Abstract
Brown adipose tissue (BAT) dissipates energy as heat to maintain optimal thermogenesis and to contribute to energy expenditure, in rodents and possibly humans. The energetic processes executed by BAT require a readily available fuel supply, which includes glucose and fatty acids (FAs). FAs become available by cellular uptake, de novo lipogenesis, and from multilocular lipid droplets in brown adipocytes. BAT also possesses a great capacity for glucose uptake and metabolism, and an ability to regulate insulin sensitivity. These properties make BAT an appealing target for the treatment of obesity, diabetes and other metabolic disorders. Recent research has revealed a better understanding of the processes of fuel utilization carried out by brown adipocytes, which is the focus of the current review.
Keywords: Brown Adipose Tissue, Energy Expenditure, Uncoupling Protein 1
Significance of Brown Fat
The main function of brown adipose tissue (BAT) is to dissipate energy in the form of heat, a property driven by the presence of the mitochondrial protein uncoupling protein 1 (UCP1) that uncouples mitochondrial respiration. BAT is also densely innervated by the sympathetic nervous system (SNS), and highly vascularized1. The thermogenic capacity of BAT may be important for heat-production in newborns, essential for rodents and hibernating mammals, and possibly helps burn excess dietary energy consumption.
Imaging studies in humans have revealed that adults possess BAT depots around the neck, clavicle and spinal cord2–8, that are metabolically active and able to take up and utilize glucose and FAs9,10. This observation has sparked interest in the possibility that human BAT manipulation might represent a target for obesity management. However, the amount of BAT and its level of activity vary greatly among people, with higher levels observed in younger, leaner people, or by season and cold-exposure2–10. Nevertheless, uptake and oxidation of glucose and FAs for heat production makes BAT an attractive target for the treatment of obesity, diabetes and other metabolic disorders (Fig. 1). In fact, two recent studies have demonstrated that cold acclimation in humans, after repeated daily cold exposure, results in an increase of BAT activity with a surge in energy expenditure11,12.
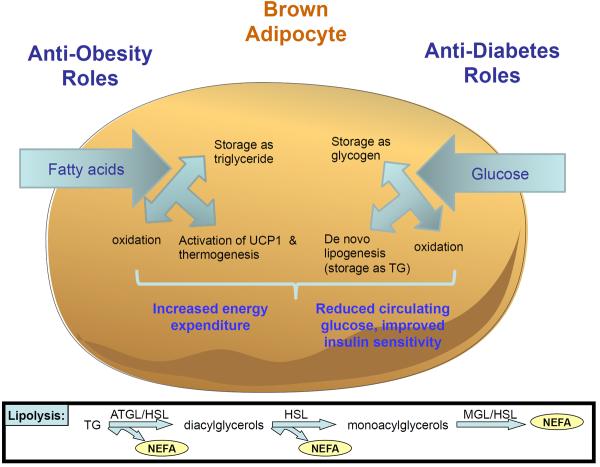
Figure 1. Glucose and FAs in BAT Combat Diabetes and Obesity.
BAT holds great promise for combating metabolic diseases such as obesity and diabetes, in part through its ability to take up and oxidize (or store) FAs and glucose. By targeting glucose and fatty acid as fuels, BAT may be able to mitigate the weight gain caused by high sugar and high fat diets. Fatty acids may be stored as TG, oxidized, or utilized to activate thermogenesis via UCP1 (Box 2). Glucose may be oxidized, stored as glycogen, or it may undergo de novo lipogenesis to provide TG for storage. Pathways involved in lipolysis are presented in the lower box. (NEFA = non-esterified fatty acids; ATGL = adipose triglyceride lipase; HSL = hormone sensitive lipase; MGL = monoglyceride lipase)
In addition to classical BAT, brown adipocytes also can be induced to appear in white adipose tissue (WAT), through a process termed `browning'. These recruitable brown adipocytes13 (also known as beige14 or brite15 adipocytes) can appear after cold-exposure or after treatment with sympathetic mimetics such as the β3-adrenergic receptor agonist CL 316, 243, as well as other agents (for review see 16–18). The induction of browning may be a novel method for increasing whole body energy expenditure and combating obesity.
An important goal in the study of BAT biology is to better understand the mechanisms underlying the uptake and utilization of FAs and glucose in energy expending processes, including the role of fuel switching (Box 1). Enabling brown adipocytes to increase these rates of catabolism may lead to greater energy expenditure in order to combat obesity and diabetes. Here we will discuss recent findings related to fuel utilization and activation of classical and recruitable brown adipocytes, including uptake and utilization of glucose and FAs, as well as what is known about the regulation of these processes.
Glucose utilization by BAT
The use of positron emission tomography - computed tomography (PET-CT) imaging with the tracer fluorodeoxyglyucose (FDG) allows imaging of metabolically active BAT in humans that readily takes up glucose. Experiments in adult humans demonstrated that the rate of cold-activated glucose uptake exceeded that of insulin-stimulated glucose uptake in skeletal muscle. Specifically, glucose uptake after cold exposure was increased by 12-fold in BAT, and was correlated with an increase in whole body energy expenditure, while insulin stimulated glucose uptake in BAT increased by 5-fold9. Interestingly, gene expression of the glucose transporter GLUT4 was higher in BAT than white adipose tissue (WAT)9 in these subjects, and in mice GLUT1 and GLUT4 are more highly expressed after cold exposure in BAT than in other tissues19, further underscoring the importance of glucose for BAT function. Previous experiments using cold-exposed mice have also shown that many of the genes up-regulated in BAT are involved in glucose uptake and catabolism,20 and activation of adrenergic signaling by cold exposure resulted in translocation of the glucose transporter GLUT1 and GLUT4 in the plasma membrane of brown adipocytes21. In obese, glucose-intolerant mice, cold exposure was able to normalize glucose tolerance and increased both glucose and FA uptake in BAT of lean and obese mice19,22,23. This increase in glucose uptake in BAT was greater than in brain, heart, liver, WAT and muscle combined19,23. Collectively, these data indicate that BAT may serve as an important glucose sink able to improve insulin sensitivity and glucose uptake after cold exposure24,25.
