Abstract
Depression is a significant public health problem, but its etiology and pathophysiology remain poorly understood. Such incomplete understanding likely arises from the fact that depression encompasses a heterogeneous set of disorders. To overcome these limitations, renewed interest in intermediate phenotypes (endophenotypes) has resurfaced, and anhedonia has emerged as one of the most promising endophenotypes of depression. Here, a heuristic model is presented postulating that anhedonia arises from dysfunctional interactions between stress and brain reward systems. To this end, we review and integrate three bodies of independent literature investigating the role of (1) anhedonia, (2) dopamine, and (3) stress in depression. In a fourth section, we summarize animal data indicating that stress negatively affect mesocorticolimbic dopaminergic pathways critically implicated in incentive motivation and reinforcement learning. In the last section, we provide a synthesis of these four literatures, present initial evidence consistent with our model, and discuss directions for future research.
Keywords: Depression, Anhedonia, Stress, Endophenotype, Dopamine
INTRODUCTION
Despite its “ignominious status as a world leader in burden of disease” (Greden 2001, p. 30) and decades of research, the etiology and pathophysiology of major depressive disorder (MDD) remain largely unknown. This lack of understanding partially stems from issues inherent in current classification systems, which define mental illness based on clusters of symptoms and clinical course rather than etiology or pathophysiology (Hyman 2007). As a consequence, the diagnosis of MDD, although reliable, might lack validity, and encompass a heterogeneous set of disorders with distinct pathophysiologies.
To overcome these limitations, a focus on narrowly defined and quantifiable phenotypes, often referred to as “endophenotypes”, has been advocated. According to Gottesman & Gould (2003), “[e]ndophenotypes provide a means for identifying the 'downstream' traits […] of clinical phenotypes, as well as the 'upstream' consequences of genes” (p. 637). In this conceptualization, intermediate phenotypes are assumed to be positioned within the causal chain between genes and disease, and thus represent a more proximal expression of biological and environmental influences than a syndrome. To be considered an endophenotype, a construct should meet the following criteria (Gottesman & Gould 2003): (1) specificity (i.e., the endophenotype is more strongly linked to a given condition than other psychiatric conditions)1; (2) heritability; (3) state-independence (i.e., the endophenotype is stable over time and independent from illness status and treatment); (4) cosegregation (i.e., the endophenotype occurs more frequently in affected, compared to unaffected, relatives of an ill individual); (5) familial association (i.e., the endophenotype is more frequent in relatives of ill individuals than the general population); and (6) biological and clinical plausibility.
In recent years, anhedonia–the loss of pleasure or lack of reactivity to pleasurable stimuli (American Psychiatric Association 2000)–has emerged as one of the most promising endophenotypes of depression (Hasler et al. 2004). As recently reviewed (Berghorst & Pizzagalli 2010), anhedonia has received significant support for the criteria of heritability, state-independence, familial association, and biological/clinical face validity. Evidence for specificity is limited, as anhedonia plays a role in other disorders, particularly schizophrenia and substance use disorders, and strong evidence for cosegregation is absent. Neverthless, the overall picture suggests validity for the anhedonic endophenotype.
Although anhedonia has long been recognized as a possible trait marker related to vulnerability to depression (Meehl 1975), little is known about associated environmental and biological factors. The overarching goal of the present review is to present a heuristic model postulating that anhedonia arises from dysfunctional interactions between stress and brain reward systems (Figure 1). To this end, the first section summarizes four bodies of literature that have developed largely independently from each other. Using Figure 1 as a roadmap, we begin by reviewing evidence indicating that blunted encoding of reward-related stimuli, reward-related decision making and reinforcement learning are core facets of depression (Link A). In the next section, we summarize evidence suggesting that MDD is characterized by blunted transmission of dopamine, the neurotransmitter most consistently linked to reward processing (Link B). Next, we briefly discuss studies emphasizing the pivotal role of stress in the emergence, maintenance, and exacerbation of depression, including work indicating that early adversities increase vulnerability to depression and stress sensitivity later in life (Link C). An explicit link between stress and anhedonic behavior is made in the fourth section, based on non-human animal data indicating that stress negatively affects mesocorticolimbic dopaminergic pathways (Link D). In the final section, we provide a synthesis of these four literatures, present initial evidence consistent with our model, and discuss both limitations of the current literature and future directions.
Figure 1.
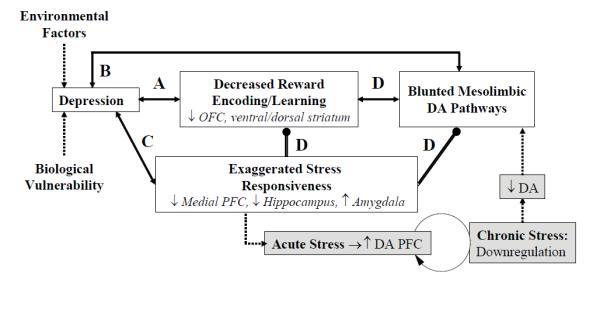
Schematic illustration of the heuristic model proposed in the current review. Double-ended arrows denote associations (no causality) between processes. Double lines with dots denote directional inhibitory links (e.g., stress response inhibits mesolimbic DA release). Dotted lines denote causal relations, and the gray boxes denote hypothesized mechanisms (e.g., chronic stress leads to lower DA release and eventually to blunted DA response). The circular arrow denotes long-term DA down-regulation with chronic stress. Letters (A-D) refer to the sections in the text. ↓: decreased, ↑: increased, NAc: nucleus accumbens, PFC: prefrontal cortex. For the sake of simplicity of the illustration, both environmental factors and biological vulnerability are graphically represented as contributing to the clinical syndrome directly; both factors likely affect, however, all of the sub-components of this heuristic model (e.g., decreased reward responsiveness, exaggerated stress responsiveness, blunted mesolimbic DA).
THE ROLE OF ANHEDONIA IN DEPRESSION
To facilitate integration between human and non-human animal findings, the following section is restricted to studies using laboratory-based measures of hedonic behavior and reinforcement learning. Due to space limitations, we also limit our review to behavioral and neuroimaging studies in MDD.
Behavioral Studies
Studies assessing perceptual/attentional processes
Perceputal Tasks
There is mixed evidence for impaired perceptual processing of positive stimuli in MDD. In face recognition studies, depressed subjects recognized happy facial expressions more slowly (e.g., Suslow et al. 2004) and less accuractely (e.g., Persad & Polivy 1993) than controls, but numerous studies have failed to replicate (e.g., Leppanen et al. 2004). Moreover, global recognition impairments extending to other facial expressions have been described, highlighting a lack of specificity (e.g., Persad & Polivy 1993).
There is a critical aspect that has been generally neglected in these early studies and might explain some of the inconsistencies. By assessing only accuracy and reaction time, these studies cannot address whether deficits might be confounded by response biases. This issue was addressed by three studies. Using signal-detection analyses, Surguladze et al. (2004) reported that MDD subjects showed a response bias away from happy expressions. In the second study, relative to controls, MDD subjects identified fewer neutral faces as happy and more neutral faces as sad, demonstrating a negative processing bias and a lack of positivity bias (Gollan et al. 2008). Finally, using morphed facial expressions, Joormann and Gotlib (2006) described that MDD subjects required greater intensity of emotional expression in order to identify happy–but not sad–faces relative to controls (Joormann & Gotlib 2006). These findings emerged within the framework of no overall group differences in accuracy. Together, these findings suggest that face recognition dysfunctions might stem from cognitive biases, rather than perceptual dysfunctions.
Attentional Tasks
Studies investigating attentional biases in depression have yielded similarly inconsistent findings, in line with the widely assumed conceptualization that MDD is characterized by impairments in elaborative, rather than attentional, processes (Williams et al. 1997). In contrast with this general assumption, some studies using the deployment of attention and dot-probe taks, in which an emotional and a neutral stimulus compete for attentional resources, suggest that MDD may be linked to attentional biases away from positive cues.
In the deployment of attention task, participants are presented with an emotional and neutral word, which are then replaced by two color bars; participants are instructed to select whichever bar appeared first. Because the two bars are presented simultaneously, preference for the bar replacing a positive word is taken as an indication that attention was captured by the positive word. Unlike healthy controls, who display a positivity bias (i.e., they attend relatively more to positive stimuli) or protective bias (i.e., they direct attention away from negative stimuli), depressed subjects perform this task in an unbiased fashion (e.g., Gotlib et al. 1988; Wang et al. 2006). Interestingly, remitted depressed subjects showed a positivity bias and/or avoidance of negative stimuli under normal conditions (McCabe et al. 2000; Wang et al. 2006), but show a lack of bias after a sad mood induction (McCabe et al. 2000).
In a study using a dot-probe paradigm, in which a neutral and emotional facial expression are presented concurrently, both currently and formerly depressed subjects selectively attended to sad faces, and failed to attend to happy faces (Joormann & Gotlib 2007). Notably, a reduced bias toward happy faces (as well as an increased bias toward sad faces) was also described in never-disordered daughters of depressed mothers after a sad mood induction (Joormann et al. 2007). Altogether, these findings raise the possibility that blunted attentional biases toward positive cues is a trait marker associated with increased vulnerability to depression.
Studies assessing encoding and retrieval
Evidence for weaker affective responses to positive cues at encoding in depression has emerged from studies investigating affective and behavioral responses to a variety of positive stimuli, although inconsistencies abound. When exposed to such stimuli, depressed subjects generally showed (1) reduced positive affect and arousal (e.g., Berenbaum & Oltmanns 1992; Rottenberg et al. 2002; Sloan et al. 2001; cf. Dichter et al. 2004), and (2) diminished behavioral (e.g., facial) responsiveness (e.g., Berenbaum & Oltmanns 1992; Sloan et al. 2001; cf. Rottenberg et al. 2002). In several studies, blunted affective and behavioral responses emerged for positive but not negative stimuli, highlighting a selective impairment (e.g., Berenbaum & Oltmanns 1992; Sloan et al. 2001).
One important question is whether decreased affective responses to positive cues might lead to impaired retrieval of such stimuli. Evidence in support of this possibility comes from early studies in which participants were asked to estimate the amount of feedback delivered during a task they had just completed. Depressed subjects were found to underestimate the frequency of positive feedback and reinforcement received, and overestimate the frequency of punishment (Nelson & Craighead 1977).
Similar findings have emerged from studies investigating estimation of the frequency of positive outcomes in real-life settings. Among children, depressive symptoms negatively correlated with ratings of the probability of positive events happening in the future, but only when these events referred to the self (Muris & van der Heiden 2006). Moreover, depressed subjects predicted fewer positive outcomes in the future, relative to both healthy controls and individuals with anxiety disorders (e.g., Miranda & Mennin 2007). Thus, depressed subjects’ perceptions of the past are characterized by an underestimation of positive reinforcements received, whereas their view of the future is colored by a reduced expectation of positive reinforcement.
Self-referential tasks
Whereas the studies reviewed above suggest that depression is characterized by blunted affective responses to positive stimuli at encoding and impaired retrieval of positive cues, they provide little insight about whether such dysfunctions might extend to self-schemas. To address this issue, several studies have examined self-referential encoding of positive and negative adjectives in depression using the self-referent encoding task (SERT).
