Abstract
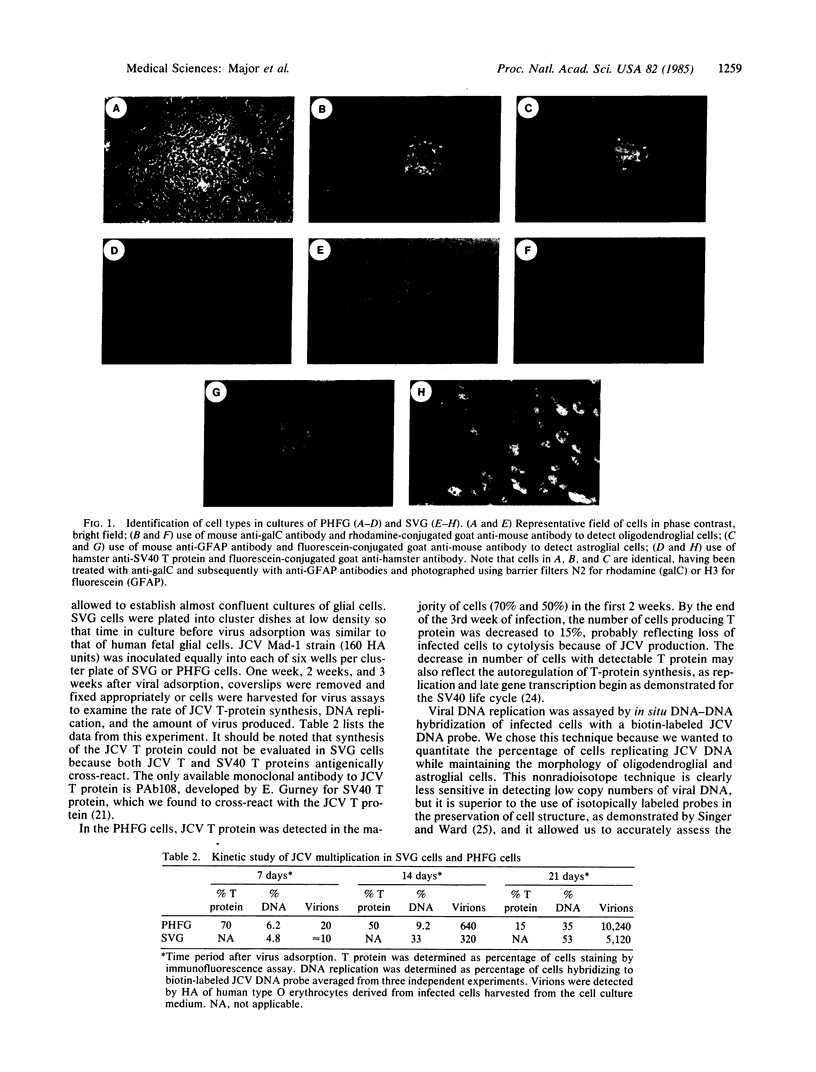
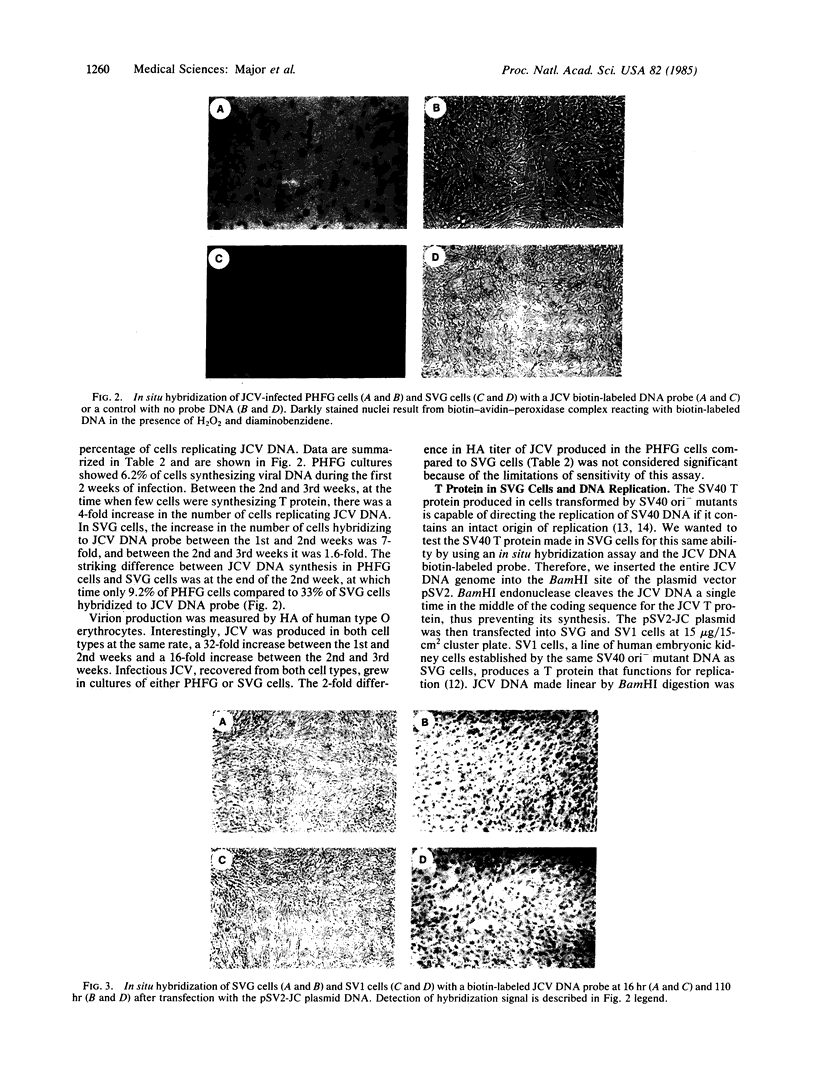
Primary cultures of human fetal brain cells were transfected with plasmid DNA pMK16, containing an origin-defective mutant of simian virus 40 (SV40). Several weeks after DNA treatment, proliferation of glial cells was evident in the culture, allowing passage of the cells at low split ratios. Initially, only 10% of the cells demonstrated nuclear fluorescence staining using a hamster tumor antibody to the SV40 T protein. By the sixth passage, however, 100% of the cells reacted positively to the same antibody. During these early passages, the cells designated SVG began growing very rapidly and acquired a homogeneous morphology. Cell division required only low serum concentrations, was not contact-inhibited, and remained anchorage dependent. These characteristics of the SVG cells have been stable through 25 passages or approximately equal to 80 cell generations. The SV40 T protein is continuously produced in the cells and can direct the replication of DNA inserts in the pSV2 vector, determined by in situ hybridization using biotin-labeled DNA probes, which contains the SV40 replication origin. More importantly, SVG cells support the multiplication of the human papovavirus JCV at levels comparable to primary cultures of human fetal glial cells, producing infectious virus as early as 1 week after viral adsorption. Their brain-cell derivation has been established as astroglial, based on their reactivity with a monoclonal antibody to glial fibrillary acid protein and lack of activity with an anti-galactocerebroside antibody, which identifies oligodendroglial cells. The SVG cells represent a unique line of continuous rapidly growing human fetal astroglial cells that synthesizes a replication-proficient SV40 T protein. Their susceptibility to JC virus (JCV) infection obviates a host restriction barrier that limited JCV studies to primary cultures of human fetal brain and thus should allow for more detailed molecular studies of human brain cells and JCV that infects them.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTROM K. E., MANCALL E. L., RICHARDSON E. P., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain. 1958 Mar;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- Boast S., La Mantia G., Lania L., Blasi F. High efficiency of replication and expression of foreign genes in SV40-transformed human fibroblasts. EMBO J. 1983;2(12):2327–2331. doi: 10.1002/j.1460-2075.1983.tb01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J. Nucleotide sequence of the region encompassing the JC virus origin of DNA replication. J Virol. 