. There is no significant difference in Simpson Diversity index in subjects on or off iron supplementation (p >0.05).
DISCUSSION
This study demonstrated that a low-dose daily iron supplement improved circulatory iron stores but failed to increase hemoglobin after 6 weeks in CF adults with hypoferremic anemia. Sputum iron, risk of CFPE according to the Akron PES, and the sputum microbiome were unaffected by iron supplementation. Moreover, hepcidin-25, the master regulator of iron metabolism, (32) emerged as a predictor of sputum iron and Akron PES.
Iron-deficiency has long been recognized in CF (26, 33). Some authors have used TSAT <15–16%, (2, 7, 26) to define hypoferremia, while others have used a combination of TSAT <16% and serum iron ≤12 µmol/l (34) or serum iron ≤12 µmol/l (4) alone. Herein, we sought to ensure hypoferremia at screening using TSAT ≤21%, the mean value for 20–39 year old Caucasian women in the NHANES III public health survey (20). Also from NHANES III, (20) Caucasian men in this age range had a higher mean TSAT (27%), which is important because most of our subjects were male. Given that the mean TSAT at screening for women and men in our study were 10 ± 4% and 13 ± 5%, respectively, and that subjects had an average age of 32 years, we contend that they were iron deficient compared to the general population and therefore, reasonable candidates for iron supplementation.
Similarly, mean hemoglobin levels for males (13.6 ± 0.9 gm/dl) and females (12.6 ± 0.7 gm/dl) at screening were lower than gender-specific means for 20–29 year old Caucasians in NHANES III (21), a cohort of comparable age and ethnicity. Therefore, in addition to being iron-deficient, subjects in this trial were mildly anemic. Like hypoferremia, CF-related anemia has been defined (1–4, 26) using different hemoglobin cutoffs in cohorts that vary by age and gender. We hope that this study focuses the discussion of hypoferremic anemia in CF.
Despite the biochemical evidence for anemia and hypoferremia in our subjects, ferrous sulfate did not increase hemoglobin. This finding could reflect type II error because we did not meet the enrollment target of 28 subjects. Non-adherence did not explain this observation. For dosing convenience and the ability to generate a matching placebo, we used ferrous sulfate, a preparation that is absorbed less efficiently than other formulations (35), raising the possibility that hemoglobin did not respond because serum iron was not sufficiently increased. Low gastric pH enhances absorption of non-heme iron like ferrous sulfate. (36) Gastric acid suppression used in CF to protect exogenous pancreatic enzymes from proteolysis may have limited iron uptake (37), and therefore, hemoglobin production in our subjects. We did not use vitamin C to enhance iron absorption out of concern for reduced adherence to a more complex regimen. Nonetheless, ferrous sulfate increased serum iron and TSAT by approximately 22% and 27%, respectively, after 6 weeks, leading us to infer that anemia might persist because iron cannot be mobilized for erythropoiesis. This conclusion is supported by our findings that reticulocyte count, a measure of red blood cell production by the bone marrow, (38) and serum erythropoietin, the salient growth factor for red blood cell precursors, (39) were unaffected by iron supplementation.
Iron is an essential nutrient for bacteria that chronically infect the lungs of CF patients, particularly P.a. (40). To ensure that iron did not cause an unintended increase in P.a. or alter the sputum microbiome, we characterized sputum microbial communities of each patient by deep sequencing at each time point in the study. Consistent with the lack of increased iron in the sputum, we saw no change in overall diversity or relative abundance of P.a. or other CF pathogens as a result of iron supplementation. Goddard et al. (41) have questioned whether sputum samples are contaminated by oropharyngeal flora, and thus, do not accurately reflect the lung microbiome. Yet, these authors reported a high concordance between deep sequencing data from CF lung tissue and clinical sputum cultures. Because we wanted to determine the effect of ferrous sulfate on the sputum microbiome within the same subjects on serial samples collected in the same manner, and because we have no reason to think that iron would differentially influence flora in the upper and lower respiratory tracts, we feel the data in Figure 4 support the conclusion that oral iron supplementation does not appear to worsen chronic bacterial infection in the CF lung.