In early studies, increased endorsement of self-referential negative adjectives in depression was found (e.g., Dobson & Shaw 1987; Serfaty et al. 2002). More recently, evidence for reduced endorsement and recall of positive traits has emerged in currently and formely depressed individuals (e.g., Gotlib et al. 2004), and the proportion of endorsed positive–but not negative–words predicted depressive symptoms 9 months later (Johnson et al. 2007). Moreover, both currently depressed (Gotlib et al. 2004; Serfaty et al. 2002) and remitted subjects undergoing a sad mood induction (Ramel et al. 2007) showed reduced recall of endorsed positive traits. Of note, in the study by Dobson & Shaw (1987), group differences after remission remained only for endorsement of positive words, raising the possibility that a reduced positive self-image might represent a vulnerability for future depressive episodes.
Similar findings have emerged from studies in pediatric samples and individuals at increased risk for depression. Reduced endorsement of positive words was observed in children of depressed mothers, but only when the SERT task was performed after a sad mood induction (Taylor & Ingram 1999). Moreover, currently depressed–but not remitted–youth endorsed significantly fewer positive traits than controls after a sad mood induction, whereas both depressed groups endorsed more negative traits (Timbremont & Braet 2004). Of note, both depressed groups recalled significantly fewer positive words compared to controls. Altogether, these findings suggest that, relative to controls, depressed subjects less frequently endorse positive information as self-descriptive and show selective recall deficits for positive information, consistent with the view that elaborative processing biases are an important aspect of depression.
Tasks assessing the effects of reward manipulations
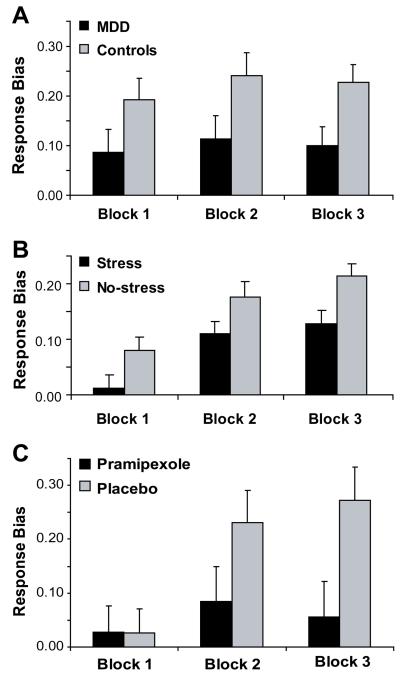
In a recognition task involving different payoff contingencies, depressed subjects showed a more conservative response bias during the reward condition relative to controls, indicating that monetary incentives failed to shift memory retrieval performance (Henriques et al. 1994). In a probabilistic reward task involving a differential reinforcement schedule, unmedicated individuals with MDD were characterized by reduced response bias towards a more frequently rewarded stimulus (Figure 2a; Pizzagalli et al. 2008b). Interestingly, trial-by-trial probability analyses revealed that MDD subjects failed to express a response bias toward the more advantageous stimulus in the absence of immediate reward. Moreover, this impairment was largest in patients reporting anhedonic symptoms, and was uniquely associated with anhedonia (rather than anxiety or general distress). Notably, blunted response bias predicted MDD chronicity after 8 weeks of antidepressant treatment (Vrieze et al. 2013) and persisted after full remission (Pechtel et al., in press). Together with evidence that elevated depressive symptoms are associated with reduced reward-based decision making (Kunisato et al. 2012), these findings indicate that depression is characterized by impaired ability to modulate behavior as a function of reinforcement, particularly when rewards are intermittent and anhedonic symptoms are elevated. Finally, MDD subjects were less willing than controls to expend effort in order to receive high monetary rewards (Treadway et al. 2012). This finding was not replicated in a study using a similar paradigm, in which MDD and healthy subjects exerted similar amounts of efforts in order to see humorous cartoons and reported similar ratings of liking; however, unlike healthy controls, levels of reward liking did not predict motivation to expend efforts for rewards in MDD subjects (Sherdell et al. 2012).
Figure 2.
Behavioral findings emerging from a probabilistic reward task involving a differential reinforcement schedule. Response bias toward the more frequently rewarded stimulus was reduced in (a) unmedicated MDD subjects (Pizzagalli et al. 2008b); (b) healthy controls performing the task under acute stressor (Bogdan & Pizzagalli 2006); and (c) healthy controls receiving a single dose of a D2/3 agonist hypothesized to reduce phasic DA signaling to reward outcomes via presynaptic DA autoreceptor activation (Pizzagalli et al. 2008a).
Interim summary
In spite of the traditional conceptualization of anhedonia, evidence for “loss of pleasure” and reduced affective/behavioral responses to positive cues in depression is mixed (see also Treadway & Zald, 2011). Moreover, although a lack of positivity bias has been described in some studies probing perceptual and attentional processes, evidence for dysfunction at early stages of the information processing flow is limited. Contrary to these inconsistent findings, MDD appears to be characterized by (1) underestimation of reinforcements received and reduced expectation of future rewards; (2) less frequent endorsement and recall of positive traits in self-referential tasks; (3) diminished ability to modulate behavior as a function of reinforcement history; (4) reduced willingness to exert effort in order to gain reward; and (5) uncoupling between “liking” and “wanting”.
Collectively, these findings provide a more nuanced understanding of anhedonia in MDD, in which motivation, reinforcement learning, and reward-based decision making–rather than the experience of pleasure per se–are impaired, and are consistent with early behavioral models postulating that depression may result from a loss of positive reinforcement (e.g., Bandura 1971). Because positive reinforcers are stimuli that increase the likelihood of a behavior, the dysfunctions reviewed above may reduce the motivation to pursue future rewards and engage in pleasurable activities, which might in turn maintain and/or exacerbate depressive symptoms. Consistent with this hypothesis, experiential sampling studies have shown that depressed subjects engage in fewer activities associated with immediate pleasure and increased likelihood of future reward (Hopko et al. 2003). Furthermore, anhedonia has been found to predict (1) depression two years later (Wardenaar et al. 2012); (2) poor outcome (e.g., McMakin et al. 2012; Spijker et al. 2001), even when adjusting for overall depression severity (Uher et al. 2012); and (3) chronic course of depression over a 10-year period (Moos & Cronkite 1999). Given these findings, it is interesting to note that behavioral treatments encouraging patients to engage in rewarding activities, reschedule activities to reintroduce positive reinforcement, and reduce avoidance have shown substantial success in alleviating depression (Ekers et al. 2008).
In spite of convincing evidence implicating facets of anhedonia in MDD, several important questions remain. First, very few studies have investigated whether these dysfunctions are state- or trait-related, and incosistencies exist. Using self-report assessments, McFarland & Klein (2009) found that while currently depressed individuals reported reduced emotional reactivity to anticipated reward, this dysfunction was not observed in formelly depressed individuals. These findings contrast with data indicating that individuals with remitted depression show reduced (1) endorsement of positive words (Dobson & Shaw 1987), (2) attentional bias to happy faces (Joormann & Gotlib 2007), and (3) ability to modulate behavior as a function of rewards (Pechtel et al., in press). Of note, reduced endorsement of positive words (Taylor & Ingram 1999) and reduced attentional bias toward happy faces (Joormann et al. 2007) were also described in unaffected offsprings of depressed mothers (after induction of a sad mood). Collectively, these findings suggest that, although self-reported affective responses might normalize after symptom remission, encoding and attentional biases away from positive cues as well blunted reinforcement learning persist after remission, particularly after a mood challenge. Future studies will be required to test this hypothesis.
Second, very few studies have investigated the predictive validity of reduced reward responsiveness, but initial evidence is promising. Among MDD inpatients, blunted reward responsiveness predicted MDD chronicity 8 weeks later, even when controlling for initial depression severity (Vrieze et al. 2013). In a pediatric sample, reduced selection of high-probability, high-reward reward options at age 11 predicted depressive–but not anxious–symptoms at age 12 (Forbes et al. 2007). Similarly, endorsement of positive–but not negative–words predicted greater reduction of depressive symptoms 9 months later (Johnson et al. 2007). Finally, among adolescent offsprings of depressed parents, low reward seeking during a gambling task predicted depressive symptoms, new onset of MDD, and reduced engagement in extracurricular activities one year later, even when controlling for baseline depressive symptoms (Rawal et al. 2013). These findings suggest that laboratory-based tasks probing reward-related decision making might be used to identify individuals at risk for future depression, opening opportunities for intervention.
Neuroimaging Studies
A growing number of studies have investigated neural activity in response to positive cues–including rewards–in MDD. Broadly speaking, two general points emerge. First, across a variety of tasks probing distinct aspect of reward processing, dysfunctions in ventral (i.e., nucleus accumbens) and dorsal (i.e., caudate, putamen) striatal regions as well as orbitofrontal cortex (OFC) are among the most replicated findings. Of primary importance, and as reviewed in more detail below, these regions have been implicated in distinct aspects of reward processing. Accordingly, these studies promise to provide further clues about which aspects of reward processing might be dysfunctional in depression. Second, these findings imply that anhedonia might be neurobiologically complex, and associated with various abnormalities. In the following section, a brief synopsis of the neural basis of incentive processing is presented, which will provide a framework for interpreting findings in depression.
Ventral striatum
A variety of findings implicate ventral striatal regions, particularly the nucleus accumbens (NAc), in coding the incentive properties of stimuli and reward prediction errors. Studies in non-human primates have shown that striatal DA neurons code reward-related prediction errors using phasic bursts of DA: when the animal receives an unpredicted reward, increased firing (and DA release) is seen (“positive-prediction error”), and learning about the behavior that led to reward is supported. Conversely, omission of an expected reward leads to transient neural (and DA) suppression (“negative-prediction error”), and the association between the action and outcome is weakened. Interestingly, after the animal learns that a given stimulus will be followed by a reward, this signal “travels” back in time and DA neurons fire in response to the reward-predicting cues (Schultz 1998).
Consistent with these animal data, functional magnetic resonance imaging (fMRI) studies have described robust activation in the human ventral striatum in response to a wide range of rewarding cues (O'Doherty 2004). Moreover, fMRI studies have described a shift in ventral striatal activation from rewards to reward-predicting cues after associative learning has occurred (e.g, O'Doherty et al. 2004). Finally, the ventral striatum has been found to be more strongly activated during the anticipation, rather than consumption, of rewards (e.g., Dillon et al. 2008). Accordingly, the ventral striatum has been strongly implicated in coding reward prediction errors and the hedonic value of outcomes, and is robustly recruited during reward anticipation.
Dorsal striatum
Similar to the NAc, putamen activation is potentiated by unexpected rewards and suppressed by omission of expected rewards, indicating that this region plays an important role in coding reward prediction errors (O’Doherty 2004). Several findings indicate, however, that dorsal striatal regions might play a particularly strong role in reward-related learning (Delgado 2007). Interestingly, whereas the ventral striatum has been implicated in stimulus-reward learning, the dorsal striatum appears to be mostly involved in stimulus-response-reward learning (O'Doherty et al. 2004), that is, in linking incentives to actions. Fitting this assumption, caudate activation was found to track the extent of behavioral adjustments during reward-based learning (Haruno et al. 2004). Similarly, putamen activation in response to monetary gains predicted behavioral adjustments in trials following reward (Wrase et al. 2007). Finally, caudate activation was particularly strong when rewards are unpredictable and participants believe that the outcome is contingent upon their action (Tricomi et al. 2004).
Orbitofrontal cortex
A large body of work implicates the OFC in stimulus-reinforcement representations, and particularly, in flexibly updating such representations to guide adaptive behavior (O'Doherty 2004). Consistent with this view, OFC lesions in humans lead to perseverative responses in reversal-learning tasks (e.g., Hornak et al. 2004), and neuroimaging studies have shown that the OFC codes incentive, rather than sensory, features of stimuli across modalities (O'Doherty 2004).