BAT can also take up glucose in an insulin-independent matter. Using a class of selective partial agonists of the non-canonical hedgehog signaling pathway, Teperino et al. demonstrated that these compounds cause robust insulin-independent glucose uptake in BAT and skeletal muscle via activation of the Smo-AMPK axis26, that involves the G protein-coupled receptor (GPCR or GPR) of the hedgehog pathway Smoothened (Smo), and AMP-activated protein kinase (AMPK), indicating that energy sensing by AMPK in BAT may regulate fuel utilization. Another hormone that might regulate BAT glucose uptake and thermogenesis is thyroid hormone. Thyroid hormone is converted from the low-activity form thyroxine (T4) to the active form 3, 3', 5-triiodothryonine (T3) by the enzymes type 1 and type 2 deiodinases (DIO1 and DIO2). BAT is a site of high expression of DIO2 and DIO2 knock-out mice have defects in both lipolysis, lipogenesis and adaptive thermogenesis, despite increased levels of UCP127,28. Additionally, DIO2 knock-out mice are insulin resistant and susceptible to diet-induced obesity (DIO), perhaps a result of defects in BAT energy expenditure29. Together, these findings indicate there may be some insulin-independent pathways which can regulate BAT glucose uptake.
BAT mass and glucose disposal
Given the ability of BAT to take up glucose, one hypothesis is that increasing BAT mass in an individual may increase their glucose disposal. Indeed, a recent rodent study utilizing BAT transplantation from donor mice into the visceral cavity of recipient mice in an effort to increase BAT mass, demonstrated improved glucose tolerance, increased insulin sensitivity, lower body weight, and decreased fat mass30. Similarly, BAT transplantation from chow-fed donor mice into the visceral cavity of high-fat diet (HFD) recipient mice resulted in complete reversal of HFD-induced insulin resistance30. Likewise, in a separate study transplantation of BAT into the interscapular region was able to reverse diet-induced obesity and improve insulin sensitivity31. In another study, subcutaneous transplantation of embryonic BAT was also able to restore euglycemia in streotozotocin (STZ)-treated type 1 diabetic mice32.
It is well established that administration of CL 316, 243 via subcutaneous miniosmotic pumps leads to an increase in BAT mass and increased basal and insulin-stimulated whole-body glucose disposal, without affecting body weight in non-obese rats33, an effect mostly mediated by WAT and BAT, and not muscle. Collectively these data suggest that manipulation of BAT mass might be a powerful way of increasing glucose disposal.
FAs as Fuel for BAT
FAs fulfill a wide variety of roles in physiology (reviewed in34 and35), including providing structural support in cell membranes, affecting the activities of certain transcription factors and activating GPCRs. After a meal, FAs and glucose are stored in the adipose as triglycerides (TG), and when energy is depleted, TGs are degraded to release FAs via the process of lipolysis. Dietary nutrients are also stored in liver, muscle and heart, but these are relatively short-lived as compared to fuel stored in adipocytes. Most cells are able to take up FAs secreted from adipose upon metabolic demand36. Upon internalization, FAs are esterified, to become available for β –oxidation, or reassembly for storage as inert TG via lipogenesis. Thus, the balance between adipose FAs and TGs is tightly regulated by the biochemical processes of lipolysis and lipogenesis. In the BAT of cold-exposed rodents, genes involved in glucose metabolism, lipogenesis, and uptake and catabolism of FAs are up-regulated as part of cold adaptation20, and fatty acids are utilized for UCP1 activation (Box 2). It is currently unclear how brown adipocytes regulate de novo lipogenesis vs. FA uptake, but it has been reported that BAT undergoes more lipogenesis than WAT37.
The high level of vascularization of BAT may also play a key role in fuel utilization. Indeed, PPARγ in the endothelium is able to regulate lipids, and may integrate vascular and metabolic responses38. Additionally, vascular endothelial growth factor B (VEGF-B) is able to control endothelial uptake of FA in heart and skeletal muscle, although the role for VEGF-B in the vasculature of BAT remains to be explored39. A newly identified transcription factor, TLE3, may act as a white and brown adipocyte switch, as it is able to interfere with PRDM16's interaction with PPARγ, in order to reduce fatty acid oxidation and thermogenesis40. Deletion of TLE3 has the opposite effect and is able to increase thermogenesis in subcutaneous adipose tissue40.
Lipolysis and Lipogenesis in BAT
Lipolysis requires lipases including adipose triglyceride lipase (ATGL; which hydrolyzes TG to diacylglycerols and NEFAs), hormone sensitive lipase (HSL; which can hydrolyze a variety of acylesters including TG, diacylglycerols and monoacylglycerols), and monoglyceride lipase (MGL; which cleaves monoacylglycerols to NEFAs)36 (Fig. 1). These processes occur intracellularly, whereas lipoprotein lipase (LPL) acts in extracellular/vascular lipolysis (see below). Mice with a whole-body deletion of ATGL have lower energy expenditure, including reduced insulin-stimulated glucose disposal in BAT, but overall the mice are protected from DIO due to increased cardiac and liver glucose clearance, despite increased lipid content in these tissues41. However, these mice are sick and their condition closely mimics neutral lipid storage disease with myopathy42. Adipose-specific deletion of ATGL converts BAT to a WAT-like tissue, with impaired thermogenesis and lower expression of UCP143. This study also clarified a mechanism for ATGL action, which involves AMPK-mediated phosphorylation to activate TG hydrolase activity43. Together, these studies indicate that ATGL's role in lipolysis is required to maintain a brown-like phenotype.
LPL is capable of chylomicron- and VLDL-TG lipolysis as well as cellular uptake of TG and other lipids44, including the uptake of lipids into BAT19. LPL is highly expressed and secreted by adipose tissues45. Cold exposure results in a decrease in triglycerides and an increase in `good' HDL-cholesterol19 in the circulation, although recent evidence indicates that while HDL cholesterol is considered beneficial, it may in fact increase cardiovascular risk46. Indeed, UCP1-dependent lipolysis induced by cold-exposure in mice genetically prone to atherosclerosis further promotes plaque growth and instability47. However, paradoxically the BAT-like perivascular adipose tissue is actually beneficial in preventing atherosclerosis48. Mice with a lack of perivascular adipose tissue, due to smooth muscle cell deletion of PPARγ, do not exhibit the inhibition of atherosclerosis by cold-exposure as was observed in wild-type mice, due to impaired lipid clearance in the mice lacking perivascular adipose48.