To investigate whether inflammation influenced trends in the iron content of serum and sputum, we measured serum hepcidin-25, a peptide hormone that reduces enteral iron absorption and increases iron sequestration within mononuclear cells in response to interleukin-6 (IL-6) (42, 43). Using serum hepcidin-25 as a biomarker for the severity of inflammation in CF is justified by our findings in patients evaluated at the onset of and recovery from CFPE that lower serum hepcidin-25 and IL-6 concentrations were associated with better lung function and higher serum and lower sputum iron levels (24). Herein, we report for the first time that serum hepcidin-25 is associated with sputum iron variation in CF (Table 2) and incremental increases in Akron PES (Figure 3), a tool that objectifies CFPE (25). The correlation between serum hepcidin-25 and Akron PES establishes a rationale to explore whether the former test can be used to predict the onset of CFPE.
There are several important limitations to our study. Ferrous sulfate might not have increased hemoglobin because anemia was mild, thereby reflecting a potential ceiling effect. The dose of ferrous sulfate was also low, but this was informed by a concern for causing constipation in a population already at risk for this problem. Because constipation occurred very rarely, a larger study using a higher ferrous sulfate dose would be needed to assess the safety of treatment in CF. The power to detect changes in Akron PES was only 42%; therefore, we cannot definitely state that iron supplementation is not associated with CFPE. Based on the observed difference and standard deviation in Akron PES under both treatment conditions, we found that 160 subjects would be needed to ensure that CFPE risk was lower for ferrous sulfate with 80% power and p <0.05. For the endpoint of sputum iron, 29 subjects would be required to detect a treatment-related difference with a power of 80% and p <0.05, but only 22 subjects completed this study. Therefore, a larger study would be required to determine whether ferrous sulfate increases sputum iron in CF and/or is associated with CPFE, as defined using the Akron PES.
In summary, this controlled trial of iron supplementation for the hypoferremic anemia of CF asked whether this practice worsened respiratory health, a concern raised by an in-vitro model of chronic P.a. lung infection (16, 17) and one that several authors have recently discussed (11, 19). We found no evidence that 325 mg of ferrous sulfate taken daily for 6 weeks increased sputum iron, increased the relative abundance of P.a. or other CF pathogens, or hastened CFPE onset according to the Akron PES. Nonetheless, a larger blinded randomized controlled trial would be needed to fully demonstrate safety.
AKNOWLEDGEMENTS
Funding Sources: Flatley Foundation of Boston, Massachusetts (GAO, BAS, AHG); NIH P20-GM103413-10 and R01-HL074175-09 (BAS); Cystic Fibrosis Foundation Research Development Program (STANTO07R0); Hitchcock Foundation (GAO); and NIH R01 AI091699 (GAO). Funding sources had no involvement in the design of this study, collection, analysis, and interpretation of data, writing of this manuscript, and publication decisions.
The authors would like to acknowledge Gordana Olbina, Ph.D., who performed the ELISAs for serum hepcidin-25 and EPO at Intrinsic LifeSciences, LLC, and Brian P. Jackson, Ph.D., Director of the Trace Metal Analysis Core Facility at Dartmouth.
Abbreviation list
- CBC
complete blood count
- CF
cystic fibrosis
- CFPE
cystic fibrosis pulmonary exacerbation
- CFRD
cystic fibrosis-related diabetes
- CFTR
cystic fibrosis transmembrane conductance regulator
- ELISA
enzyme-linked immunosorbent assay
- FEV1%
percent-predicted forced expiratory volume in one second
- IL-6
interleukin-6
- IRB
institutional review board
- OTU
operational taxonomic unit
- P.a.
Pseudomonas aeruginosa
- PEG
polyethylene glycol
- PES
Pulmonary Exacerbation Score
- SDI
Simpson diversity index
- sTfR
soluble transferrin receptor
- TSAT
transferrin saturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Alex H. Gifford, M.D., Diana M. Alexandru, D.O., Zhigang Li, Ph.D., Dana B. Dorman, R.N., M.S.N., Lisa A. Moulton, R.N., Katherine E. Price, Ph.D., Thomas H. Hampton, M.S., Mitchell L. Sogin, Ph.D., Jonathan B. Zuckerman, M.D., H. Worth Parker, M.D., Bruce A. Stanton, Ph.D., and George A. O’Toole, Ph.D., each declare that he/she does not have a personal or financial interest in the subject matter of this manuscript.