Functional neuroimaging studies assessing reward processing in MDD
In one of the first fMRI studies directly assessing reward processing in depression, Forbes and coworkers (2006) presented a task involving choices linked to varying magnitude and probabilities of reward and punishment to a pediatric sample. Compared to controls, depressed children showed reduced activation in various reward-related regions (e.g., caudate, OFC) to rewards. In an extension of this work, this group reported that depressed youth showed reduced anticipatory caudate responses immediately following a winning trial, indicating that reward anticipation was blunted after rewards in MDD (Olino et al. 2011).
Our group used a monetary incentive delay task to investigate neural substrates implicated in anticipation and consumption of monetary gains in depression (Pizzagalli et al. 2009). Relative to controls, unmedicated MDD subjects showed reduced activation in the left nucleus accumbens and bilateral caudate in response to partially unpredictable rewards as well as reduced activation in the left putamen to reward-predicting cues (Pizzagalli et al. 2009; see also Stoy et al. 2012). Reduced putamen activation was also associated with impaired reward-related reversal learning in MDD (Robinson et al. 2012), and blunted putamen/caudate activation during reward antiticipation normalized after antidepressant treatment (Stoy et al. 2012) or psychotherapy (Dichter et al. 2009). When seen in the framework of prior studies (e.g., Delgado 2007; O’Doherty et al. 2004; Wrase et al. 2007), these findings highlight neural patterns pointing to impaired hedonic coding (NAc), reward-related learning (caudate), and reward prediction (putamen) in MDD.
Direct evidence of neural abnormalities associated with reward-related learning in depression emerged from two elegant studies that combined neuroimaging and mathematical modeling of reward prediction errors. In the first, Steele and coworkers showed that, unlike controls, medicated MDD participants failed to speed up reaction time and activate ventral striatum regions after receiving positive feedback (Steele et al. 2007). Notably, lack of RT changes after positive (and negative) feedback correlated with anhedonic symptoms. In the second study, the same group investigated neural correlates of reinforcement learning in treatment-resistant MDD subjects using a Pavlovian reward learning paradigm, in which abstract pictures were probabilistically associated with delivery of water (participants were mildly water-deprived; Kumar et al. 2008). Through modeling of reward prediction errors, the authors showed that depressed subjects were characterized by smaller reward-learning signals in the ventral striatum relative to controls. Because reward-learning signals have been strongly implicated in reinforcement learning and goal-directed behavior, these findings provide important information about incentive dysfunction in depression.
Although findings reviewed above have uncovered blunted striatal activation during reward encoding and reinforcement learning, it is unclear whether such dysfunction relates to affect in daily life. This important issue was addressed in a study that combined fMRI and ecological sampling techniques in adolescents with MDD (Forbes et al. 2009). Relative to controls, youngsters with MDD showed weaker caudate activation during the anticipation and outcome of rewards, in line with findings seen in adults (Pizzagalli et al. 2009). Notably, blunted caudate activation to both anticipation and consumption of reward correlated with reduced subjective positive affect in natural settings four days before the scan.
Functional neuroimaging studies assessing responses to other appetitive stimuli in MDD
Additional evidence for reduced encoding of incentive cues in depression comes from studies that have described diminished neural responses to positive stimuli (e.g., happy facial expressions, pictures of positive social interaction) in regions implicated in reward processing, such as the ventral striatum (e.g., Epstein et al. 2006), the caudate (e.g., Elliott et al. 1998), and the OFC (e.g., Schaefer et al. 2006). Three sets of additional findings deserve mention. First, reduced reward-related ventral and dorsal striatal activation has been observed in asymptomatic children of parents with MDD as well as remitted individuals with a history of MDD (Gotlib et al. 2010; McCabe et al. 2009; Monk et al. 2008), consistent with the assumption that blunted encoding of reward-related cues might be associated with increased MDD vulnerability. Second, negative correlations between ventral striatal activation to positive cues and anhedona have been reported (Epstein et al. 2006; Keedwell et al. 2005). Third, deep brain stimulation of the NAc has antidepressant effects in highly treatment-resistant depressed subjects that persists up to four years, highlighting a possible causal link between ventral striatal dysfunction and depression (Bewernick et al. 2012).
Interim summary
Across a variety of reward tasks, depression has been associated with blunted activation of striatal regions, and less consistently, OFC. In light of prior evidence, ventral striatal dysfunctions in MDD might reflect dysfunction in coding the motivational significance of stimuli and updating predictions about expected reward. Caudate dysfunction might be linked to deficient learning of action-reward contingencies, leading to diminished positive reinforcement. Finally, OFC dysfunctions might be associated with impairments in representing the motivational value of stimuli and in updating stimulus-outcome representations. Collectively, these findings suggest that distinct psychological processes and neural abnormalities might contribute to anhedonic phenotypes. Surprisingly, no study has investigated neural correlates associated with decreased recollection of positive cues, underestimation of reinforcements received, or reduced expectation of future rewards. Given evidence for such dysfunction in the behavioral literature reviewed above, future neuroimaging studies should address these important questions.
THE ROLE OF DOPAMINE IN DEPRESSION
Decades of neuroscience research have shown that dopamine (DA) plays a critical role in reinforcement learning (Schultz 1998) and incentive motivation (Berridge 2007). Owing these findings, interest in the role of DA in the pathophysiology of depression has resurfaced (e.g., Dunlop & Nemeroff 2007). In particular, possible dysfunctions within mesolimbic DA pathways, which originate from ventral tegmental area neurons and projects to the NAc, have attracted substantial attention.
Studies Investigating DA Metabolites
One of the first indications of blunted DA transmission in depression came from reports that MDD subjects displayed reduced levels of homovanillic acid (HVA), one of the major metabolites of DA, in cerebrospinal fluid (CSF) (e.g., Mitani et al. 2006). However, several studies have failed to replicate this finding (e.g., Sher et al. 2003), whereas others reported that reduced HVA levels were present only in patients with melancholic depression (Roy et al. 1985). One possible reason for these inconsistencies is that lumbar CSF concentration may not reliably index brain DA. This issue was circumvented by Lambert and coworkers (2000), who directly measured central nervous system HVA levels through catheters placed in the internal jugular vein. Using this technique, the authors were able to confirm reduced levels of HVA, providing the strongest evidence to date of reduced DA metabolites in depression.
Studies Using DA Depletion or Challenge Paradigms
Evidence of a potential causal link between reduced DA levels and depression comes from studies that acutely lowered DA synthesis through catecholamine depletion. Miller et al. (1996) reported that administration of a tyrosine hydroxylase inhibitor (α-methylparatyrosine, AMPT) increased depressive (including anhedonic) symptoms in remitted subjects. These findings were replicated by Berman et al. (1999), who showed that AMPT administration in euthymic subjects with MDD history led to a transient but marked re-instantiation of depressive symptoms. In addition, AMPT-induced relapse of depressive symptoms was observed in two additional samples with MDD history (Bremner et al. 2003; Hasler et al. 2008).
These findings have been extended by two positron emission tomography (PET) studies, which have shown that DA depletion affects reward-related brain regions. In the first, AMPT-induced increases in depressive symptoms were accompanied by decreased activation in regions receiving strong catecholamine innervations, including the OFC (Bremner et al. 2003). More recently, Hasler and coworkers reported that AMPT administration reduced OFC but increased ventral striatal metabolism in both control and remitted depressed subjects (Hasler et al. 2008). Interestingly, ventral striatal increases were larger in remitted subjects. Moreover, among the remitted subjects, larger metabolic increases in the ventral striatum were positively correlated with anhedonic symptoms. At first glance this latter finding appears paradoxical, but it can be explained by the fact that, in the striatum, DA inhibits release of glutamate, the main excitatory transmitter in the brain. Thus, AMPT-induced reduction in DA transmission might have led to disinhibition of striatal regions, resulting in increased PET activation. Accordingly, remitted subjects reporting the strongest anhedonic effects might have had the largest reduction in DA transmission.
Similar findings emerged from a study in which administration of dextroamphetamine (a psychostimulant that induces DA release) was used to probe reward pathways in MDD. After receiving dextroamphetamine, MDD subjects displayed reduced activation in various reward-related brain regions (OFC, caudate, and putamen) in response to pleasant pictures (Tremblay et al. 2005). Behaviorally, MDD subjects also showed potentiated affective responses (e.g., euphoria) to dextroamphetamine (Tremblay et al. 2002; 2005). Interestingly, the rewarding effects of the DA agonist were largest in anhedonic subjects (Tremblay et al. 2002). Findings of increased hedonic responses and reduced activation in reward-related brain areas in response to dextroamphetamine can be explained by the DA-glutamate interactions mentioned above. Thus, DA release after dextroamphetamine might have inhibited glutamate in striatal regions, resulting in decreased striatal activation and fMRI signals. Both the behavioral and fMRI findings were interpreted as reflecting a hypofunctional reward system in MDD.
Although findings reviewed thus far suggest that MDD is characterized by reduced DA synthesis, the interpretation is complicated by null findings that AMPT administration did not modulate depressive symptoms in currently depressed, drug-free subjects (Berman et al. 2002; Miller et al. 1996), and by the fact that AMPT depletes both DA and norepinephrine. Although the lack of findings in currently depressed subjects might be explained by floor effects, acute lowering of DA synthesis through administration of an amino-acid mixture lacking DA precursors (phenylalanine and/or tyrosine) did not worsen depressive symptoms in two remitted depressed samples (McTavish et al. 2005; Roiser et al. 2005). Intererstingly, DA depletion significantly reduced betting in a gambling task in the remitted sample (Rosier et al. 2005), indicating that behavioral manifestation of reduced reward responsiveness can emerge in the absence of self-reported effects.
Molecular Imaging Studies
Findings from imaging studies using radioactive tracers to probe putative DA dysfunctions in MDD have not painted a coherent picture. However, some evidence consistent with reduced DA tone has emerged in the form of reduced dopamine transporter (DAT) density and increased density of D2 post-synaptic receptors.2
Increased D2-receptor density in depression?
Early studies using single photon emission computerized tomography (SPECT) in conjunction with the [123I]-IBZM tracer described increased striatal D2 binding in MDD (e.g., Shah et al. 1997), a finding suggesting possible compensatory up-regulation of D2 receptors due to low DA transmission. However, later studies failed to replicate this finding (e.g., Ebert et al. 1996), raising the possibility that clinical heterogeneity might contribute to these inconsistencies. In line with this, Ebert et al. (1996) found that increased D2 binding was restricted to patients with psychomotor retardation, whereas D2 receptor up-regulation was reduced in patients responding to pharmacology (Ebert et al. 1996). Similarly, reduced striatal D2 binding was found in recovered depressed subjects treated with selective serotonin reuptake inhibitors, suggesting that remission may be linked to increased endogenous DA release and/or reduced D2 receptor expression (Montgomery et al. 2007).
Decreased DAT density in depression?
Studies assessing DAT density in MDD have yielded similarly inconsistent findings, with some studies describing down-regulation of DAT densities (e.g., Sarchiapone et al. 2006), and others describing opposite results (e.g., Brunswick et al. 2003). These inconsistencies might reflect the fact that these studies have used tracers (e.g., β-CIT) that have similar affinities for the DAT and serotonin transporter, which complicates interpretations. In contrast, post-mortem studies have provided compelling evidence of reduced DAT levels in striatal regions (caudate, putamen, NAc) of depressed subjects (Bowden et al. 1997; Klimek et al. 2002).