Through a mechanism requiring the scavenger receptor cluster of differentiation 36 (CD36) and LPL, BAT is capable of taking up TG, and the overall rate of FA uptake by BAT during cold exposure is greater than that of skeletal muscle and requires LPL action19. Higher activity of LPL results in greater adiposity and insulin resistance49. Conversely, loss of LPL selectively from adipose tissue and not skeletal muscle resulted in an increase in lipogenesis in BAT and WAT. Despite previous findings showing that lipogenesis is important for BAT function, removal of LPL to prevent its lipolysis and promote de novo lipogenesis, did not activate BAT or induce browning of WAT in response to high fat diet or ADRB3 stimulation50. Adipocyte-specific deletion of LPL leads to an increase of de novo lipogenesis products, such as palmitoleate (C16n1–7)50, however these mice are not protected from metabolic disease and do not undergo adipose tissue browning, thus increasing lipogenesis alone is not sufficient to induce browning.
LPL is transported across capillary endothelial cells by the GPI-anchored glycosylphosphatidylinositol anchored high density lipoprotein binding protein 1 (GPIHBP1; reviewed in51,52, which can bind LPL and chylomicrons53. The movement of LPL and GPIHBP1 across endothelial cell membranes is bidirectional51, and GPIHBP1 knockout mice display mislocalized LPL (including in brown adipose tissue)54, decreased adipose tissue TG and defective lipolysis55.
FAs consisting of 16 carbon atoms or less are synthesized by fatty acid synthase (FAS), but elongation of these FAs to very-long-chain-fatty acids (VLCFAs) is done via the elongation activities of very long-chain fatty acid enzymes (ELOVL). ELOVL3 is highly expressed in BAT after cold exposure56, and mice lacking ELOVL3 are only able to survive the cold by shivering, as they lack the FA elongation activity needed to supply fuel for BAT thermogenesis, although the mice are able to eventually adapt and restore their elongation abilities in BAT57. Paradoxically, Elovl3−/− mice are resistant to DIO due to increased energy expenditure, not reduction of food intake. These mice also display reduced hepatic lipogenesis and triglyceride formation58. Conversely, adipose-specific ablation of FAS resulted in increased energy expenditure and browning of subcutaneous WAT59. The pattern of FA composition is different in BAT versus WAT and between lean and obese mice60, and the profile can be altered by transplantation of BAT from obese mice to lean mice, with the graft adopting the FA composition of the host60. Interestingly, loss of adipose LPL also changes the FA composition of both BAT and WAT, with a tendency for an increase in products of de novo lipogenesis and an accompanying increase in expression of genes for lipogenesis, as well as a decrease in polyunsaturated fatty acids (PUFAs)61. These findings are consistent with the role of LPL in releasing PUFAs from triglyceride-rich lipoproteins, which cannot be synthesized by the cells. Notably, one of the up-regulated FAs, palmitoleate (C16:1n-7), has been shown to have insulin-sensitizing and anti-inflammatory effects, thereby acting as a `lipokine', allowing adipose tissue to communicate with other tissues regarding metabolic status60,62. Palmitoleate was identified in the serum of fatty acid binding proteins (FABP) −/− mice, and is released from adipocytes in response to physiological stimuli in order to communicate with liver and skeletal muscle62. Therefore, not only is the oxidation and storage of lipids influenced by BAT, but activation of BAT could also have beneficial effects on whole body lipid composition, independent of weight loss.
De novo lipogenesis in adipose tissue, producing palmitoleate, is thought to be beneficial, whereas excessive lipogenesis in liver is linked with metabolic disease63. In brown adipocytes, PPARα and carbohydrate response element binding protein (ChREBP), which has also been linked to liver lipogenesis, are part of a feedback loop involved in BAT lipogenesis64. Recently, PPARδ has also been implicated in liver de novo lipogenesis, producing a serum lipid (phosphatidylcholiune 18:0/18:1) which communicates with skeletal muscle to increase FA utilization, and in mice is regulated by diurnal changes in the activity of hepatic PPARδ65. Whether this new lipokine can communicate with fat is currently unknown.
Transporters and sensors
Sympathetic activation of BAT results in recruitment of enzymes needed for uptake, mobilization and oxidation of lipids, as well as turning on a thermogenic gene expression profile; these effects are synergistically enhanced by thyroid hormone66. Therefore, given the unwanted side effects of sympathomimetics in humans67, identifying other pathways which can mimic these effects specifically in BAT may increase energy expenditure more safely. For instance, targeting FA sensing and transport into BAT may be an appealing option, along with a safer level of BAT activation.
FAs are essential for proper function and energy expenditure of brown adipocytes. FA uptake in adipocytes is mainly regulated by the fatty acid translocase CD36, as well as certain isoforms of fatty acid transport proteins (FATPs) such as FATP1 and FATP4 (reviewed in34), which bring FAs across the cell membrane (Fig. 2). Once inside the cell, FAs may either be stored as TG in lipid droplets or transported into the mitochondria for oxidation. CD36 variants have been associated with BMI in humans68, and loss of CD36 function renders mice unable to survive the cold due to lack of availability of fats for combustion in brown fat thermogenesis19. Similarly, lack of the FATP member FATP1 leaves mice unable to defend body temperature due to lack of FA transport into brown adipocytes for thermogenesis69. CD36 may also act as a FA sensor as well as a transporter, which has been shown in taste buds of mice, where CD36 and GPR120 act together70 in fat sensing.
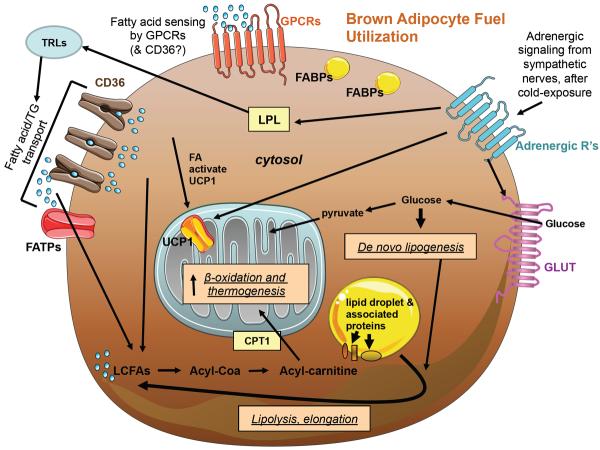
Figure 2. Fuel Utilization in Brown Adipocytes.