Author contributions
AHG was the principal investigator and responsible for all aspects of the investigation including the final content of the manuscript.
JL, KEP, and THH were responsible for statistical analyses, preparation, and review of the manuscript.
DMA, DBD, LAM, JBZ, and HWP were responsible for data collection, preparation, and review of the manuscript.
MLS was responsible for sputum microbiome analyses and review of the manuscript.
BAS was responsible for study design, preparation, and review of the manuscript.
GO was responsible for study design, measurement of sputum iron, preparation, and review of the manuscript.
Contributor Information
Alex H. Gifford, Email: Alex.H.Gifford@hitchcock.org.
Diana M. Alexandru, Email: ALEXAD1@mmc.org.
Zhigang Li, Email: Zhigang.Li@dartmouth.edu.
Dana B. Dorman, Email: Dana.B.Dorman@hitchcock.org.
Lisa A. Moulton, Email: Lisa.A.Moulton@hitchcock.org.
Katherine E. Price, Email: Katherine.E.Price@dartmouth.edu.
Thomas H. Hampton, Email: Thomas.H.Hampton@dartmouth.edu.
Mitchell L. Sogin, Email: sogin@mbl.edu.
Jonathan B. Zuckerman, Email: jzuckerman@cmamaine.com.
H. Worth Parker, Email: H.Worth.Parker@hitchcock.org.
Bruce A. Stanton, Email: bas@dartmouth.edu.
George A. O’Toole, Email: georgeo@dartmouth.edu.
REFERENCES
- 1.Fischer R, Simmerlein R, Huber RM, Schiffl H, Lang SM. Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatric pulmonology. 2007;42(12):1193–1197. doi: 10.1002/ppul.20717. Epub 2007/10/20. [DOI] [PubMed] [Google Scholar]
- 2.Pond MN, Morton AM, Conway SP. Functional iron deficiency in adults with cystic fibrosis. Respiratory medicine. 1996;90(7):409–413. doi: 10.1016/s0954-6111(96)90114-6. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 3.von Drygalski A, Biller J. Anemia in cystic fibrosis: incidence, mechanisms, and association with pulmonary function and vitamin deficiency. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2008;23(5):557–563. doi: 10.1177/0884533608323426. Epub 2008/10/14. [DOI] [PubMed] [Google Scholar]
- 4.Gifford AH, Miller SD, Jackson BP, Hampton TH, O'Toole GA, Stanton BA, et al. Iron and CF-related anemia: expanding clinical and biochemical relationships. Pediatric pulmonology. 2011;46(2):160–165. doi: 10.1002/ppul.21335. Epub 2010/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrighting DM, Andrews NC. Iron homeostasis and erythropoiesis. Current topics in developmental biology. 2008;82:141–167. doi: 10.1016/S0070-2153(07)00006-3. Epub 2008/02/20. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochimica et biophysica acta. 2010;1800(8):760–769. doi: 10.1016/j.bbagen.2010.03.011. Epub 2010/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keevil B, Rowlands D, Burton I, Webb AK. Assessment of iron status in cystic fibrosis patients. Annals of clinical biochemistry. 2000;37(Pt 5):662–665. doi: 10.1258/0004563001899708. Epub 2000/10/12. [DOI] [PubMed] [Google Scholar]
- 8.Khalid S, McGrowder D, Kemp M, Johnson P. The use of soluble transferrin receptor to assess iron deficiency in adults with cystic fibrosis. Clinica chimica acta; international journal of clinical chemistry. 2007;378(1–2):194–200. doi: 10.1016/j.cca.2006.11.021. Epub 2007/01/27. [DOI] [PubMed] [Google Scholar]
- 9.Chang J, Bird R, Clague A, Carter A. Clinical utility of serum soluble transferrin receptor levels and comparison with bone marrow iron stores as an index for iron-deficient erythropoiesis in a heterogeneous group of patients. Pathology. 2007;39(3):349–353. doi: 10.1080/00313020701329732. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G, Goodnough LT. Anemia of chronic disease. The New England journal of medicine. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. Epub 2005/03/11. [DOI] [PubMed] [Google Scholar]