Interim Summary
Investigations of possible DA dysfunction in MDD have received renewed interest. Findings emerging from studies assessing DA metabolites, utilizing DA depletion paradigms or neuroimaging techniques converge in suggesting reduced DA transmission in depression. In neuroimaging studies, the most replicated finding is decreased DAT binding, a dysfunction that can be explained by blunted endogenous DA transmission. Despite this evidence, it is important to emphasize that direct evidence of reduced DA release, particularly during reward tasks, is missing. When seen within the framework of prior animal studies, disruption of DA signaling can explain behavioral findings of abnormal prediction errors and blunted reinforcement learning in MDD (Kumar et al. 2008; Pizzagalli et al. 2008b).
THE ROLE OF STRESS IN DEPRESSION
The role of stress in the development, expression, and exacerbation of depression is well established (Brown & Harris 1978). In community samples, up to 70-80% of Major Depressive Episodes (MDEs) are preceded by major life events, particularly in the 1-3 months before MDE onset, and it has been estimated that stressors are approximately 2.5 times more frequent in the period before an MDE relative to a comparable period in controls (Hammen 2005; Mazure 1998). In addition, chronic stressors have been linked to poorer prognosis and more frequent relapse (e.g. Lethbridge & Allen 2008), symptom deterioration following therapy (Hawley et al. 2007), treatment resistance (Amital et al. 2008), and higher depressive symptoms in both depressed and remitted samples (Leskela et al. 2006). Recently, several reviews summarizing links between stress and depression have appeared (e.g., Hammen 2005), so the goal of the following section is not to provide an exhaustive summary. Instead, we highlight selected findings that have contributed to a more nuanced understanding of stress-depression links, and are particularly relevant to the theoretical integration presented here.
First, although severe stressors have been generally linked to increased risk of depression, chronic stressors and events characterized by a perceived (1) lack of control, (2) inability to escape or resolve the aversive situation (e.g., entrapment), or (3) loss of status (e.g., humiliation) appear to be particularly depressogenic (e.g., Brown & Harris 1978; Kendler et al. 2003). Findings emphasizing the uncontrollability component of stressors are consistent with data indicating that perceived control over stressors is a key modulator of physiological stress responses (Dickerson & Kemeny 2004). Notably, Haeffel et al. (2008) reported that stressors interacted with a cognitive vulnerability (negative inferential style) to predict decreases in self-reported goal-directed behavior, and that this effect was mediated by hopelessness. This suggests that in an ongoing stressful situation, perceived lack of control may interact with the expectation that a desired outcome will not occur to “shut down” goal-directed behavior.
Second, stressors play a stronger role in triggering first episodes of depression than recurrences (e.g., Daley et al. 2000), and the association between stressors and depression becomes weaker with increasing number of episodes (Kendler et al. 2000). These findings have been interpreted as supporting the “kindling/sensitization” theory proposed by Post (1992), which postulates that neurobiological changes occurring in response to depression and stress might “sensitize” individuals, and thus increase risk for future depressive episodes in the absence of stressors. Based on the animal literature reviewed in the next section, future studies should investigate whether dysfunctions within mesocortical and mesolimbic DA pathways might contribute to these sensitization effects.
Finally, individuals with stress-sensitized systems (e.g., individuals exposed to early adversity or with recurrent MDD) appear to be particularly affected by minor stressors later in life. For example, MDEs were triggered by smaller amounts of stress in children or adolescents exposed to early childhood adversities compared to youngsters without such histories (e.g., Hammen et al. 2000). In addition, retrospective (e.g., Gladstone et al. 1999) and prospective (Widom et al. 2007) studies reveal that early adversities (e.g., physical abuse and neglect) not only increase rates of depression, but also accelerate the emergence of depression. Interestingly, severe childhood adversities have been associated with higher rates of anhedonic symptoms (Lumley & Harkness 2007).
Interim Summary
Severe stressors, particularly those deemed uncontrollable and inescapable, play an important etiological role in depression, especially for first episodes. With increasing numbers of MDEs, the link between stress and depression becomes weaker, arguably due to kindling/sensitization processes that increase vulnerability to future episodes. Initial evidence indicates that such processes might be potentiated in individuals with a history of early adversity, which has been linked to the emergence of anhedonic symptoms. As will be discussed in the next section, these data show intriguing parallels with animal models of depression, in which chronic uncontrollable stressors induce anhedonic-like behavior and profound dysfunction within brain reward pathways.
THE EFFECTS OF STRESS ON DOPAMINERGIC PATHWAYS
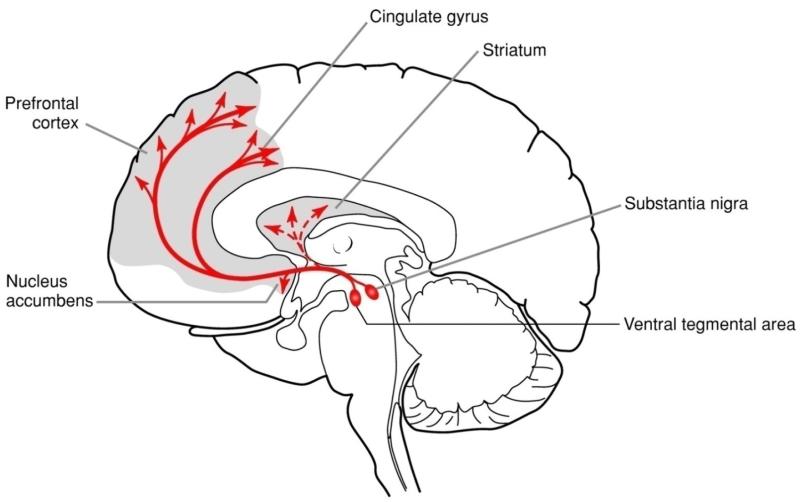
The vast majority of cell bodies producing DA can be found in various nuclei in the midbrain, including the retro-rubro field, the substantia nigra pars compacta, and the ventral tegmental area (VTA). DA-producing neurons in the VTA innervate the mesocortical and mesolimbic DA pathways, which are particularly relevant for the purpose of the present review. Originating from the VTA, the mesocortical pathway primarily projects to the prefrontal and anterior cingulate cortex (Figure 3), and plays an important role in executive function. The mesolimbic pathways originate from the VTA but project mostly to ventral striatal regions (particularly the NAc), the amygdala, and the hippocampus, among other regions (Figure 3). Functionally, the mesolimbic DA pathway has been implicated in incentive motivation and reinforcement learning. Moreover, a variety of findings indicate that DA might be particularly important for coding the incentive salience (“wanting”), rather than hedonic aspects (“linking”), of stimuli, and might thus encode motivational aspects of rewards (Berridge 2007). In the following sections, the effects of acute and chronic stressors on mesolimbic and mesocortical DA pathways will be reviewed.
Figure 3.
Schematic illustration of mesocortical and mesolimbic DA pathways. The diagram shows DA nuclei within the ventral tegmental area projecting to the nucleus accumbens and prefrontal cortex, and within the substantia nigra projecting to the dorsal striatum (caudate and putamen). Figure adapted with permission from Hyman et al. (2006).
The Effects of Acute Stressors on Motivated Behavior and Dopaminergic Transmission
Over decades, a multitude of animal studies have shown that acute mild stressors (e.g., foot shock, physical restraint) quickly and robustly activate mesocortical DA neurons, leading to a substantial increase in DA levels in the medial prefrontal cortex (mPFC) (e.g., Abercrombie et al. 1989). If animals are exposed to more severe and sustained stressors, increased DA levels are also observed in mesolimbic pathways, particularly the NAc (Cabib & Puglisi-Allegra 2012). Interestingly, stress-induced DA release starts earlier and is larger in the mPFC (95% increase of DA outflow) than in NAc (39% increase) or striatum (25% increase) (Abercrombie et al. 1989; Chrapusta et al. 1997). Behaviorally, enhanced mesolimbic DA release in the face of acute stressors has been found to promote behavioral activation and active coping (Cabib & Puglisi-Allegra 2012).
Interestingly, the mesocortical and mesolimbic DA systems respond in opposite ways when animals face inescapable or uncontrollable acute stressors. In the NAc, inhibition of mesolimbic DA release has been described when animals face sustained uncontrollable and inescapable stressors and show behavioral evidence of coping failures (Cabib & Puglisi-Allegra 2012; Rossetti et al. 1993). In an early rat study, for example, Imperato and coworkers (1992) reported that acute restraint stress led to increased extracellular DA in the NAc in the first three days of testing, whereas no DA responses were seen after the fourth day. These findings contrast with observations of increased DA release in the NAc in response to short, novel, and/or controllable aversive conditions (Chrapusta et al. 1997). Altogether, these findings indicate that blunting in mesolimbic DA release is associated with “coping failure”, including learned helplessness and behavioral “despair” (Cabib & Puglisi-Allegra 2012). Notably, antidepressant administration before exposure to uncontrollable stress prevented mesolimbic DA depletion (Rossetti et al. 1993).
Exposure to an inescapable stressor generally results in higher mPFC DA release compared to exposure to an escapable stressor of identical intensity and duration (Cuadra et al. 1999). Furthermore, prior exposure to chronic stress amplifies the response of mesocortical DA neurons in response to a subsequent acute stressor, highlighting possible sensitization effects consistent with the kindling hypothesis (Post 1992). Because DA exerts inhibitory effects on mPFC function, DA release in the mPFC in the face of uncontrollable stressors may reduce mPFC-mediated behavior, including regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Maier et al. 2006). In addition, since PFC DA transmission exerts a regulatory (inhibitory) control over DA activity in the NAc (e.g., Del Arco & Mora 2008), potentiated stress-related responses in the mesocortical DA system are expected to blunt DA release in mesolimbic pathways, and maintain depression-like behavior.
Fitting this hypothesis, when exposed to acute stressors, an inbred line of rats displaying elevated levels of “despair” showed rapid activation of the mesocortical DA system and concurrent inhibition of the mesolimbic DA system (Cabib et al. 2002). Notably, in this susceptible strain, depletion of mesocortical DA as well as chronic antidepressants reversed the stress-induced mesolimbic inhibition and eliminated stress-induced “despair” behavior (Ventura et al. 2002). Based on these findings, the authors proposed that vulnerability to depression might involve a susceptibility to “stress-induced activation of [the] mesocortical DA system leading to inhibition of the mesolimbic DA system” (Ventura et al. 2002, p. 999).3
The Effects of Chronic Stressors on Motivated Behavior and Dopaminergic Transmission
Behavioral and physiological effects
Based on the observation that early adversities and chronic stressors increase vulnerability to depression in humans (see prior section), animal models of depression soon started investigating behavioral and neurobiological sequelae of prolonged exposure to stressors. Katz was among the first to develop a stress-induced animal model of depression, in which rats were subjected to various severe stressors for a period of three weeks (Katz 1982). Notably, chronically stressed animals decreased intake of palatable solutions, suggesting a possible decreased sensitivity to reward.
This approach was subsequently modified by Willner, who developed the chronic mild stress (CMS) model, in which animals are exposed to a variety of relatively mild stressors over a prolonged period of time (Willner 2005). A multitude of rodent studies have shown that exposure to the CMS leads to: (1) decreased intake of and preference for palatable liquids (e.g., Bekris et al. 2005), which can last one month after stress termination (Elizalde et al. 2008), (2) reduced place preference conditioning (i.e., reduced approach to a place previously paired with reward; Papp et al. 1991) (3) increased threshold for brain stimulation reward in the VTA (Moreau et al. 1992); (4) reduced DA release in the NAc in response to palatable food (Di Chiara et al. 1999); and (5) reduced basal striatal DA activity but increased basal PFC DA activity (e.g., Bekris et al. 2005; Mangiavacchi et al. 2001).
