Abstract
Background
Randomized trials of complex, non-pharmacologic interventions implemented in home and community settings, such as the University of Southern California (USC)–Rancho Los Amigos National Rehabilitation Center (RLANRC) Pressure Ulcer Prevention Study (PUPS), present unique challenges with respect to: (a) participant recruitment and retention, (b) intervention delivery and fidelity, (c) randomization and assessment, and (d) potential inadvertent treatment effects.
Purpose
We describe the methods employed to address the challenges confronted in implementing PUPS. In this randomized controlled trial, we are assessing the efficacy of a complex, preventive intervention in reducing the incidence of, and costs associated with, the development of medically serious pressure ulcers in people with spinal cord injury.
Method
Individuals with spinal cord injury recruited from RLANRC were assigned to either a 12-month preventive intervention group or a standard care control group. The primary outcome is the incidence of serious pressure ulcers with secondary endpoints including ulcer-related surgeries, medical treatment costs, and quality of life. These outcomes are assessed at 12 and 24 months after randomization. Additionally, we are studying the mediating mechanisms that account for intervention outcomes.
Results
PUPS has been successfully implemented, including recruitment of the target sample size of 170 participants, assurance of the integrity of intervention protocol delivery with an average 90% treatment adherence rate, and enactment of the assessment plan. However, implementation has been replete with challenges. To meet recruitment goals, we instituted a five-pronged approach customized for an underserved, ethnically diverse population. In intervention delivery, we increased staff time to overcome economic and cultural barriers to retention and adherence. To ensure treatment fidelity and replicability, we monitored intervention protocol delivery in accord with a rigorous plan. Finally, we have overcome unanticipated assessment and design concerns related to: (a) determining pressure ulcer incidence/severity, (b) randomization imbalance, and (c) inadvertent potential control group contamination.
Limitations
We have addressed the most daunting challenges encountered in the recruitment, assessment, and intervention phases of PUPS. Some challenges and solutions may not apply to trials conducted in other settings.
Conclusions
Overcoming challenges has required a multifaceted approach incorporating individualization, flexibility, and persistence as well as the ability to implement needed mid-course corrections.
Keywords: Spinal cord injury, pressure ulcer prevention, RCTs of multicomponent individualized interventions, underserved minority research participants
Introduction
Randomized controlled trials (RCTs), generally accepted as the most reliable method for determining intervention efficacy, frequently are employed to evaluate health interventions when the intervention protocol is straightforward and highly structured [1–3]. However, a pressing need exists for rigorous evaluation of the efficacy of complex, non-pharmacologic interventions, such as rehabilitation or health promotion programs, which are multi-faceted and often individualized [1,3–7]. In practice, RCTs with these features are labor-intensive and pose considerable challenges that can become more pronounced when the intervention is delivered to underserved, diverse populations in the home or community [1,4,8]. In addition to difficulties in standardizing intervention content and delivery [5], socioeconomic factors may require unanticipated modifications in approach, and participant adherence may be poor [7,9,10]. Finally, when the intervention targets a medical rehabilitation patient population that tends to be heterogeneous within diagnostic categories, other methodological concerns may arise [2,11].
We present key methodological challenges encountered in implementing the University of Southern California (USC)–Rancho Los Amigos National Rehabilitation Center (RLANRC) Pressure Ulcer Prevention Study (PUPS), a prospective, single-blind RCT. Its aims are to: (1) assess the efficacy of a lifestyle intervention, the Pressure Ulcer Prevention Program (PUPP), in preventing medically serious (Stage III or IV) pressure ulcers and related surgeries in adults with spinal cord injury; (2) document the intervention’s potential medical cost savings and effects on quality of life; and (3) model the intervening process mechanisms that mediate any intervention effects.