The schematic shows a summary of FA sensing, uptake and oxidation pathways, as well as glucose uptake and downstream metabolism pathways in a typical brown adipocyte. Shown are glucose uptake by GLUT transporters, including GLUT translocation stimulated by adrenergic signaling, and the fate of glucose in de novo lipogenesis, storage as glycogen, or conversion to pyruvate and mitochondrial oxidation. FAs are sensed by GPCRs and possibly also CD36, and are taken up by CD36 and FATPs. LPL produced and secreted by adipocytes after adrenergic stimulation is also able to break down triglyceride-rich lipoproteins (TRLs), providing additional lipid fuel for uptake. FAs activate mitochondrial UCP1, as does activation from the sympathetic nervous system and adrenergic signaling, enabling energy expenditure via thermogenesis. FAs also become available from the lipolysis of lipid droplets (which is partially under regulation by lipid droplet-associated proteins). FAs may be elongated and/or converted to acyl-carnitine for transport into the mitochondria via the carnitine shuttle, where they become fuel for β-oxidation. FABPs also contribute to intracellular FA handling.
GPCRs are widely regarded as the fat sensors of the cell. As a family, these seven-transmembrane receptors are found on various cell types and are activated by various ligands, including FAs. In adipose tissues, FA sensing GPR41, 43 and 120 have been identified, and are known to play roles in leptin secretion, lipolysis, adipogenesis and glucose uptake via GLUT4 translocation (reviewed in35). This latter example provides a hint at the potential cross-talk between FA and glucose uptake in the adipocyte. However, much of this work has been done in white, and not brown, adipose tissues. Determining how FAs are sensed and transported in brown adipocytes will go a long way in elucidating the mechanisms of fuel utilization in BAT.
FABPs in adipocytes
FABPs are important for FA trafficking and act as lipid chaperones to transport lipids to certain cell compartments or outside of the cell. Brown adipose tissue expresses a cohort of FABPs that are also expressed in WAT or muscle, including FABP3 (or heart type; also expressed in muscle and other tissues) and FABP4 (or adipocyte type; also called aP2) (reviewed in71). After cold exposure all ten known FABP isoforms were assessed in BAT of rats, and it was found that FABPs 3–5 were all expressed in BAT of room-temperature and cold-exposed animals, with FABP4 being the most abundant and FABP3 having the highest (10-fold) induction by cold exposure72. FABP3 knock-out mice display reduced fatty acid uptake (reviewed in71) and FABP3 expression in BAT is increased in UCP1 knock-out mice and with diet induced obesity, both correlating with an increased demand for adaptive thermogenesis73., While FABP4 has been regarded as a marker for mature adipocytes, it has recently been identified as a marker for adipocyte progenitors in WAT and BAT, which reside in the stem cell niche and express known progenitor markers74. Importantly, this finding indicates that in addition to aP2-Cre expression in mature adipocytes, this driver also targets adipocyte progenitor cells, making it a useful genetic tool for investigation of adipogenesis.
FA oxidation in mitochondria
FAs are released in cells in part by the action of acyl-coA thioesterases (Acots) which hydrolyze fatty acyl-CoAs. Recently, Acot11 (also known as thioesterase superfamily 1, or Them1), which is highly expressed in BAT, has been shown to decrease energy expenditure and promote conservation of calories. In particular, mice lacking Them1 were protected against DIO and had increased FA oxidation in BAT, as well as improved whole-body glucose homeostasis75. Carnitine palmityl-transferase1 (CPT1) is the rate limiting enzyme in the carnitine shuttle (Fig. 2), which transports FAs into the mitochondria for oxidation. Mice lacking the muscle-form of CPT1 (mCPT1; which along with the liver form are expressed in BAT) die during cold exposure due to their inability to undergo thermogenesis, consistent with the expression of CPT1 in BAT and muscle76. In Sprague Dawley rats, BAT has the highest CPT1 activity and palmitate oxidation rate of the tissues examined77. In addition to FA coordination in the adipose tissue itself, lipolysis in WAT which releases FAs into circulation, as well as FA flux in the liver, are also important78 but not necessary79 for thermogenesis in rodents, as long as a sufficient supply of dietary nutrients is sustained. Fasted animals rely heavily/essentially on flux from WAT and liver to BAT and muscle for thermogenesis. Therefore, targeting FA oxidation in BAT mitochondria by increasing fuel availability or flux through mitochondrial pathways is another appealing method for increasing BAT energy expenditure.
FA activation of PPARs; lipid droplet proteins in BAT
In addition to FA oxidation in the mitochondria, FAs may also act as signaling molecules (reviewed in80), including the ability to act as ligands for the family of nuclear receptors, peroxisome proliferator-activated receptors (PPARs), which includes PPAR α, δ and γ. In response to adrenergic signaling activation, cyclic adenosine monophosphate (cAMP) activates protein kinase A (PKA) which in turn activates the co-activator cAMP response element binding protein (CREB), which increases transcription of genes such as peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) and UCP1. PKA also activates p38 MAPK signaling, which is known to regulate UCP1 and PGC1α expression81. The genes for UCP1 and PGC1α contain binding sites for PPARs, and together these genes are able to regulate FA oxidation and lipid storage. In parallel to this pathway, PKA also activates lipolysis via ATGL and HSL (summarized in82). PPAR γ activation in mice and humans initiates TG uptake into adipose tissue via LPL83 without any significant changes in glucose or insulin. Treatment with the PPAR γ agonist rosiglitazone increases de novo lipogenesis in WAT without increasing glucose uptake, but it is unknown if the same effects would be observed in BAT84. Overall, PPAR γ interaction with FAs in BAT may provide an important link between fuel availability and energy expending processes.
Recently, it has been demonstrated that adrenergic activation of thermogenic genes in BAT requires lipolysis, and that increased levels of FAs activating PPAR α and δ were sufficient to increase FA oxidation82. Ligands for PPAR α and δ are created at the lipid droplet surface within minutes of stimulation82. The lipid droplet is an important storage component for TG in adipose tissues, shielding the other cell organelles from the potential lipotoxicity of FA, and providing a surface area that is able to be acted upon by water-soluble lipases in the cytosol. The lipid droplet also stores cholesterol esters, preventing cholesterol lipotoxicity. The surface of the lipid droplet is comprised of phospholipids and proteins (Fig. 2), which help regulate the process of lipolysis (reviewed in85), and many of which are now known to play a role in the phenotype of brown adipocytes, especially given that brown adipocytes contain more lipid droplet surface area due to their multilocularity. Lipid droplet proteins include the Plin family (such as Plin1, or perilipin), the Cide family (such as CIDEC or Fsp27), and several others, with the majority being more highly expressed in BAT than WAT, indicating an important role for lipid droplet access and utilization in brown adipocytes86. Nearly all the lipid-droplet proteins are up-regulated in cold-exposed subcutaneous white fat, which is known to readily undergo browning, but this was not seen in mesenteric white fat86, which does not regularly undergo browning. Lipid droplet remodeling may represent a mechanism for the proposed process of transdifferentiation, whereby white adipocytes can directly transform into UCP1-positive brown adipocytes86.