In rats, CMS exposure potentiated decreases in sucrose preference elicited by a later stressor (restraint), raising the possibility that early exposure to uncontrollable stressors increases vulnerability for developing anhedonia after encountering novel stressors (Zurita et al. 2000). Highlighting promising validity, CMS-induced anhedonic-like symptoms and accumbens DA blunting could be reversed (e.g., Bekris et al. 2005; Elizalde et al. 2008) or prevented (e.g., Di Chiara et al. 1999) by antidepressant pretreatments.
In addition to the CMS model, anhedonic-like behavior and decreased motivation have emerged in other paradigms involving chronic stressors. Prolonged restraint stress, for example, has been associated with a 50% reduction in motivated behavior in an appetitive operant conditioning paradigm (Kleen et al. 2006). In a particularly innovative study, stress-induced anhedonia was greater, appeared earlier, and lasted longer in rats with a (pre-stress) “pessimistic” rather than “optimistic” trait, which was operationalized as the tendency to respond to an ambiguous tone with physical properties in-between tones previously associated with a negative (electric shock) vs. positive (food pellet) outcome, respectively (Rygula et al. 2013).
Along similar lines, sustained post-natal deprivation and maternal separation produced adult phenotypes characterized by reduced (1) motivation to obtain sucrose reward (Ruedi-Bettschen et al. 2005), (2) social motivation (Mintz et al. 2005), and (3) acquisition and expression of Pavlovian appetitive conditioning (Matthews & Robbins 2003). Notably, Pryce and coworkers (2004) found that adult monkeys subjected to early maternal separation displayed diminished motivation to obtain reward despite normative consummatory behavior. These findings fit prior reports indicating that administration of DA antagonists reduces approach motivation without affecting consumption (Pfaus & Phillips 1991), and with theories emphasizing the role of DA in incentive motivation (“wanting”; Berridge 2007).
Chronic social stress (typically induced by chronic social isolation or a resident-intruder paradigm leading to social defeat) also produced behavioral indices of anhedonia (diminished preference for sucrose solution) and motivational deficits (decreased exploratory behavior) (e.g., Grippo et al. 2007; Rygula et al. 2008). In rats, social defeat followed by isolation reduced anticipatory behavior for up to three months post-defeat (Von Frijtag et al. 2000). Notably, post-defeat social housing (Von Frijtag et al. 2000) as well as antidepressant treatment (Rygula et al. 2008) abolished anticipatory reward deficits of socially defeated animals. Similarly, three months of Pavlovian “behavioral training” involving receipt of a predictable reward restored anticipatory responses to a sucrose reward in rats that had previously been exposed to social defeat and isolation (van der Harst et al. 2005).
Long-term neurobiological effects
Physiologically, prolonged exposure to chronic unavoidable stressors leads to three abnormalities that are particularly relevant to this review: (1) downregulation of mesolimbic DA pathways; (2) reduced DAT levels; and (3) sensitization of mesocortical DA responses to novel stressors.
First, a large body of work indicates that chronic stressors lead to profound and long-lasting changes within mesolimbic DA pathways (Cabib & Puglisi-Allegra 2012). For example, chronic unavoidable stressors produced a 64% reduction in the number of spontaneously active DA neurons in the VTA (Moore et al. 2001) and reduced DA output in the NAc up to 14 days post-stress (Gambarana et al. 1999). Notably, stress-induced reduction in DA transmission has been observed in the shell of the NAc (a sub-region primarily involved in motivational processes), and reduction in mesolimbic DA was closely related to coping failures (e.g., escape deficit) and maintenance of depression-like behaviors (Mangiavacchi et al. 2001); such blunting was normalized by antidepressant treatment (Mangiavacchi et al. 2001).
Second, prolonged exposure to chronic stressors results in decreased DAT levels along mesoaccumbens DA pathways, a marker indicative of blunted mesolimbic DA release. Specifically, reduced DAT has been observed in the NAc, caudate, or putamen of adult animals exposed to (1) early maternal separation (Brake et al. 2004); (2) chronic psychosocial stress (Isovich et al. 2000; Lucas et al. 2004); and (3) prolonged immobilization stress (Lucas et al. 2007). Intriguingly, as reviewed in the section on the role of DA in depression, lowered DAT binding has been described in MDD patients (Bowden et al. 1997; Klimek et al. 2002). Reduced DAT has been also observed in rats bred for increased vulnerability to depression-like behavior, and in those displaying reduced motivation for reinforcements (e.g., Jiao et al. 2003), strengthening the hypothesis that DA dysfunctions play an important role in the pathophysiology of depression.
Of note, reduced DAT in the NAc of chronically stressed animals was observed three weeks after stress termination (Lucas et al. 2004). Conversely, successful acquisition of appetitive behavior, which had been previously shown to induce DA in the NAc (Masi et al. 2001), prevented stress-induced DAT reductions (Nanni et al. 2003). These latter findings are particularly important because they show that acquisition of appetitive behavior can partially protect against the deleterious effects of later stressors on DAT function, and highlight intriguing parallel to data on the efficacy of behavioral activation treatments for depression (Ekers et al. 2008). Of note, mice that did not succumb to a psychosocial stressor (i.e., did not show decreased sucrose consumption after social defeat) were characterized by increased plasticity and gene expression within the VTA and NAc (Krishnan et al. 2007). Moreover, in socially isolated rats, DA concentration in the ventral striatum was positively correlated with sucrose intake (Brenes & Fornaguera 2008). Together, these findings are consistent with the hypothesis that a stable and responsive brain reward system might characterize resilience (Southwick et al. 2005).
Third, various findings indicate that prior exposure to chronic stress sensitizes mesocortical DA response to novel stressors. Thus, chronically stressed rats show a potentiated mPFC DA release in response to a later stressor (Cuadra et al. 1999). Sensitized DA response in the PFC (but reduced NAc release) was also seen in rats exposed to an acute stressor 14 days after termination of chronic stress (Chrapusta et al. 1997). Because mesocortical DA neurons are hypothesized to inhibit responses of DA terminals in the NAc (King et al. 1997), this stress-induced sensitization of mesocortical DA neurons might contribute to the maintenance of anhedonic behavior.
Interim Summary
Decades of animal research have shown that early adverse events (e.g., maternal separation) as well as prolonged exposure to uncontrollable stressors leads to a downregulation of mesolimbic DA pathways and reduced responsiveness to rewarding stimuli. In light of (1) robust links between stress and depression (see section The Role of Stress in Depression), (2) evidence of DA dysfunction in depression (see section The Role of Dopamine in Depression), and (3) data highlighting a key role of phasic DA responses in the acquisition and expression of motivated behavior (this section), these preclinical findings suggest that dysfunctions in mesolimbic DA pathways might subserve disrupted positive reinforcement learning and lack of reactivity to pleasurable stimuli seen in depression. As elaborated in more detail below, these data also raise the possibility that the well-established link between stress and depression might be partially mediated by the emergence of anhedonic phenotypes.
Of primary relevance to depression research however, this preclinical work has clearly shown that these behavioral and physiological effects are dependent on the nature of the stressor (uncontrollable vs. controllable), early experiences (normal vs. adverse rearing environments), genetic make-up (Cabib & Puglisi-Allegra 2012; Ventura et al. 2002), and pre-existing “cognitive biases” (Rygula et al. 2013). The ability of an organism to exert control over a stressful situation, in particular, has been found to have profound effects on biological and behavioral processes. This feature parallels clinical literature in which the individual’s appraisal of his/her ability to cope with a stressor moderates depressogenic effects (see section The Role of Stress in Depression).
ARE DEPRESSION AND ANHEDONIA PATHOLOGICAL CONDITIONS ARISING FROM DYSFUNCTIONAL INTERACTIONS BETWEEN STRESS AND THE BRAIN REWARD SYSTEM? A SYNTHESIS
The main goals of the present paper were twofold. The first goal was to review three large bodies of literature that have emphasized the prominent roles of anhedonia, DA, and stress in depression. More importantly, in light of the fact that these three fields of inquiry have evolved largely independently from each other, the second goal was to integrate these literatures, and advance the hypothesis that depression and anhedonia are pathological conditions arising from dysfunctional interactions between stress and the brain reward system. To this end, we reviewed a fourth body of preclinical work that convincingly shows that chronic, uncontrollable stressors induce an anhedonic phenotype as well as profound and long-lasting neurobiological changes within mesocortical and mesolimbic pathways. Because these animal data are rarely considered in clinical science, we identified several similarities between human and preclinical findings, cognizant of the limitations of translating animal findings to humans. Based on this convergence, we propose that stress induces anhedonic behavior by causing dysfunction in mesolimbic DA pathways implicated in incentive motivation and reinforcement learning; stress-induced anhedonia may, in turn, strengthten the relationship between stress and depression. In the next sections, empirical data supporting this proposition in humans will be presented, followed by a discussion of future directions.
Several sets of findings provide correlational evidence in favor of links among stress, DA, and depression in humans. First, the melancholic subtype of depression, in which anhedonia plays a key role (American Psychiatric Association 2000), has been associated with both hypercortisolemia (Gold & Chrousos 1999) and increased perceived severity of stressors (Willner et al. 1990). Second, individuals with elevated anhedonic symptoms reported higher perceived stress relative to controls despite similar exposure to stressors (Horan et al. 2007). Along similar lines, we found that subjects appraising their life as being unpredictable, uncontrollable, and overwhelming showed decreased reward responsiveness (Pizzagalli et al. 2007). A recent study using experience sampling procedures further extended these findings by showing that MDD subjects were characterized by increased stress sensitivity and reduced reward experience (Wichers et al. 2008). Antidepressant treatment normalized both effects. Interestingly, increased reward experience–but not reduction in stress sensitivity–from baseline to week six predicted treatment response, suggesting that normalization of hedonic capacity is key for fostering symptom remission.
These correlational findings have been complemented by three sets of data that have provided important evidence that stressors can indeed decrease hedonic capacity and/or reward responsiveness in humans. First, both acute (Al'Absi et al. 2012) and chronic (Berenbaum & Connelly 1993) stress reduced self-reported rating of pleasure. Notably, these effects were largest in individuals reporting a familial history of depression (Berenbaum & Connelly 1993), suggesting that stress-induced hedonic blunting may be strongest in individuals at increased risk for depression. Second, both acute laboratory (Bogdan & Pizzagalli 2006; Bogdan et al. 2011; Figure 2b) and naturalistic (Nikolova et al. 2012) stressors reduced participants’ ability to modulate behavior as a function of rewards, providing important empirical evidence that stress reduces reinforcement learning. Interestingly, stress-induced reductions reward responsiveness were largest in individuals with elevated anhedonic symptoms (Bogdan & Pizzagalli 2006) or carrying variants of the corticotrophin-releasing hormone type 1 receptor (CRHR1) gene previously linked to stress regulation (Bogdan et al. 2011). When seen within the framework of preclinical evidence, these findings indicate that both objective stressors and perceived lack of control over stressors might reduce hedonic capacity and reinforcement learning. Whether these behavioral effects are accompanied by perturbation within mesolimbic and mesocortical DA pathways remains to be tested.