Studies such as PUPS are crucial because ecologically valid, cost-effective interventions need to be provided after discharge into community settings for populations vulnerable to secondary medical complications [12–16]. The occurrence of pressure ulcers is the most frequent of these conditions among adults with spinal cord injury [15–18], with an annual incidence of 25% to 66% [19]. Such ulcers are associated with annual treatment costs estimated between $2.2 and $3.6 billion within the United States [20] and their occurrence can be life-threatening or otherwise diminish quality of life [21–25]. People with spinal cord injury remain at high risk for pressure ulcers even after receiving education in prevention techniques (such as routine skin checks) [26,27]. Based on findings from our previous research [28–33], we hypothesized that an intervention focusing on prevention in daily life contexts could reduce the risk of pressure ulcers in community-dwelling people with spinal cord injury [14–16,34].
Methods
Figure 1 provides an overview of the PUPS design. At the time of study enrollment, participants were classified into one of three strata based on current pressure ulcer status and pressure ulcer history: (1) low risk (≤1 medically serious pressure ulcers in the past two years; n=100); (2) high risk (≥2 medically serious pressure ulcers in the past two years; n=67); or (3) current stable or healing Stage III pressure ulcer (n=3; pre-existing Stage III ulcers were excluded from analysis). Within each stratum, participants were randomized to the intervention group (n=83) or control group (n=87). The conceptual model to be tested is depicted in Figure 2. In the model, the degree to which participants comply with the intervention, and utilize its key components, is hypothesized to have a positive effect on each of the mediators of successful prevention.
Figure 1.
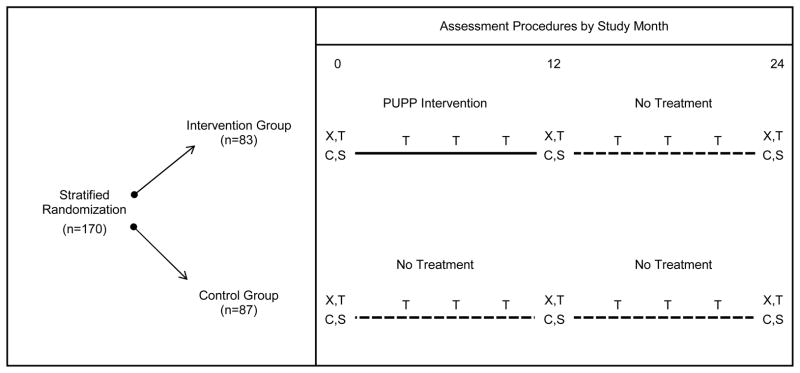
Assessment Procedures by Study Month
PUPP = Pressure Ulcer Prevention Program
X = Administration of assessment battery and skin checks
T = Healthcare utilization telephone interview
C = Chart review
S = Skin check
Figure 2.
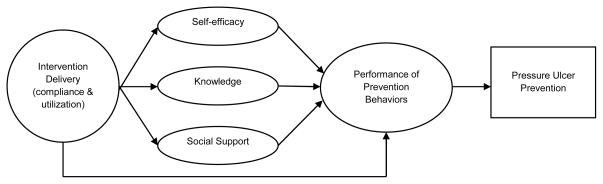
Mediation Model of Intervention Effects
PUPS participants
Sample size calculations indicated that the inclusion of 160 participants would produce sufficient power (91%) to detect a 50% reduction in the incidence of pressure ulcers (from a mean of 1.33 per year, based on a preliminary study, to a mean of .67 per year [d = .578]), given 30% attrition [35]. However, as PUPS progressed, to counteract the possibility of a baseline ulcer rate lower than our original estimate, we enrolled 170 participants (144 men and 26 women). Eligible participants were non-ambulatory, cognitively intact, English- or Spanish-speaking adults with a history of traumatic spinal cord injury at least six months prior to the date we assessed eligibility. They were required to have had at least one medically serious pressure ulcer within the past five years, and to have no worsening Stage III or any Stage IV ulcer present.