Cell death-inducing DNA fragmentation factor-α-like effector A (CIDEA) is a lipid droplet protein and an inhibitor of lipolysis that is also highly expressed in the mitochondria of BAT. CIDEA can directly interact with UCP1 and suppress its activity87. Mice lacking CIDEA exhibit higher energy expenditure, increased lipolysis in BAT, and are resistant to HFD-induced obesity87. Along these lines, CIDEA is down-regulated (mRNA and protein) after cold-exposure through a mechanism involving adrenergic signaling88. CIDEA is also expressed around lipid droplets in BAT, and its expression and promoter activity can be repressed by receptor-interacting protein 140 (RIP140) and induced by PGC1α89. A more recently described BAT lipid droplet protein in the CIDEA family is CIDEC or Fat-specific protein 27 (Fsp27). During adipogenesis, levels of Fsp27 are greatly increased, and it appears to act opposite of CIDEA by promoting lipid accumulation through interaction with perilipin90, another well-described lipid droplet protein. Fsp27 is also positively correlated with obesity, and is upregulated in adipose and liver of ob/ob mice91. The Fsp27−/− mice have reduced adiposity and increased browning of WAT, with improved insulin sensitivity that may be attributed to both of these changes. Interestingly, Fsp27−/− mouse embryonic fibroblasts (MEFs) obtained from these mice also showed an increased likelihood to differentiate to brown adipocytes91.
While deletion of Fsp27 leads to browning of WAT, overexpression of perilipin leads to the same effect92, revealing a complex regulatory network of lipid droplet-associated proteins. Normally, perilipin acts to suppress lipolysis in the absence of PKA stimulation, and perilipin transgenic mice are resistant to a HFD93, through a mechanism now known to involve browning of WAT, increased energy expenditure, and a reduction of Fsp27 expression in WAT92. Whether lipid droplet protein function differs between WAT and BAT remains to be determined, but it is likely that lipid droplet proteins in BAT play a role in regulating fuel availability and may be a target for increasing BAT energy expenditure.
Signaling Pathways Regulating BAT Fuel Utilization
In order for BAT to be effective at burning extra calories, it needs a readily available fuel supply, as well as activation of its energy-expending capacity for β-oxidation and thermogenesis. The classic method of activating BAT energy expenditure is via the SNS, and although this pathway and its synergistic activation of BAT with the thyroid hormone system are well characterized, recently several novel factors and signaling pathways (such as fibroblast growth factor 21 (FGF21), cardiac naturietic peptides, and bone morphogenetic proteins (BMPs)) have been described which can affect fuel availability, activation of energy expenditure in BAT, or both. These factors and pathways have been nicely reviewed elsewhere17,94.
Given the importance of the SNS in innervating and activating BAT, it is no surprise that adrenergic signaling controls hydrolysis of TG and the regulation of the enzymatic machinery for lipolysis and fuel utilization81. Recently, the β1- isoform of the adrenergic receptor has been shown to be instrumental for this purpose95, although the β3- isoform is the highest expressed in BAT and WAT of mice, with lesser expression in WAT, BAT and intestine of humans96,97.
In humans, few factors or treatments aside from cold-exposure have been shown to directly activate BAT. Indeed, it has recently been shown that ephedrine activates the SNS without activating BAT67, although others have reported that ephedrine, when used at a higher dosage, activates BAT in lean but not obese subjects98. Overall, adrenergic agonists used in humans represent several challenges, necessitating the development of sympathomimetics with fewer adverse side effects.
In mice, more factors have been identified which activate BAT. For example, BMP7 is able to activate mitochondrial activity of brown adipocytes in culture by a mechanism that involves SMAD and p38 signaling, and upregulation of FA transporters CPT1 and CD3699. In mice, systemic BMP7 treatment also acts to lower RER and increase energy expenditure99. FGF21 can directly increase PGC1α protein levels and thereby inducing the expression of thermogenic genes in BAT and WAT in an autocrine/paracrine manner100,101. Accordingly, FGF21 deficient mice display an impaired response to chronic cold challenges101.
Concluding remarks and future perspectives
Given that obesity results from an energy imbalance leading to caloric overload, WAT must appropriately respond by storing excess energy in lipid droplets. The ability of adipose tissue to undertake this role, or the `adipose expandability' of a given person102, may be a factor predisposing to the metabolic disturbances with obesity, which include ectopic lipid deposition in tissues such as skeletal muscle and liver, and leading to lipotoxicity. If agents can 1) specifically increase SNS activation of lipolysis in WAT to provide FA for thermogenesis and β -oxidation in BAT, 2) maximally activate the available BAT or 3) increase BAT mass, then perhaps the energy imbalance can be righted by increasing energy expenditure. As summarized in this review, given the ability of BAT to take up FA and glucose, it could help ameliorate the excess circulating levels of these fuels, thereby preventing adverse physiological effects.
As described above, while we are beginning to understand the responsiveness of activated BAT to affect insulin and glucose homeostasis as well as sensing and taking up lipids, a better understanding of these processes may provide novel opportunities for the development of therapies for obesity and metabolic disease. Additionally, the ability to target adipose thermogenesis specifically, without adverse effects in other tissues, either by developing specific adrenergic agonists acting only on adipose tissues or by utilizing other chemical uncouplers to mimic the actions of UCP1, would be important steps in increasing whole body energy expenditure. As described in Box 3, we do not yet understand the relative importance of glucose vs FAs as fuel for BAT, how WAT lipolysis regulates BAT fuel supply, or what role the SNS plays in regulating fuel availability to BAT. We also do not yet fully understand the complement of circulating factors coming from adipose and non-adipose tissues that may drive BAT energetics. Finally, the lipid droplet proteins making stored fuel available for catabolism are not fully identified or understood yet, and may provide additional clues for how to increase lipolysis and FA oxidation. Overall, the energy expending capacity of BAT is great, and targeting BAT catabolic processes is an important potential tool for reducing adiposity.