Finally, recent findings indicate that early adversities are associated with behavioral and neural evidence of reduced reward processing. In a sample of depressed adolescents, childhood maltreatment correlated with adult anhedonic symptoms (Lumley & Harkness 2007), and such link was mediated by themes of loss and worthlessness. In a “wheel-of-fortune” task, maltreated children with depressive disorders showed a conservative decision-making strategy during high-risk trials (Guyer et al. 2006). We extended these findings by showing that (1) women with a history of MDD and childhood maltreatment were characterized by neural and behavioral deficits in utilizing previous reinforcement to optimize decision making (Pechtel & Pizzagalli 2013), and (2) euthymic young adults with a history of childhood adversity showed blunted striatal activation during reward–but not penalty–anticipation (Dillon et al. 2009). This selective dysfunction was hypothesized a priori due to preclinical data indicating that (1) early adverse events have long-lasting effects on mesolimbic DA pathways (Ruedi-Bettschen et al. 2005; Pryce et al. 2004); and (2) DA has been primarily implicated in the anticipatory phase (“wanting”) rather than consummatory phase (“liking”) of reward processing (Berridge 2007).
In sum, multiple lines of evidence converge to suggest that both chronic stressors and early childhood adversities might increase risk for depression by reducing hedonic capacity, incentive motivation, and reinforcement learning, providing promising leads for therapeutic interventions. Whether these deficits in humans are associated with reduction in mesolimbic DA signaling, however, remains unknown. In an initial attempt to elucidate this relationship, we performed a pharmacological challenge study in psychiatrically healthy subjects and found that administration of a single low dose of a D2/D3 agonist (pramipexole), which is hypothesized to decrease phasic DA due to presynaptic autoreceptor activation, reduced reward responsiveness (Figure 2c; Pizzagalli et al. 2008a). Critically, this blunted reward responsiveness was qualitatively similar to the pattern observed in both unmedicated MDD subjects (Pizzagalli et al. 2008b; Figure 2a) and healthy controls under acute stress (Bogdan & Pizzagalli 2006; Figure 2b), providing promising convergence. We expect that similar pharmacological challenges in both control and MDD subjects, particularly when combined with functional neuroimaging and computational modeling (e.g., Kumar et al. 2008), will provide important insights into mechanisms underlying blunted hedonic bahaviors and incentive motivation in depression.
FUTURE DIRECTIONS AND CAVEATS
There are several unanswered questions concerning the etiology and pathophysiology of depression that require further attention. First, with the exception of few recent studies describing reduced reward-related striatal (NAc, putamen) and OFC activation in individuals at increased risk for depression (offspring of parents with MDD; Gotlib et al. 2010; McCabe et al. 2012; Monk et al. 2008), most neuroimaging studies have investigated symptomatic subjects during an MDE. Along similar lines, although reduced DAT density might arise as a compensatory mechanism linked to reduced DA transmission, no studies can exclude the alternative hypothesis that decreased DAT levels constitute the primary abnormality that increases MDD vulnerability. Consequently, studies investigating at-risk samples, including monozygotic twins discordant for depression, individuals with cognitive vulnerability or carrying genetic variants associated increased depression liability, before the onset of the first MDE, will be needed to investigate whether striatal or DAT dysfunctions is a consequence or cause of MDD.
Second, although evidence indicates that behavioral activation treatments are effective in alleviating depressive symptoms (Ekers et al. 2008), it is currently unknown whether such treatment might be particularly useful for MDD subjects characterized by reinforcement learning deficits and/or mesolimbic DA blunting. Such knowledge would be important for moving us closer to personalized treatments.
Third, with the exception of few recent attempts aiming at parsing distinct components of reward processing in MDD (e.g., Pizzagalli et al. 2008b; Sherdell et al. 2012; Treadway et al. 2002), little attention has been devoted to investigating the possibility that anhedonia is psychologically and neurobiologically complex. Identifying environmental and biological factors associated with different manifestations of anhedonia might enrich our understanding of the etiology and pathophysiology of depressive phenotypes.
Fourth, in spite of the focus in this review on mesocorticolimbic DA pathways, depression has been clearly associated with dysfunction in other key regions implicated in affect and emotion regulation, including the PFC, amygdala, and hippocampus (Pizzagalli & Treadway, in press). Although a summary of this literature is beyond the scope of this review, it is important to emphasize that these regions have been implicated in the regulation of stress responses and coping, albeit in different ways. Thus, whereas the mPFC and hippocampus provide inhibitory control over stress responses, the amygdala has been implicated in potentiating stress responses (Diorio et al. 1993). Elegant work by Maier and coworkers, in particular, has shown that detection of control within a stressful situation activates the mPFC, which in turn inhibits stress-induced activation of brainstem and limbic regions (Maier et al. 2006). Intriguingly, experience of stress controllability has been found to modify mPFC responses to future uncontrollable stressors, which might contribute to increases in resilience. Based on this evidence, we hypothesize that in MDD, mPFC, hippocampal, and amygdala dysregulation is associated with increased stress responsiveness (Figure 1).
Fifth, despite compelling evidence that stressors have profound effects on mesocorticolimbic DA pathways, it is not immediately clear how specific this link is. Is DA particularly susceptible to the deleterious effects of stressors, especially uncontrollable ones? Or, are other neurotransmitters equally affected by such perturbations, with the emphasis on DA simply the consequence of a strong focus on reward processing?
Finally, although we have emphasized the effects of chronic stressors, including early rearing environments, on reward processing and mesolimbic DA pathways in both the animal and human literature, it is clear that such stressors have long-lasting effects on other processes, including enhanced perceptual sensitivity to threat-related cues, exaggerated HPA responses, and hippocampal dysfunction, among others (for review, see Pechtel & Pizzagalli 2011). Future human research should pay particular attention to the role of epigenetic effects4 in increasing risk for MDD, especially in the context of early adversity. Seminal work by Meaney, Turecki and others has shown that low levels of maternal care and chronic stress in rodents was associated with adult phenotypes characterized by potentiated behavioral and endocrinological responses to stress, reduced appetitive behavior, and increased vulnerability to stress-induced learned helplessness (for reviews, see Zhang et al. 2013; Luzt & Turecki 2013). Critically, these phenotypes were associated with increased methylation of the promoter region of the glucocorticoid receptor (GR) gene in hippocampal neurons, which in turn reduced gene expression and thus the number of GR in the hippocampus. These alterations ultimately resulted in blunted glucocorticoid negative feedaback sensitivity and exaggerated corticotrophin-releasing factor (CRF) levels. Initial findings of increased DNA methylation in the hippocampus of suicide victims with a history of childhood adversity (McGowan et al. 2009; Labonté et al. 2012) as well as evidence of methylation in the promoter region of the GR gene in volunteers reporting childhood maltreatment (Tyrka et al. 2012) suggest that similar processes might occur in humans. Collectively, this emerging evidence indicates that epigenetic-mediated alterations in the expressions of genes implicated in stress regulation could be a mechanism through which the environment can confer increased vulnerability to MDD. Clearly, these mechanisms deserve further scrutiny, since they might open important avenues for prevention.
CONCLUSION
In conclusion, depression is etiologically and pathophysiologically complex and heterogeneous. In the present review, we have focused on a promising endophenotype of depression–anhedonia–and proposed that this cardinal symptom of and risk factor for depression might arise due to the detrimental effects of stressors on mesocorticolimbic DA pathways. Although limited by a focus on anhedonia, the present review proposes a working model that makes precise hypotheses about behavioral (e.g., decreased reward encoding and reinforcement learning), neurochemical (e.g., blunted mesolimbic DA transmission), and neurobiological (e.g., functional and structural striatal dysfunctions) abnormalities that might characterize links between stress and depression. We expect that approaches focusing on intermediate depressive phenotypes will help overcome the limitations of current classification systems, will propel the field toward a better understanding of this debilitating disease, and will facilitate the development of much needed prevention and treatment approaches.
ACKNOWLEDGMENTS
The author is deeply indebted to the many members of his laboratory for their critical contributions and thoughtful discussions over the years, which fueled the work described here. A special thanks to Daniel G. Dillon, Ryan Bogdan, and Sunny Dutra for commenting on early versions of this manuscript. The author’s work has been supported by grants from the National Institute of Mental Health (NIMH; R01 MH068376, R01 MH095809, R21 MH078979), National Center for Complementary & Alternative Medicine (NCCAM; R21 AT002974) and a NARSAD Independent Investigator Award.
LIST OF ACRONYMS
Ten most important acronyms used in text:
- CMS
chronic mild stress
- DA
dopamine
- DAT
dopamine transporter
- fMRI
functional magnetic resonance imaging
- HVA
homovanillic acid
- MDD
major depressive disorder
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- OFC
orbitofrontal cortex
- VTA
ventral tegmental area
Additional acronyms:
- AMPT
α-methylparatyrosine
- CSF
cerebrospinal fluid
- SERT
self-referent encoding task
- PET
positron emission tomography
Footnotes
DISCLOSURE STATEMENT
Over the past 3 years, Dr Pizzagalli has received consulting fees from ANT North America (Advanced Neuro Technology), AstraZeneca, Shire, Servier and Ono Pharma USA and honoraria from AstraZeneca for projects unrelated to the present review.
FOOTNOTES
1. In light of concerns that current definitions of psychiatric diseases are not necessarily biologically valid, the specificity criterion might not be essential.
2. An important point emerging from basic neuroscience research is that the DA system shows a high degree of plasticity depending on DA availability. Pharmacologically-induced depletion of DA, for example, leads to down-regulation of the dopamine transporter (DAT) and up-regulation of post-synaptic D2 receptors (e.g., Gordon et al. 1996). Consequently, reduced DAT, for example, would be expected to lead to lower re-uptake of DA into pre-synaptic terminals, allowing DA to act longer within the synaptic cleft. Accordingly, in MDD, both decreased DAT density and up-regulation of D2 receptors in striatal regions might represent compensatory mechanisms due to blunted endogenous DA transmission.
3. Recent reviews have emphasized that adolescence – a period characterized by a dramatic increase in the onset of depression and the emergence of gender differences – is characterized by the lowest levels of DA in striatal regions and highest levels of DA in prefrontal regions throughout ontogeny (e.g., Spear 2000). Based on the evidence reviewed above, this imbalance toward PFC DA might leave the adolescent brain more vulnerable to the effects of stress and what Spear called a “mini-reward deficiency syndrome” (p. 446) characterized by reduced reports of positive affect and reactivity to mildly pleasurable cues, which might increase risk for depression and motivate adolescents to compensate through risk-taking behavior or experimentation with drugs.
4. Epigenetics refers to “functionally relevant modifications to the genome that do not involve a change in nucleotide sequence” (i.e., DNA) (p. 752, Bagot & Meaney 2010). Examples of epigenetics modification include histone methylation, histone acetylatin/deacetylatin, and DNA methylation. Importantly, promoter methylation has been typically linked to “silencing” of gene transcription. Studies in rodents have demonstrated that early adverse experiences and chronic stress can “program” stable changes in gene expression, which in turn shape individual differences in behavioral and physiological responses to stress in adulthood (Bagot & Meaney 2010; Lutz & Turecki 2013).