Participants were enrolled between February 11, 2009 and November 11, 2011 and include 83 Hispanics/Latinos, 54 African-Americans, 22 Whites, and 11 people of mixed or other ethnicities. Consistent with data indicating that individuals with disabilities are more likely to be living in poverty than non-disabled individuals, 54% reported monthly household incomes below $1,000, less than one-fourth of the 2011 national median monthly income of $4,171 [36,37]. Individuals with low income and racial/ethnic minority group members are at elevated risk for pressure ulcers and therefore represent an appropriate group to receive preventive intervention [38]. A data and safety monitoring board (DSMB) was formed to monitor the safety and rights of the research participants. All participants completed the informed consent process, approved by the RLANRC Institutional Review Board, prior to study enrollment.
Intervention and control group protocols
PUPP is a complex intervention aimed at incorporating sound, personally relevant pressure ulcer prevention practices into participants’ daily routines [39–42]. The PUPP intervention, which incorporates both fixed and variable (customizable) elements within six modular content areas, is summarized in Table 1. The inclusion of an individualized component is consistent with theoretical work stressing the agentic role of individuals in managing their lives [15,16,43–45], the importance of self-determination and patient-centeredness [46–48], and the potential for intervention tailoring to affect health outcomes positively [49]. Key aspects of the intervention include identification of personally chosen goals, intervener-facilitated problem solving, motivational interviewing [50], traditional rehabilitation strategies [51–53], presentation of practical knowledge in response to real-world dilemmas, and an emphasis on making sustainable, long-term lifestyle changes.
Table 1.
Intensive Phase Content of the Intervention Protocol
| Modules | Fixed Elements | Variable (Optional) Elements |
|---|---|---|
| Module 1 Understanding Lifestyle and Pressure Ulcer Risk |
Importance of Lifestyle Prevention Practices in Daily Life Personal Risk Profile Development of Personal Prevention Plan |
Activity vs. Health; Life Events; Exercise; Nutrition & Weight; Smoking; Alcohol/Substance Abuse; Prevention Techniques; Pressure Reliefs; Pressure & Shearing; Stages of Pressure Ulcers; Response to Emerging Ulcers |
| Module 2 Advocacy |
Attendant Care Partnering with Your Healthcare Professional Self-Advocacy Fine-Tuning of Personal Prevention Plan |
Access to Health Care; Medical Treatments; Medical Administration Issues; Medical Complications; Selecting/Managing Care Attendants; Emotions, Attitudes, & Self-Efficacy; Decision Making |
| Module 3 Equipment and the Physical Environment |
Equipment Transportation and Use of Environmental Options Further Refinement of Personal Prevention Plan |
Personally Relevant Transportation Options; Overcoming Environmental Barriers; Detail on Specific Equipment Options; Living Situations; Safety In and Outside the Home |
| Module 4 Social Support |
Social Support Family and Intimate Relationships Review of Current Use of Personal Prevention Plan |
Developing Friendships; Social Networking; E-Mailing; Dealing with Family Problems; Job Issues; Social Contact and Ulcer Risk; Overcoming Loneliness |
| Module 5 Happiness and Personal Well-Being |
Accomplishing a Sense of Well-Being Relation of Mental Health to Ulcer Risk Further Refinement of Personal Prevention Plan |
Coping Strategies; Managing Pain; Depression; Stress; Risk Taking; Alcohol & Drugs; Healthy Activity; Maintaining a Positive Outlook |
| Module 6 Planning the Future |
Successfully Anticipating Change Making Healthy Habits Permanent Review of Personal Prevention Plan |
Aging Skin; Finances; Strategizing for Continued Success; Personal Organization Skills; Aging and Spinal Cord Injury |
The intervention was delivered by occupational therapists in consultation with registered nurses who made wound care recommendations for pre-existing or emergent pressure ulcers. All interveners were blind to the study design and hypotheses. Participants in the intervention group received preplanned weekly contacts including 9 home visits and 15 telephone calls during an intensive phase (months 1–6), followed by a tapered phase (months 7–12) in which contacts were bi-weekly, with 2 home visits and 9 telephone calls. Intervention participants were instructed to contact their therapist for immediate assistance whenever they detected a possible new ulcer or experienced an event that heightened their pressure ulcer risk. Finally, up to $400 in prevention-related equipment was provided to each participant.