- 11.Smith DJ, Anderson GJ, Lamont IL, Masel P, Bell SC, Reid DW. Accurate assessment of systemic iron status in cystic fibrosis will avoid the hazards of inappropriate iron supplementation. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2012 doi: 10.1016/j.jcf.2012.10.001. Epub 2012/10/23. [DOI] [PubMed] [Google Scholar]
- 12.Nairz M, Schroll A, Sonnweber T, Weiss G. The struggle for iron - a metal at the host-pathogen interface. Cellular microbiology. 2010;12(12):1691–1702. doi: 10.1111/j.1462-5822.2010.01529.x. Epub 2010/10/23. [DOI] [PubMed] [Google Scholar]
- 13.Lamont IL, Konings AF, Reid DW. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2009;22(1):53–60. doi: 10.1007/s10534-008-9197-9. Epub 2009/01/09. [DOI] [PubMed] [Google Scholar]
- 14.Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(31):11076–11081. doi: 10.1073/pnas.0504266102. Epub 2005/07/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pusztaszeri M, Aubert JD, Braunschweig R, Mihaescu A. Pseudomonas aeruginosa biofilms in a bronchoalveolar lavage. Diagnostic cytopathology. 2009;37(11):825. doi: 10.1002/dc.21014. Epub 2009/02/05. [DOI] [PubMed] [Google Scholar]
- 16.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O'Toole GA, et al. The DeltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. American journal of physiology Lung cellular and molecular physiology. 2008;295(1):L25–L37. doi: 10.1152/ajplung.00391.2007. Epub 2008/03/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreau-Marquis S, O'Toole GA, Stanton BA. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. American journal of respiratory cell and molecular biology. 2009;41(3):305–313. doi: 10.1165/rcmb.2008-0299OC. Epub 2009/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonelli M, Midulla F, Tancredi G, Salvatori FM, Bonci E, Cimino G, et al. Bronchial artery embolization for the management of nonmassive hemoptysis in cystic fibrosis. Chest. 2002;121(3):796–801. doi: 10.1378/chest.121.3.796. Epub 2002/03/13. [DOI] [PubMed] [Google Scholar]
- 19.Hoo ZH, Wildman MJ. Intravenous iron among cystic fibrosis patients. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2012;11(6):560–562. doi: 10.1016/j.jcf.2012.05.002. Epub 2012/06/22. [DOI] [PubMed] [Google Scholar]
- 20.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer C. Hematological and iron-related analytes--reference data for persons aged 1 year and over: United States, 1988–94. Vital and health statistics Series 11, Data from the national health survey. 2005;247:1–156. [PubMed] [Google Scholar]
- 21.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–1750. doi: 10.1182/blood-2005-07-3046. Epub 2005/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubinstein S, Moss R, Lewiston N. Constipation and meconium ileus equivalent in patients with cystic fibrosis. Pediatrics. 1986;78(3):473–479. Epub 1986/09/01. [PubMed] [Google Scholar]
- 23.Polk RE, Healy DP, Sahai J, Drwal L, Racht E. Effect of ferrous sulfate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrobial agents and chemotherapy. 1989;33(11):1841–1844. doi: 10.1128/aac.33.11.1841. Epub 1989/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gifford AH, Moulton LA, Dorman DB, Olbina G, Westerman M, Parker HW, et al. Iron Homeostasis during Cystic Fibrosis Pulmonary Exacerbation. Clinical and translational science. 2012;5(4):368–373. doi: 10.1111/j.1752-8062.2012.00417.x. Epub 2012/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraynack NC, McBride JT. Improving care at cystic fibrosis centers through quality improvement. Seminars in respiratory and critical care medicine. 2009;30(5):547–558. doi: 10.1055/s-0029-1238913. Epub 2009/09/18. [DOI] [PubMed] [Google Scholar]
- 26.Ater JL, Herbst JJ, Landaw SA, O'Brien RT. Relative anemia and iron deficiency in cystic fibrosis. Pediatrics. 1983;71(5):810–814. Epub 1983/05/01. [PubMed] [Google Scholar]