LITERATURE CITED
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J. Neurochem. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Al'Absi M, Nakajima M, Hooker S, Wittmers L, Cragin T. Exposure to acute stress is associated with attenuated sweet taste. Psychophysiology. 2012;49:96–103. doi: 10.1111/j.1469-8986.2011.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Press; Washington, DC: 2000. text revision. [Google Scholar]
- Amital D, Fostick L, Silberman A, Beckman M, Spivak B. Serious life events among resistant and non-resistant MDD patients. J. Affect. Disord. 2008;110:260–264. doi: 10.1016/j.jad.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bagot RC, Meaney MJ. Epigenetics and the biological basis of Gene x Environment interactions. J. Am. Acad. Child Adol. Psychiatry. 2010;49:752–71. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bandura A. The Nature of Reinforcement. Academic Press, Inc; New York and London: 1971. [Google Scholar]
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav. Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J. The effect of stress on hedonic capacity. J. Abnorm. Psychol. 1993;102:474–81. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J. Abnorm. Psychol. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghorst L, Pizzagalli DA. Defining depression endophenotypes. In: Beyer CE, Stahl SA, editors. In Next Generation Antidepressants. Moving Beyond Monoamines To Discover Novel And Differentiated Treatment Strategies For Mood Disorders. Cambridge University Press; Cambridge, UK: 2010. pp. 70–89. [Google Scholar]
- Berman RM, Narasimhan M, Miller HL, Anand A, Cappiello A, et al. Transient depressive relapse induced by catecholamine depletion: Potential phenotypic vulnerability marker? Arch. Gen. Psychiatry. 1999;56:395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- Berman RM, Sanacora G, Anand A, Roach LM, Fasula MK, et al. Monoamine depletion in unmedicated depressed subjects. Biol. Psychiatry. 2002;51:469–73. doi: 10.1016/s0006-3223(01)01285-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: The case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: Implications for depression. Biol. Psychiatry. 2006;60:1147–54. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J. Neurosci. 2011;31:13246–54. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden C, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW. Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res. 1997;769:135–40. doi: 10.1016/s0006-8993(97)00692-6. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur. J. Neurosci. 2004;19:1863–74. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, et al. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: Implications for the neural circuitry of depression. JAMA. 2003;289:3125–34. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes JC, Fornaguera J. Effects of environmental enrichment and social isolation on sucrose consumption and preference: Associations with depressive-like behavior and ventral striatum dopamine. Neurosci. Lett. 2008;436:278–82. doi: 10.1016/j.neulet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris T. Social origins of depression: A study of psychiatric disorder in women. Free Press; New York: 1978. [Google Scholar]
- Brunswick DJ, Amsterdam JD, Mozley PD, Newberg A. Greater availability of brain dopamine transporters in major depression shown by [99m Tc]TRODAT-1 SPECT imaging. Am. J. Psychiatry. 2003;160:1836–41. doi: 10.1176/appi.ajp.160.10.1836. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci. Biobehav. Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Cabib S, Ventura R, Puglisi-Allegra S. Opposite imbalances between mesocortical and mesoaccumbens dopamine responses to stress by the same genotype depending on living conditions. Behav. Brain Res. 2002;129:179–85. doi: 10.1016/s0166-4328(01)00339-4. [DOI] [PubMed] [Google Scholar]
- Chrapusta SJ, Wyatt RJ, Masserano JM. Effects of single and repeated footshock on dopamine release and metabolism in the brains of Fischer rats. J. Neurochem. 1997;68:2024–31. doi: 10.1046/j.1471-4159.1997.68052024.x. [DOI] [PubMed] [Google Scholar]
- Cuadra G, Zurita A, Lacerra C, Molina V. Chronic stress sensitizes frontal cortex dopamine release in response to a subsequent novel stressor: Reversal by naloxone. Brain Res. Bull. 1999;48:303–8. doi: 10.1016/s0361-9230(98)00179-8. [DOI] [PubMed] [Google Scholar]
- Daley SE, Hammen C, Rao U. Predictors of first onset and recurrence of major depression in young women during the 5 years following high school graduation. J. Abnorm. Psychol. 2000;109:525–33. [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol. Biochem. Behav. 2008;90:226–35. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol. Psychiatry. 1999;46:1624–33. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early- and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–40. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol. Psychiatry. 2009;66:886–97. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol. Psychiatry. 2009;66:206–13. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson KS, Shaw BF. Specificity and stability of self-referent encoding in clinical depression. J. Abnorm. Psychol. 1987;96:34–40. doi: 10.1037//0021-843x.96.1.34. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression-striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology (Berl) 1996;126:91–4. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Ekers D, Richards D, Gilbody S. A meta-analysis of randomized trials of behavioural treatment of depression. Psychol. Med. 2008;38:611–23. doi: 10.1017/S0033291707001614. [DOI] [PubMed] [Google Scholar]
- Elizalde N, Gil-Bea FJ, Ramirez MJ, Aisa B, Lasheras B, et al. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology (Berl) 2008;199:1–14. doi: 10.1007/s00213-007-1035-1. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ. Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol. Med. 1998;28:559–71. doi: 10.1017/s0033291798006709. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry. 2006;163:1784–90. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, et al. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. J. Child. Psychol. Psychiatry. 2006;47:1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol. Psychiatry. 2007;61:633–9. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Tagliamonte A, Scheggi S, Ghiglieri O, De Montis MG. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: A microdialysis study. J. Neurochem. 1999;72:2039–46. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- Gladstone G, Parker G, Wilhelm K, Mitchell P, Austin MP. Characteristics of depressed patients who report childhood sexual abuse. Am. J. Psychiatry. 1999;156:431–7. doi: 10.1176/ajp.156.3.431. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. The endocrinology of melancholic and atypical depression: relation to neurocircuitry and somatic consequences. Proc. Assoc. Am. Physicians. 1999;111:22–34. doi: 10.1046/j.1525-1381.1999.09423.x. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Res. 2008;159:18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur. J. Pharmacol. 1996;298:27–30. doi: 10.1016/0014-2999(95)00770-9. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. J. Abnorm. Psychol. 2004;113:386–98. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McLachlan A, Katz AN. Biases in visual attention in depressed and nondepressed individuals. Cogn. Emot. 1988;2:185–200. [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch. Gen. Psychiatry. 2010;67:380–7. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greden JF. The burden of disease for treatment-resistant depression. J. Clin. Psychiatry. 2001;62(Supplement 16):26–31. [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom. Med. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, et al. Behavioral alterations in reward system function: The role of childhood maltreatment and psychopathology. J. Am. Acad. Child. Adolesc. Psychiatry. 2006;45:1059–67. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM, Deyoung CG, et al. Association between polymorphisms in the dopamine transporter gene and depression: Evidence for a gene-environment interaction in a sample of juvenile detainees. Psychol. Sci. 2008;19:62–9. doi: 10.1111/j.1467-9280.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu. Rev. Clin. Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. J. Consult. Clin. Psychol. 2000;68:782–87. [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, et al. A neural correlate of reward-based behavioral learning in caudate nucleus: A functional magnetic resonance imaging study of a stochastic decision task. J. Neurosci. 2004;24:1660–5. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch. Gen. Psychiatry. 2008;65:521–31. doi: 10.1001/archpsyc.65.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley L, Ho RM, Zuroff D, Blatt S. Stress reactivity following brief treatment for depression: Differential effects of psychotherapy and medication. J. Consult. Clin. Psychol. 2007;75:244–56. doi: 10.1037/0022-006X.75.2.244. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J. Abnorm. Psychol. 1994;103:460–6. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Armento ME, Cantu MS, Chambers LL, Lejuez CW. The use of daily diaries to assess the relations among mood state, overt behavior, and reward value of activities. Behav. Res. Ther. 2003;41:1137–48. doi: 10.1016/s0005-7967(03)00017-2. [DOI] [PubMed] [Google Scholar]
- Horan WP, Brown SA, Blanchard JJ. Social anhedonia and schizotypy: The contribution of individual differences in affective traits, stress, and coping. Psychiatry Res. 2007;149:147–56. doi: 10.1016/j.psychres.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J. Cogn. Neurosci. 2004;16:463–78. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Can neuroscience be integrated into the DSM-V? Nat. Rev. Neurosci. 2007;8:725–32. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–9. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- Isovich E, Mijnster MJ, Flugge G, Fuchs E. Chronic psychosocial stress reduces the density of dopamine transporters. Eur. J. Neurosci. 2000;12:1071–8. doi: 10.1046/j.1460-9568.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt S. Strain differences in the distribution of dopamine transporter sites in rat brain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:913–9. doi: 10.1016/S0278-5846(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Joormann J, Gotlib IH. Does processing of emotional stimuli predict symptomatic improvement and diagnostic recovery from major depression? Emotion. 2007;7:201–6. doi: 10.1037/1528-3542.7.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J. Abnorm. Psychol. 2006;115:705–14. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol. 2007;116:80–5. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J. Abnorm. Psychol. 2007;116:135–43. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Katz RJ. Animal model of depression: Pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 1982;16:965–8. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch. Gen. Psychiatry. 2003;60:789–96. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the "kindling" hypothesis. Am. J. Psychiatry. 2000;157:1243–51. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- King D, Zigmond MJ, Finlay JM. Effects of dopamine depletion in the medial prefrontal cortex on the stress-induced increase in extracellular dopamine in the nucleus accumbens core and shell. Neuroscience. 1997;77:141–53. doi: 10.1016/s0306-4522(96)00421-6. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav. Neurosci. 2006;120:842–51. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: A postmortem study. Biol. Psychiatry. 2002;52:740–8. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131:2084–93. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Ueda K, Onoda K, Okada G, et al. Effects of depression on reward-based decision making and variability of action in probabilistic learning. J. Behav. Ther. Exp. Psychiatry. 2012;43:1088–94. doi: 10.1016/j.jbtep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Labonté B, Suderman M, Maussion G, Navaro L, Yerko V, et al. Genome-wide epigenetic regulation by early-life trauma. Arch. Gen. Psychiatry. 2012;69:722–31. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Agren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: Evidence in support of the catecholamine hypothesis of mood disorders. Arch. Gen. Psychiatry. 2000;57:787–93. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Res. 2004;128:123–33. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Leskela U, Rytsala H, Komulainen E, Melartin T, Sokero P, et al. The influence of adversity and perceived social support on the outcome of major depressive disorder in subjects with different levels of depressive symptoms. Psychol. Med. 2006;36:779–88. doi: 10.1017/S0033291706007276. [DOI] [PubMed] [Google Scholar]
- Lethbridge R, Allen NB. Mood induced cognitive and emotional reactivity, life stress, and the prediction of depressive relapse. Behav. Res. Ther. 2008;46:1142–50. doi: 10.1016/j.brat.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, et al. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–57. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Wang CJ, McCall TJ, McEwen BS. Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 2007;1155:108–15. doi: 10.1016/j.brainres.2007.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley MN, Harkness KL. Specificity in the relations among childhood adversity, early madladaptive schemas, and symptom profiles in adolescent depression. Cogn. Ther. Res. 2007;31:639–57. [Google Scholar]