The control group did not receive any study-related intervention or comparable telephone or in-person contacts. However, individuals in this group could access usual care at RLANRC during the study period. Following study completion, they received up to $400 in prevention-related equipment, and a one-hour consultation with an occupational therapist and registered nurse.
To ensure that the intervention protocol was implemented validly [54,55], we followed a multi-faceted plan consistent with guidelines for establishing fidelity for complex interventions [6,56,57]. This plan included: (a) 30 hours of standardized intervener training; (b) one monitoring session per month in which each intervener’s adherence to the protocol was assessed using a specialized rating scale; and (c) weekly supervisory protocol adherence meetings.
Data collection
At baseline, 12 months, and 24 months after randomization, assessments are undertaken of demographic variables, potential mediating variables, and primary and secondary outcome variables via: (a) medical record review, (b) skin examinations performed by specially trained nurses; and (c) validated questionnaires administered by trained graduate students. Additionally, all participants complete quarterly telephone interviews to assess healthcare utilization and pressure ulcer status. All personnel administering assessments are blinded to the group assignment of participants. To reinforce adherence to the study protocol, participants are compensated for completing assessments.
The primary outcome, incidence of serious pressure ulcers, is assessed via the Bates-Jensen Wound Assessment Tool (BWAT) [58]. Because pressure ulcer assessment can be complicated (for example, a wound could appear and heal within less than a 12-month assessment period), medical/billing records and quarterly telephone interviews are used to supplement the BWAT findings.
Table 2 presents an overview of scales used to measure secondary outcomes and mediating variables [13,26,59–66]. For each construct, we attempted to identify a questionnaire that was brief, sensitive to change, available in English and Spanish, and previously validated, preferably with the spinal cord injury population. Health care utilization is assessed through review of medical/billing records from RLANRC and outside facilities, supplemented by self-reported quarterly telephone interviews. Data on the direct costs of the intervention are captured through logs maintained by the interveners.
Table 2.
Existing Measures of Secondary Outcomes in the USC Pressure Ulcer Prevention Study
| Construct | Instrument | Total number of items |
|---|---|---|
| Social Support | Interpersonal Support Evaluation List (ISEL) [61] | 6 |
| Knowledge of Ulcer Prevention | Pressure Ulcer Knowledge Test [13] | 14 |
| Performance of Preventive Behaviors | Garber Procedure for Assessing Performance of Preventive Behaviors [26] | 10 |
| Health-Related Quality of Life | Adapted Research and Development (RAND) 36-Item Health Survey 1.0 [62] | 36 |
| Life Satisfaction | Satisfaction with Life Scale (SWLS) [63] | 5 |
| Thinking and Memory | Short Portable Mental Status Questionnaire (SPMSQ) [59] | 10 |
| Readiness to Change | Adapted Stages of Change Measure [60] | 6 |
| Self-Efficacy | Adapted Moorong Self Efficacy Scale [64] | 27 |
| Drug and Alcohol Use | Adapted Cut Down, Annoyed, Guilty, and Eye Opener (CAGE) Questionnaire [65] | 11 |
| Depression | Quasi-Adaptive Short Form for the Patient Reported Outcomes Measurement Information System (PROMIS) version 1 Depression Item Bank [66] | 5 |
Statistical analyses
All randomized participants will be included in the intent-to-treat analysis. The primary test of intervention efficacy will involve Poisson regression analysis of between-group differences in the number of serious ulcers over the 12-month intervention phase of the study. Secondary analyses will examine the annual incidence of ulcer-related surgeries, quality of life, and indicators of the mediating constructs included in Figure 2. All statistical analyses will be repeated using data obtained through the 24-month follow-up period.