- 27.Senn S. Cross-over Trials in Clinical Research. 2nd ed. Chichester, England: John Wiley and Sons, Ltd.; 2002. [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. Epub 1999/01/05. [DOI] [PubMed] [Google Scholar]
- 29.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. doi: 10.1182/blood-2008-02-139915. Epub 2008/08/12. [DOI] [PubMed] [Google Scholar]
- 30.Heck JE, Andrew AS, Onega T, Rigas JR, Jackson BP, Karagas MR, et al. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environmental health perspectives. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. Epub 2010/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, et al. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. Journal of bacteriology. 2012;194(17):4709–4717. doi: 10.1128/JB.00566-12. Epub 2012/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–772. doi: 10.1126/science.1224577. Epub 2012/11/10. [DOI] [PubMed] [Google Scholar]
- 33.Ehrhardt P, Miller MG, Littlewood JM. Iron deficiency in cystic fibrosis. Archives of disease in childhood. 1987;62(2):185–187. doi: 10.1136/adc.62.2.185. Epub 1987/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid DW, Withers NJ, Francis L, Wilson JW, Kotsimbos TC. Iron deficiency in cystic fibrosis: relationship to lung disease severity and chronic Pseudomonas aeruginosa infection. Chest. 2002;121(1):48–54. doi: 10.1378/chest.121.1.48. Epub 2002/01/18. [DOI] [PubMed] [Google Scholar]
- 35.Bovell-Benjamin AC, Viteri FE, Allen LH. Iron absorption from ferrous bisglycinate and ferric trisglycinate in whole maize is regulated by iron status. The American journal of clinical nutrition. 2000;71(6):1563–1569. doi: 10.1093/ajcn/71.6.1563. Epub 2000/06/06. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Current gastroenterology reports. 2010;12(6):448–457. doi: 10.1007/s11894-010-0141-0. Epub 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barraclough M, Taylor CJ. Twenty-four hour ambulatory gastric and duodenal pH profiles in cystic fibrosis: effect of duodenal hyperacidity on pancreatic enzyme function and fat absorption. Journal of pediatric gastroenterology and nutrition. 1996;23(1):45–50. doi: 10.1097/00005176-199607000-00009. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Piva E, Brugnara C, Chiandetti L, Plebani M. Automated reticulocyte counting: state of the art and clinical applications in the evaluation of erythropoiesis. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2010;48(10):1369–1380. doi: 10.1515/CCLM.2010.292. Epub 2010/07/30. [DOI] [PubMed] [Google Scholar]
- 39.Fried W. Erythropoietin and erythropoiesis. Experimental hematology. 2009;37(9):1007–1015. doi: 10.1016/j.exphem.2009.05.010. Epub 2009/06/09. [DOI] [PubMed] [Google Scholar]
- 40.Vasil ML, Ochsner UA. The response of Pseudomonas aeruginosa to iron: genetics, biochemistry and virulence. Molecular microbiology. 1999;34(3):399–413. doi: 10.1046/j.1365-2958.1999.01586.x. Epub 1999/11/17. [DOI] [PubMed] [Google Scholar]
- 41.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(34):13769–13774. doi: 10.1073/pnas.1107435109. Epub 2012/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mena NP, Esparza A, Tapia V, Valdes P, Nunez MT. Hepcidin inhibits apical iron uptake in intestinal cells. American journal of physiology Gastrointestinal and liver physiology. 2008;294(1):G192–G198. doi: 10.1152/ajpgi.00122.2007. Epub 2007/10/27. [DOI] [PubMed] [Google Scholar]
- 43.Theurl I, Fritsche G, Ludwiczek S, Garimorth K, Bellmann-Weiler R, Weiss G. The macrophage: a cellular factory at the interphase between iron and immunity for the control of infections. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine. 2005;18(4):359–367. doi: 10.1007/s10534-005-3710-1. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]