BOX1: Fuel Switching.
The exact metabolic controls and consequences for fuel switching from carbohydrate to lipid or vice versa are currently poorly understood. Measurement of respiratory exchange ratio (RER) is one indication of fuel switching, where RER is calculated as the ratio of VCO2/VO2, and RER closer to 1 indicates greater carbohydrate utilization and closer to 0.7 is greater fat utilization. One hope is that switching from glucose to greater lipid oxidation would result in increased energy expenditure and could be utilized as an obesity treatment. However, it is incorrect to believe that increasing substrate into the mitochondria alone would necessarily increase energy expenditure (reviewed in 103). The ability of FAs to enter the mitochondria via the carnitine shuttle is essential for their availability to be oxidized, however they will not be utilized until the cell requires the energy. Metabolism of lipids requires the presence of ADP for this purpose. Additionally, NADH and FADH2 feed the electron transport chain as products from both glucose or lipid metabolism, and therefore switching fuel source would not necessarily increase energy expenditure overall103. Therefore, in obesity where there is an overabundance of FAs as fuel from the diet and WAT lipolysis, the oxidation of these fats would need to be stimulated in addition to transportation of the fuel to the mitochondria, in order to raise energy expenditure. Chemical uncouplers which mimic the actions of activated UCP1 may be one way to achieve this103, in addition to activation of UCP1 in existing brown fat depots, enabling more fuel oxidation for thermogenesis.
Fuel switching in humans occurs on a daily basis, with greater carbohydrate usage after eating, and greater utilization of lipids during fasting or sleep. During these changes, lipid flux into and out of the adipose tissue needs to be tightly regulated. The ability to make this switch, termed `metabolic flexibility', can be measured by RER and has been shown to be blunted in subjects with a family history of type 2 diabetes, which may be a factor predisposing to insulin resistance104.
One factor that may regulate fuel switching in BAT is lipocalin prostaglandin D synthase (L-PGDS), which is capable of both synthesizing D-series prostaglandins as well as carrying lipophilic molecules105. L-PGDS KO mice exposed to cold had lower basal metabolic rates, despite the same thermogenic capacity. The KO mice also displayed lower lipid utilization and increased carbohydrate utilization and improved glucose tolerance on a high-fat diet, indicating that this synthase is important for fuel preference105. The flexibility of BAT in terms of fuel switching requires further investigation, but given the ability of BAT to utilize glucose and fatty acids as fuel, the tissue holds great promise for increasing metabolic flexibility.
BOX 2: Fatty Acid-Dependent and Independent Regulation of UCP1 Activity and Thermogenesis.
UCP1 is a mitochondrial proton channel that is able to catalyze a proton leak, leading to uncoupling of oxidative phosphorylation from ATP production, and instead producing heat as a by-product. UCP1-activity is thought to involve activation by FAs106,107, as well as inhibition by purine nucleotides, such as ATP or GDP108. Specifically, LCFAs released by lipolysis of BAT lipid droplets upon adrenergic stimulation, activate UCP1-mediated thermogenesis107. These mechanisms were largely unknown, but several hypotheses exist including: 1) allosteric binding of LCFAs to an H+ or OH-uniporter channel, 2) LCFAs binding to the pore of UCP1 and regulating its function, or 3) UCP1 as an LCFA anion carrier bringing them outside the mitochondria to bind protons, or 4) FAs induce a conformational change in UCP1 (summarized in109). A recent approach used patch-clamp to measure the UCP1 currents in the native inner mitochondrial membrane of BAT, and found that UCP1 has no constitutive activity (likely due to purine inhibition) until it is activated by LCFA generated within the inner mitochondrial membrane, which bind to the cytosolic side of UCP1106. There is also a difference in H+ transport by UCP1 depending on the pKa of the LCFA that is activating it, indicating that the LCFA anions directly bind and carry H+ as they are transported by UCP1, and that this LCFA activation overcomes the purine inhibition. Fuel substrate and uncoupling state regulate the state of BAT mitochondria, which involves pyruvate/malate coupling with GDP, while oleate and succinate promote uncoupling108. Related to the ability of fuel status to influence UCP1 activity, is the concept of diet-induced thermogenesis110, or the increased energy expenditure conferred by intake of certain diets, which is lost after UCP1 ablation in mice housed at thermoneutrality111. In humans, however, a 24hr period of overfeeding was not found to activate BAT 112. While the concept remains controversial 113, numerous studies report findings of diet-induced thermogenesis 95.
BOX 3: Outstanding Questions.
Outstanding questions regarding the control of energy expenditure and fuel utilization in BAT
What are the relative contribution and importance of glucose versus FAs as a fuel source for the energy expending processes of BAT, and is there a master `switch' that regulates changes in fuel supply in BAT?
What role does the SNS play in the regulation of fuel supply, sensing or uptake in BAT? Do other cell types in BAT provide noradrenergic stimulation?
How does BAT regulate de novo lipogenesis versus beta-oxidation?
What physiological or pathophysiological states induce secretion of factors from non-adipose tissues (skeletal muscle, cardiac muscle, liver, gut, and other locations) that may regulate adipose tissue thermogenesis and fuel utilization?
What is the complete milieu of lipid droplet-associated proteins and how do they act together or independently to regulate availability of stored lipid and overall cellular energetics?
Highlights
BAT is a very energy expending tissue, undergoing high levels of thermogenesis and β-oxidation.
Brown adipose tissue utilizes both glucose and fatty acids as fuel.
The regulation of these fuels occurs via availability, sensing, uptake and utilization.
All of the above may be potential targets for increasing BAT activation and combating obesity.