We will conduct analyses using one-tailed tests (with the expectation of a beneficial intervention effect); we will consider probabilities of 0.05 or less to be statistically significant. In all analyses, we will adjust for randomization strata as well as background variables found to be effect modifiers. We will assess possible medical cost savings through analysis of variance of individual patient medical costs, and regression models of medical costs as a function of group assignment. To analyze cost effectiveness, we will compare the cost per quality adjusted life year [67] and the net health benefit [68] between intervention and control groups. Finally, we will use structural equation modeling to identify which variables mediate the effects of the PUPP intervention.
Results
To date the study is progressing successfully. The target accrual of 170 participants has been met. Twelve-month and 24-month assessments have been completed for 145 and 129 of all participants, respectively. However, in implementing PUPS, we have been confronted with a series of methodological challenges.
Recruitment and retention challenges
Obtaining the needed sample size for a study of community-dwelling individuals with spinal cord injury was difficult. Spinal cord injury affects only a small fraction of the general population, with approximately 250,000 prevalent cases in the United States [69]. To achieve our stipulated accrual goal, we implemented a five-component recruitment plan. First, we hired a bilingual Hispanic “lead” recruiter to oversee the recruitment team. Second, other members of the recruitment team shared characteristics (e.g., language, ethnicity, having spinal cord injury) with the target population. Third, in addition to printed recruitment materials, recruiters attended the RLANRC pressure ulcer management clinic each week and met face-to-face with prospective participants. Fourth, we developed strong rapport with the medical personnel who staffed the clinics. Fifth, recruitment materials were translated and adapted to be culturally appropriate for the targeted population.
As PUPS progressed, we noted that recruitment lagged behind schedule and that a number of participants were excluded solely due to the presence of a Stage III ulcer. We concluded that this exclusion criterion could be relaxed without jeopardizing the integrity of the study. After receiving IRB approval for the change, we enrolled three participants with a current healing or stable Stage III ulcer. However, no additional potential participants had ulcers in this category. In hindsight, our recruitment goal could have been met without amending this exclusion criterion.
Various background characteristics of the sample contributed to challenges in retention. In addition to a high proportion of the sample having income below poverty levels, 36% had less than a high school education and 17% had unstable housing or had recently been homeless. Additionally, individuals engaged in high-risk activities are overrepresented in the spinal cord injury population, as the leading causes of spinal cord injury nationally are motor vehicle accidents and acts of violence [69]. The population in Los Angeles County comprises an even higher-risk group, as 43% of spinal cord injuries locally are caused by gunshot wounds [RH Adkins, Co-Director, Regional SCI Care System of Southern California, personal communication, 1 Oct 2013]. A significant proportion of our participants, as represented by the intervention group, reported past or current participation in high-risk activities, including substance abuse or dependence (32%), participation in illegal activity (31%), incarceration or criminal justice system involvement (19%), and gang activity (15%) [9].
Thus, we had many challenges to successful retention. Nevertheless, through persistence, flexible scheduling, prioritizing the convenience of participants, development of “back up” strategies, and the development of rapport over time, we retained 83.5% of participants at 12 months, and 77% at 24 months, better than our predicted 70% retention rate. We estimate that activities aimed at combating attrition, such as making follow-up telephone calls or repeated home visits, required 50% more staff time than originally projected. Moreover, we implemented explicit measures to avoid turnover of staff who interacted with participants by building a shared sense of community (e.g., holiday parties, celebration of recruitment milestones). Finally, as previously noted, all participants were compensated for completing assessments, and received progressively more compensation at the 12 and 24 month testing sessions as compared to baseline.