1983 Apr;46(1):170–176. doi: 10.1128/jvi.46.1.170-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Matsumura P. Human embryonic kidney cells: stable transformation with an origin-defective simian virus 40 DNA and use as hosts for human papovavirus replication. Mol Cell Biol. 1984 Feb;4(2):379–382. doi: 10.1128/mcb.4.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Mourrain P., Cummins C. JC virus-induced owl monkey glioblastoma cells in culture: biological properties associated with the viral early gene product. Virology. 1984 Jul 30;136(2):359–367. doi: 10.1016/0042-6822(84)90172-7. [DOI] [PubMed] [Google Scholar]
- Martin J. D., Padgett B. L., Walker D. L. Characterization of tissue culture-induced heterogeneity in DNAs of independent isolates of JC virus. J Gen Virol. 1983 Oct;64(Pt 10):2271–2280. doi: 10.1099/0022-1317-64-10-2271. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Transformation of human skeletal muscle cells by simian virus 40. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6581–6585. doi: 10.1073/pnas.80.21.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Papovaviral persistent infections. Microbiol Rev. 1982 Dec;46(4):384–425. doi: 10.1128/mr.46.4.384-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Rogers C. M., Walker D. L. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect Immun. 1977 Feb;15(2):656–662. doi: 10.1128/iai.15.2.656-662.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoli D., Wroblewska Z., Gilden D. H., Girardi A., Koprowski H. Human brain in tissue culture. III. PML-SV40-induced transformation of brain cells and establishment of permanent lines. J Comp Neurol. 1975 Jun 1;161(3):317–328. doi: 10.1002/cne.901610304. [DOI] [PubMed] [Google Scholar]
- Shein H. M. Propagation of human fetal spongioblasts and astrocytes in dispersed cell cultures. Exp Cell Res. 1965 Dec;40(3):554–569. doi: 10.1016/0014-4827(65)90234-x. [DOI] [PubMed] [Google Scholar]
- Shein H. M. Transformation of astrocytes and destruction of spongioblasts induced by a simian tumor virus (SV40) in cultures of human fetal neuroglia. J Neuropathol Exp Neurol. 1967 Jan;26(1):60–76. doi: 10.1097/00005072-196701000-00005. [DOI] [PubMed] [Google Scholar]
- Silverman L., Rubinstein L. J. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathol. 1965 Nov 18;5(2):215–224. doi: 10.1007/BF00686519. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Ward D. C. Actin gene expression visualized in chicken muscle tissue culture by using in situ hybridization with a biotinated nucleotide analog. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7331–7335. doi: 10.1073/pnas.79.23.7331. [DOI] [PMC free article] [PubMed] [Google Scholar]