- Luzt P-E, Turecki G. DNA methylation and childhood maltreatment: From animal models to human studies. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.07.069. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymos S, Labonté B, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associated with childhood abuse. Nat. Neurosci. 2009;12:342–6. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin. Neurosci. 2006;8:397–406. doi: 10.31887/DCNS.2006.8.4/smaier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana C. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J. Neurochem. 2001;79:1113–21. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- Masi F, Scheggi S, Mangiavacchi S, Tolu P, Tagliamonte A, De, et al. Dopamine output in the nucleus accumbens shell is related to the acquisition and the retention of a motivated appetitive behavior in rats. Brain Res. 2001;903:102–9. doi: 10.1016/s0006-8993(01)02442-8. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci. Biobehav. Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Mazure CM. Life stressors as risk factors in depression. Clin. Psychol. Sci. Pract. 1998;5:291–313. [Google Scholar]
- McCabe C, Woffindale C, Harmer CJ, Cowen PJ. Neural processing of reward and punishment in young people at increased familial risk of depression. Biol. Psychiatry. 2012;72:588–94. doi: 10.1016/j.biopsych.2012.04.034. [DOI] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 2009;205:667–77. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SB, Gotlib IH, Martin RA. Cognitive vulnerability for depression: Deployment of attention as a function of history of depression and current mood state. Cogn. Ther. Res. 2000;24:427–44. [Google Scholar]
- McFarland BR, Klein DN. Emotional reactivity in depression: Diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress. Anxiety. 2009;26:117–22. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Mannie ZN, Harmer CJ, Cowen PJ. Lack of effect of tyrosine depletion on mood in recovered depressed women. Neuropsychopharmacology. 2005;30:786–791. doi: 10.1038/sj.npp.1300665. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J. Am. Acad. Child. Adolesc. Psychiatry. 2012;51:404–11. doi: 10.1016/j.jaac.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: Some conjectures. Bull. Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- Miller HL, Delgado PL, Salomon RM, Berman R, Krystal JH, et al. Clinical and biochemical effects of catecholamine depletion on antidepressant-induced remission of depression. Arch. Gen. Psychiatry. 1996;53:117–28. doi: 10.1001/archpsyc.1996.01830020031005. [DOI] [PubMed] [Google Scholar]
- Mintz M, Ruedi-Bettschen D, Feldon J, Pryce CR. Early social and physical deprivation leads to reduced social motivation in adulthood in Wistar rats. Behav. Brain Res. 2005;156:311–20. doi: 10.1016/j.bbr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Miranda R, Mennin DS. Depression, generalized anxiety disorder, and certainty in pessimistic predictions about the future. Cogn. Ther. Res. 2007;31:71–82. [Google Scholar]
- Mitani H, Shirayama Y, Yamada T, Kawahara R. Plasma levels of homovanillic acid, 5-hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:531–4. doi: 10.1016/j.pnpbp.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165:90–8. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Stokes P, Kitamura Y, Grasby PM. Extrastriatal D2 and striatal D2 receptors in depressive illness: Pilot PET studies using [11C]FLB 457 and [11C]raclopride. J. Affect. Disord. 2007;101:113–22. doi: 10.1016/j.jad.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001;24:410–9. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Moos RH, Cronkite RC. Symptom-based predictors of a 10-year chronic course of treated depression. J. Nerv. Ment. Dis. 1999;187:360–8. doi: 10.1097/00005053-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic unpredictable mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behavior in rats. Eur. Neuropsychopharmacol. 1992;2:43–9. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Muris P, van der Heiden S. Anxiety, depression, and judgments about the probability of future negative and positive events in children. J. Anxiety Disord. 2006;20:252–61. doi: 10.1016/j.janxdis.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Nanni G, Scheggi S, Leggio B, Grappi S, Masi F, et al. Acquisition of an appetitive behavior prevents development of stress-induced neurochemical modifications in rat nucleus accumbens. J. Neurosci. Res. 2003;73:573–80. doi: 10.1002/jnr.10685. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Craighead WE. Selective recall of positive and negative feedback, self-control behaviors, and depression. J. Abnorm. Psychol. 1977;86:379–88. doi: 10.1037//0021-843x.86.4.379. [DOI] [PubMed] [Google Scholar]
- Nikolova Y, Bogdan R, Pizzagalli DA. Perception of a naturalistic stressor interacts with 5-HTTLPR/rs25531 genotype and gender to impact reward responsiveness. Neuropsychobiology. 2012;65:45–54. doi: 10.1159/000329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Silk JS, et al. "I won, but I'm not getting my hopes up": depression moderates the relationship of outcomes and reward anticipation. Psychiatry Res. 2011;194:393–5. doi: 10.1016/j.pscychresns.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl) 1991;104:255–59. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl) 2011;214:55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Disrupted reinforcement learning and maladaptive behavior in women with a history of childhood sexual abuse: a high-density event-related potential study. JAMA Psychiatry. 2013;70:499–507. doi: 10.1001/jamapsychiatry.2013.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J. Psychiatr. Res. 2013 doi: 10.1016/j.jpsychires.2013.08.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad SM, Polivy J. Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J. Abnorm. Psychol. 1993;102:358–68. doi: 10.1037//0021-843x.102.3.358. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav. Neurosci. 1991;105:727–43. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Bogdan R, Ratner KG, Jahn AL. Increased perceived stress is associated with blunted hedonic capacity: Potential implications for depression research. Behav. Res. Ther. 2007;45:2742–53. doi: 10.1016/j.brat.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: Behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008a;196:221–32. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated subjects with Major Depressive Disorder. Am. J. Psychiatry. 2009;166:702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in Major Depressive Disorder: Evidence from a probabilistic reward task. J. Psychiatr. Res. 2008b;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Treadway MT. Neuroimaging approaches to Major Depressive Disorder – From “where?” to “why?”. In: Gotlib IH, Hammen CL, editors. In Handbook of Depression. 3rd. Guilford; New York: in press. [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am. J. Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol. Psychiatry. 2004;56:72–9. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biol. Psychiatry. 2007;61:231–9. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Rawal A, Collishaw S, Thapar A, Rice F. 'The risks of playing it safe': a prospective longitudinal study of response to reward in the adolescent offspring of depressed parents. Psychol. Med. 2013;43:27–38. doi: 10.1017/S0033291712001158. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am. J. Psychiatry. 2012;169:152–9. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiser JP, McLean A, Ogilvie AD, Blackwell AD, Bamber DJ, et al. The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology. 2005;30:775–85. doi: 10.1038/sj.npp.1300659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti ZL, Lai M, Hmaidan Y, Gessa GL. Depletion of mesolimbic dopamine during behavioral despair: Partial reversal by chronic imipramine. Eur. J. Pharmacol. 1993;242:313–5. doi: 10.1016/0014-2999(93)90257-i. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–46. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Roy A, Pickar D, Linnoila M, Doran AR, Ninan P, Paul SM. Cerebrospinal fluid monoamine and monoamine metabolite concentrations in melancholia. Psychiatry Res. 1985;15:281–92. doi: 10.1016/0165-1781(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav. Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Ruther E, Hiemke C, et al. Pharmacological validation of a chronic social stress model of depression in rats: Effects of reboxetine, haloperidol and diazepam. Behav. Pharmacol. 2008;19:183–96. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Rygula R, Papciak J, Popik P. Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.116. 2013 May 10. doi: 10.1038/npp.2013.116. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchiapone M, Carli V, Camardese G, Cuomo C, Di Giuda D, et al. Dopamine transporter binding in depressed patients with anhedonia. Psychiatry Res. 2006;147:243–8. doi: 10.1016/j.pscychresns.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schaefer HS, Putnam KM, Benca RM, Davidson RJ. Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol. Psychiatry. 2006;60:974–86. doi: 10.1016/j.biopsych.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Serfaty MA, Bothwell R, Marsh R, Ashton H, Blizard R, Scott J. Event-related potentials and cognitive processing of affectively toned words in depression. J. Psychophysiol. 2002;16:56–66. [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol. Med. 1997;27:1247–56. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- Sher L, Oquendo MA, Li S, Huang YY, Grunebaum MF, et al. Lower CSF homovanillic acid levels in depressed patients with a history of alcoholism. Neuropsychopharmacology. 2003;28:1712–9. doi: 10.1038/sj.npp.1300231. [DOI] [PubMed] [Google Scholar]
- Sherdell L, Waugh CE, Gotlib IH. Anticipatory pleasure predicts motivation for reward in major depression. J. Abnorm. Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J. Abnorm. Psychol. 2001;110:488–93. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spijker J, Bijl RV, de Graaf R, Nolen WA. Determinants of poor 1-year outcome of DSM-III-R major depression in the general population: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta. Psychiatr. Scand. 2001;103:122–30. doi: 10.1034/j.1600-0447.2001.103002122.x. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–74. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J. Psychopharmacol. 2012;26:677–88. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–8. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Suslow T, Dannlowski U, Lalee-Mentzel J, Donges US, Arolt V, Kersting A. Spatial processing of facial emotion in patients with unipolar depression: A longitudinal study. J. Affect. Disord. 2004;83:59–63. doi: 10.1016/j.jad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Taylor L, Ingram RE. Cognitive reactivity and depressotypic information processing in children of depressed mothers. J. Abnorm. Psychol. 1999;108:202–10. doi: 10.1037//0021-843x.108.2.202. [DOI] [PubMed] [Google Scholar]
- Timbremont B, Braet C. Cognitive vulnerability in remitted depressed children and adolescents. Behav. Res. Ther. 2004;42:423–7. doi: 10.1016/S0005-7967(03)00151-7. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol. 2012;121:553–8. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Arch. Gen. Psychiatry. 2002;59:409–16. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch. Gen. Psychiatry. 2005;62:1228–36. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. Plos ONE. 2012;7(1):e30148. doi: 10.1371/journal.pone.0030148. Doi:10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol. Med. 2012;42:967–80. doi: 10.1017/S0033291711001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Harst JE, Baars AM, Spruijt BM. Announced rewards counteract the impairment of anticipatory behaviour in socially stressed rats. Behav. Brain Res. 2005;161:183–9. doi: 10.1016/j.bbr.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Puglisi-Allegra S. Genetic susceptibility of mesocortical dopamine to stress determines liability to inhibition of mesoaccumbens dopamine and to behavioral 'despair' in a mouse model of depression. Neuroscience. 2002;115:999–1007. doi: 10.1016/s0306-4522(02)00581-x. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav. Brain Res. 2000;117:137–46. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry. 2013;73:639–45. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CE, Brennen T, Holte A. Decreased approach motivation in depression. Scand. J. Psychol. 2006;47:505–11. doi: 10.1111/j.1467-9450.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- Wardenaar KJ, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Symptom dimensions as predictors of the two-year course of depressive and anxiety disorders. J. Affect. Disord. 2012;136:1198–203. doi: 10.1016/j.jad.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Barge-Schaapveld DQ, Nicolson NA, Peeters F, de Vries M, et al. Reduced stress-sensitivity or increased reward experience: The psychological mechanism of response to antidepressant medication. Neuropsychopharmacology. 2008;34:923–31. doi: 10.1038/npp.2008.66. [DOI] [PubMed] [Google Scholar]
- Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch. Gen. Psychiatry. 2007;64:49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive Psychology and Emotional Disorders. 2nd Wiley; Chichester, UK: 1997. [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Wilkes M, Orwin A. Attributional style and perceived stress in endogenous and reactive depression. J. Affect. Disord. 1990;18:281–7. doi: 10.1016/0165-0327(90)90080-r. [DOI] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, et al. Different neural systems adjust motor behavior in response to reward and punishment. Neuroimage. 2007;36:1253–62. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Labonté B, Wen XL, Turecki G, Meaney MJ. Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology Rev. 2013;38:111–23. doi: 10.1038/npp.2012.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita A, Martijena I, Cuadra G, Brandao ML, Molina V. Early exposure to chronic variable stress facilitates the occurrence of anhedonia and enhanced emotional reactions to novel stressors: Reversal by naltrexone pretreatment. Behav. Brain Res. 2000;117:163–71. doi: 10.1016/s0166-4328(00)00302-8. [DOI] [PubMed] [Google Scholar]