Intervention delivery and fidelity challenges
Participants’ life circumstances also impeded their implementation of PUPP prevention strategies [9]. Such obstacles included tenuous family dynamics, deleterious aspects of the physical environment, and unforeseen life events. For example, participants commonly engaged family members as caregivers, paid through government assistance programs, to supplement their household income. However, some family caregivers faced competing demands from work or family obligations or had health problems themselves, which created caregiving challenges that were not addressed explicitly in the PUPP intervention protocol. Participants’ living environments, including sleeping surfaces and bathing and toileting facilities, were frequently suboptimal for maintaining skin integrity. For example, one participant lived in a garage and showered with a hose in the backyard. In such instances, a significant portion of intervention time was devoted to securing resources for home modifications and equipment purchases. In some cases, changes in participants’ life circumstances pre-empted planned intervention activities altogether and shifted the focus to crisis management, such as when a participant was incarcerated or became homeless during the intervention period.
Because some participants were involved in high-risk activities or resided in high-crime neighborhoods, safety concerns added a layer of complexity to study execution. Our team addressed these concerns by sending staff to participants’ homes in pairs whenever possible, scheduling appointments during times (morning and early afternoon) when crime rates are lower, and having on-call support available whenever staff were in the field. These strategies, though effective, required additional time and personnel to execute.
Delivery of complex interventions such as PUPP, particularly when they are individualized and permit a degree of flexibility, can be difficult to standardize. A balance between protocol adherence and clinical judgment must be maintained [70]. We achieved this requirement through two strategies. First, the six intervention modules served as a toolbox demarcating the core components of the intervention and the range of appropriate content, and provided troubleshooting guidelines. Second, interveners were expected to adhere to a set of theoretically grounded, overarching principles related to pressure ulcer risk when tailoring the sessions to be participant- and situation-specific [40]. As an illustration, one principle specifies that risk is heightened by change or disruption in routine [39, 40]. Accordingly, interveners were trained to anticipate, notice, and attend to events such as equipment breakdowns, resignations of attendants, or sudden homelessness, and then incorporate the appropriate module content in intervention delivery in a responsive and timely manner.
Although a labor-intensive effort was required to ensure that the PUPP intervention protocol was delivered in a consistent manner, our ongoing fidelity monitoring facilitated 90% participant adherence to the number of treatment sessions, and satisfactory adherence of interveners to the protocol in 98% of observed treatment sessions. When satisfactory adherence was not achieved, an intervention supervisor provided individualized feedback to the intervener to counteract further protocol drift. Additionally, protocol requirements continually were reinforced at interveners’ weekly supervisory meetings.
Finally, given the ethnic composition of the participants, the intervention components had to be translated into Spanish and be culturally sensitive. Therefore, we employed systematic steps that replicated the approach taken in our previous studies [10,71] in which culturally-relevant themes and considerations were embedded in the intervention protocol. For example, as many Hispanic participants expressed guilt (“pena”) about receiving an entitlement, they were reluctant to question a health care worker. The therapeutic strategy for promoting self-advocacy therefore was modified so that a culturally acceptable style of interaction for such situations could be practiced and eventually enacted in clinical encounters.
Randomization and assessment challenges
The first methodological challenge pertaining to randomization and outcome assessment resulted from the fact that the spinal cord injury population is heterogeneous; factors for which there is variation often are correlated with outcomes of interest. For example, whether a spinal cord injury is complete (i.e., the spinal cord is fully severed, with no sensation or movement below the level of injury) or incomplete (i.e., the injury is partial and some sensation and/or movement is retained below the level of injury) is one factor which significantly influences pressure ulcer risk [72]. Periodic monitoring for participant characteristics revealed that, despite the randomization process, after 104 participants had been randomized those with incomplete injuries were highly overrepresented in the control group (p<0.005 after Bonferroni adjustment). Because unbalanced distributions of known outcome determinants can create a risk for significant error in RCTs [73], after consulting with our DSMB and receiving IRB approval, we altered the randomization scheme in accordance with guidelines from Kendall [74] such that new participants with complete spinal cord injury were assigned to the control group with a higher probability than to the intervention group. This adjustment resulted in intervention and control groups that did not differ significantly on any baseline variable (all p-values > 0.10). It should be noted that in data analysis we will use bootstrapping to ensure robustness of the stratified outcomes to account for this change.