Acknowledgements
The authors wish to thank E. Caniano for administrative assistance, the reviewers for thoughtful and thorough comments, and Y.M. Kwon for editing of the manuscript. Elements of some figures were produced using Servier Medical Art, www.servier.com. This work was supported in part by NIH grants R01 DK077097 (Y.-H.T.), and Joslin Diabetes Center's Diabetes Research Center (DRC; P30 DK036836 from the NIDDK), a research grant from the American Diabetes Foundation and by funding from the Harvard Stem Cell Institute (to Y.-H.T.). K.L.T was funded by NIH T32-DK007260-33 and NIH F32-DK091996.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Townsend KL, Tseng YH. Brown adipose tissue: recent insights into development, metabolic function, and therapeutic potential. Adipocyte. 2012;1:13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hany TF, et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 3.Nedergaard J, et al. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 6.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 7.Zingaretti MC, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orava J, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Ouellet V, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneshiro T, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Lans AA, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enerback S. The origins of brown adipose tissue. N. Engl. J. Med. 2009;360:2021–2023. doi: 10.1056/NEJMcibr0809610. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovic N, et al. Chronic PPAR{gamma} Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct From Classical Brown Adipocytes. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarroya J, et al. An endocrine role for brown adipose tissue? Am. J Physiol Endocrinol Metab. 2013;305:E567–E572. doi: 10.1152/ajpendo.00250.2013. [DOI] [PubMed] [Google Scholar]
- 17.Schulz TJ, Tseng YH. Brown adipose tissue: development, metabolism and beyond. Biochem. J. 2013;453:167–178. doi: 10.1042/BJ20130457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 19.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 20.Yu XX, et al. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J. 2002;16:155–168. doi: 10.1096/fj.01-0568com. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu Y, et al. Effects of noradrenaline on the cell-surface glucose transporters in cultured brown adipocytes: Novel mechanism for selective activation of GLUT1 glucose transporters. Biochem. J. 1998;330:397–403. doi: 10.1042/bj3300397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams KJ, Fisher EA. Globular warming: how fat gets to the furnace. Nat Med. 2011;17:157–159. doi: 10.1038/nm0211-157. [DOI] [PubMed] [Google Scholar]
- 23.Nedergaard J, et al. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13:238–240. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Vallerand AL, et al. Cold exposure potentiates the effect of insulin on in vivo glucose uptake. Am J Physiol. 1987;253:E179–E186. doi: 10.1152/ajpendo.1987.253.2.E179. [DOI] [PubMed] [Google Scholar]
- 25.Skarulis MC, et al. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J Clin Endocrinol Metab. 2010;95:256–262. doi: 10.1210/jc.2009-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teperino R, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 27.de Jesus LA, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoffolete MA, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncoupling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- 29.Marsili A, et al. Mice with a targeted deletion of the type 2 deiodinase are insulin resistant and susceptible to diet induced obesity. PLoS. ONE. 2011;6:e20832. doi: 10.1371/journal.pone.0020832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford KI, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Souza CJ, et al. CL-316,243, a beta3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes. 1997;46:1257–1263. doi: 10.2337/diab.46.8.1257. [DOI] [PubMed] [Google Scholar]
- 34.Glatz JF, et al. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90:367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 35.Vinolo MA, et al. G-protein-coupled receptors as fat sensors. Curr. Opin. Clin Nutr Metab Care. 2012;15:112–116. doi: 10.1097/MCO.0b013e32834f4598. [DOI] [PubMed] [Google Scholar]
- 36.Lass A, et al. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011;50:14–27. doi: 10.1016/j.plipres.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts JL, et al. Brown adipose tissue triacylglycerol fatty acids of obese and lean mice: in situ and in transplants. Lipids. 1986;21:195–201. doi: 10.1007/BF02534821. [DOI] [PubMed] [Google Scholar]
- 38.Kanda T, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagberg CE, et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature. 2012;490:426–430. doi: 10.1038/nature11464. [DOI] [PubMed] [Google Scholar]
- 40.Villanueva CJ, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoy AJ, et al. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152:48–58. doi: 10.1210/en.2010-0661. [DOI] [PubMed] [Google Scholar]
- 42.Liang WC, Nishino I. Lipid storage myopathy. Curr Neurol. Neurosci. Rep. 2011;11:97–103. doi: 10.1007/s11910-010-0154-y. [DOI] [PubMed] [Google Scholar]
- 43.Ahmadian M, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg IJ, et al. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am. J Physiol Endocrinol Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 46.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat. Med. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 47.Dong M, et al. Cold Exposure Promotes Atherosclerotic Plaque Growth and Instability via UCP1-Dependent Lipolysis. Cell Metab. 2013;18:118–129. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgibbons TP, et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ. Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duivenvoorden I, et al. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 2005;54:664–671. doi: 10.2337/diabetes.54.3.664. [DOI] [PubMed] [Google Scholar]
- 50.Bartelt A, et al. Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim. Biophys. Acta. 2013;1831:934–942. doi: 10.1016/j.bbalip.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Davies BS, et al. Assessing mechanisms of GPIHBP1 and lipoprotein lipase movement across endothelial cells. J Lipid Res. 2012;53:2690–2697. doi: 10.1194/jlr.M031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young SG, et al. GPIHBP1, an endothelial cell transporter for lipoprotein lipase. J Lipid Res. 2011;52:1869–1884. doi: 10.1194/jlr.R018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beigneux AP, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davies BS, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein MM, et al. Reciprocal metabolic perturbations in the adipose tissue and liver of GPIHBP1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:230–235. doi: 10.1161/ATVBAHA.111.241406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tvrdik P, et al. Cig30, a mouse member of a novel membrane protein gene family, is involved in the recruitment of brown adipose tissue. J Biol Chem. 1997;272:31738–31746. doi: 10.1074/jbc.272.50.31738. [DOI] [PubMed] [Google Scholar]
- 57.Westerberg R, et al. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol. Chem. 2006;281:4958–4968. doi: 10.1074/jbc.M511588200. [DOI] [PubMed] [Google Scholar]
- 58.Zadravec D, et al. Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J. 2010;24:4366–4377. doi: 10.1096/fj.09-152298. [DOI] [PubMed] [Google Scholar]
- 59.Lodhi IJ, et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell Metab. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts JL, et al. Brown adipose tissue triacylglycerol fatty acids of obese and lean mice: in situ and in transplants. Lipids. 1986;21:195–201. doi: 10.1007/BF02534821. [DOI] [PubMed] [Google Scholar]
- 61.Bartelt A, et al. A new, powerful player in lipoprotein metabolism: brown adipose tissue. J Mol Med (Berl.) 2012 doi: 10.1007/s00109-012-0858-3. [DOI] [PubMed] [Google Scholar]
- 62.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eissing L, et al. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat. Commun. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iizuka K, et al. Feedback looping between ChREBP and PPARalpha in the regulation of lipid metabolism in brown adipose tissues. Endocr J. 2013;60:1145–1153. doi: 10.1507/endocrj.ej13-0079. [DOI] [PubMed] [Google Scholar]
- 65.Liu S, et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502:550–554. doi: 10.1038/nature12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 67.Cypess AM, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad Sci. U. S. A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bokor S, et al. Single-nucleotide polymorphism of CD36 locus and obesity in European adolescents. Obesity. (Silver. Spring) 2010;18:1398–1403. doi: 10.1038/oby.2009.412. [DOI] [PubMed] [Google Scholar]
- 69.Wu Q, et al. Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes. 2006;55:3229–3237. doi: 10.2337/db06-0749. [DOI] [PubMed] [Google Scholar]
- 70.Martin C, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS. ONE. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto T, et al. Quantitative evaluation of the effects of cold exposure of rats on the expression levels of ten FABP isoforms in brown adipose tissue. Biotechnol. Lett. 2011;33:237–242. doi: 10.1007/s10529-010-0444-0. [DOI] [PubMed] [Google Scholar]
- 73.Yamashita H, et al. Induction of fatty acid-binding protein 3 in brown adipose tissue correlates with increased demand for adaptive thermogenesis in rodents. Biochem. Biophys. Res. Commun. 2008;377:632–635. doi: 10.1016/j.bbrc.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 74.Shan T, et al. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013;27:277–287. doi: 10.1096/fj.12-211516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, et al. Targeted deletion of thioesterase superfamily member 1 promotes energy expenditure and protects against obesity and insulin resistance. Proc. Natl. Acad Sci. U. S. A. 2012;109:5417–5422. doi: 10.1073/pnas.1116011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji S, et al. Homozygous carnitine palmitoyltransferase 1b (muscle isoform) deficiency is lethal in the mouse. Mol. Genet. Metab. 2008;93:314–322. doi: 10.1016/j.ymgme.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doh KO, et al. Interrelation between long-chain fatty acid oxidation rate and carnitine palmitoyltransferase 1 activity with different isoforms in rat tissues. Life Sci. 2005;77:435–443. doi: 10.1016/j.lfs.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 78.Hauton D, et al. The role of the liver in lipid metabolism during cold acclimation in non-hibernator rodents. Comp Biochem. Physiol B Biochem. Mol. Biol. 2006;144:372–381. doi: 10.1016/j.cbpb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 79.Hauton D, et al. Both substrate availability and utilisation contribute to the defence of core temperature in response to acute cold. Comp. Biochem. Physiol A. Mol Integr. Physiol. 2009;154:514–522. doi: 10.1016/j.cbpa.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Wymann MP, Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 81.Collins S, et al. Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. Int. J. Obes. (Lond. ) 2010;34(Suppl 1):S28–S33. doi: 10.1038/ijo.2010.180. [DOI] [PubMed] [Google Scholar]
- 82.Mottillo EP, et al. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. J Biol. Chem. 2012;287:25038–25048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laplante M, et al. PPAR-gamma activation mediates adipose depot-specific effects on gene expression and lipoprotein lipase activity: mechanisms for modulation of postprandial lipemia and differential adipose accretion. Diabetes. 2003;52:291–299. doi: 10.2337/diabetes.52.2.291. [DOI] [PubMed] [Google Scholar]
- 84.Festuccia WT, et al. Depot-specific effects of the PPARgamma agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J Lipid. Res. 2009;50:1185–1194. doi: 10.1194/jlr.M800620-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Girousse A, Langin D. Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int. J Obes. (Lond) 2012;36:581–594. doi: 10.1038/ijo.2011.113. [DOI] [PubMed] [Google Scholar]
- 86.Barneda D, et al. Dynamic changes in lipid droplet-associated proteins in the “browning” of white adipose tissues. Biochim. Biophys. Acta. 2013;1831:924–933. doi: 10.1016/j.bbalip.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Z, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat. Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu T, Yokotani K. Acute cold exposure-induced down-regulation of CIDEA, cell death-inducing DNA fragmentation factor-alpha-like effector A, in rat interscapular brown adipose tissue by sympathetically activated beta3-adrenoreceptors. Biochem. Biophys. Res. Commun. 2009;387:294–299. doi: 10.1016/j.bbrc.2009.06.147. [DOI] [PubMed] [Google Scholar]
- 89.Hallberg M, et al. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol. Cell Biol. 2008;28:6785–6795. doi: 10.1128/MCB.00504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol. Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 91.Toh SY, et al. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sawada T, et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS One. 2010;5:e14006. doi: 10.1371/journal.pone.0014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyoshi H, et al. Perilipin overexpression in mice protects against diet-induced obesity. J Lipid Res. 2010;51:975–982. doi: 10.1194/jlr.M002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 95.Ueta CB, et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol. 2012;214:359–365. doi: 10.1530/JOE-12-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krief S, et al. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Collins S. ß-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrin. (Lausanne) 2012;2:102. doi: 10.3389/fendo.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carey AL, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 99.Townsend KL, et al. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid. Redox. Signal. 2012 doi: 10.1089/ars.2012.4536. doi:10.1089/ars.2012.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hondares E, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Virtue S, Vidal-Puig A. It's not how fat you are, it's what you do with it that counts. PLoS. Biol. 2008;6:e237. doi: 10.1371/journal.pbio.0060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Geisler JG. Targeting energy expenditure via fuel switching and beyond. Diabetologia. 2011;54:237–244. doi: 10.1007/s00125-010-1932-4. [DOI] [PubMed] [Google Scholar]
- 104.Ukropcova B, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007;56:720–727. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- 105.Virtue S, et al. A new role for lipocalin prostaglandin d synthase in the regulation of brown adipose tissue substrate utilization. Diabetes. 2012;61:3139–3147. doi: 10.2337/db12-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fedorenko A, et al. Mechanism of Fatty-Acid-Dependent UCP1 Uncoupling in Brown Fat Mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 108.de Meis L, et al. Brown adipose tissue mitochondria: modulation by GDP and fatty acids depends on the respiratory substrates. Biosci. Rep. 2012;32:53–59. doi: 10.1042/BSR20100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Divakaruni AS, et al. Fatty acids change the conformation of uncoupling protein 1 (UCP1) J Biol. Chem. 2012;287:36845–36853. doi: 10.1074/jbc.M112.381780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 111.Feldmann HM, et al. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 112.Schlogl M, et al. Overfeeding over 24 hours does not activate brown adipose tissue in humans. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]