A second challenge arose from inconsistencies in how pressure ulcers routinely are assessed and documented clinically. Although we expected identification of serious (Stage III or IV) ulcers to be straightforward, previous research indicated that staging accuracy is fairly poor [34]. Additionally, chart reports sometimes did not distinguish between pressure-related and diabetic wounds, designate whether ulcers were new or previously documented, or indicate clearly where on the body ulcers were located. We also found that participants frequently underreported the presence or severity of an ulcer because they had not detected it, could not accurately gauge its severity, or deliberately refrained from disclosure.
To ameliorate these problems, we initiated a process through which two blinded, study-employed nurse wound specialists independently integrate the various data sources to obtain a single, comprehensive ulcer index. Their assessments are reviewed by our Ulcer Reconciliation Team of three blinded PhD-level evaluators. Occasionally cases are referred to a leading wound care specialist for additional evaluation. The final assessment is based on a unanimous decision.
Potential inadvertent contamination of the control group
In designing the study, we had not considered that the quarterly telephone interviews, in and of themselves, might function as an “intervention” in that asking questions could alert control group participants to pay increased attention to the integrity of their skin and to act when such integrity was compromised. However, in preparing our DSMB reports, we noticed that the overall rate of serious pressure ulcers (we were blind to group allocation) was much lower than expected, 0.53/participant/year rather than the 1.33 calculated from our preliminary chart review of the ulcer rate in the target population. One possible explanation for this discrepancy was that the quarterly telephone interviews inadvertently were reducing the incidence of ulcers in the control group. Previous studies provide evidence that interventions delivered primarily via telephone can produce behavioral change [75]. This hypothesis was supported by feedback the Project Manager received, which indicated that participants whom she knew were in the control group were thanking telephone interviewers for the ongoing “assistance” they provided to participants.
To understand the extent to which telephone calls were operating as an “intervention”, we initiated a concomitant prospective cohort study of 70 participants comparable to those in PUPS, in whom we are evaluating serious pressure ulcer incidence during a 12-month period with no interim study contact. Thus, we expect to be able to determine whether there is a significant difference in incidence between the cohort sample, PUPS intervention group, and PUPS control group.
Discussion
We confronted many challenges while conducting a methodologically rigorous RCT of a complex individualized intervention with underserved and ethnically diverse participants in a community-based setting. Challenges we have discussed, along with the solutions we adopted, included recruitment, randomization, retention, intervention delivery and fidelity, outcome assessment, and inadvertent contamination of the control group. Given the human and economic costs of preventable conditions such as pressure ulcers, and the dearth of ecologically valid, cost-effective interventions to address these and many other health concerns, such studies are an important undertaking.
Factors to consider in implementing trials of individualized, multicomponent interventions include selection of intervention components; procedures for individual tailoring; strategies for fidelity monitoring; approaches to blinding investigators and staff; and analytic plans to evaluate the underlying mechanisms of the intervention [7,76]. Careful forethought with regard to these issues, and real-time responsiveness as the study unfolds, should help to enhance the methodological rigor of the study as well as its external validity and potential for future translation and dissemination.
Lessons Learned
A unique strength of PUPS is its inclusion of a large racial/ethnic minority, socioeconomically disadvantaged sample, in response to a call to reduce health disparities in the United States by including underrepresented minorities, underserved and typically higher-risk groups in controlled studies [77,78]. The National Center for Medical Rehabilitation Research launched an initiative to encourage participation of individuals from racial/ethnic minorities who have disabilities in clinical trials [3]. While research on underserved and minority populations is sorely needed, there can be difficulties in recruitment and retention of these populations. Therefore, we employed recruitment strategies identified as effective in systematic evidence-based best practice reviews [78–80]. As it has been observed that minorities are overrepresented among study dropouts [81], we sought to minimize attrition by employing a multifaceted approach consistent with a comprehensive retention plan developed in our previous research [82]. This plan contained numerous evidence-based strategies to address individual, cultural, and general factors likely to affect retention [78].
As observed by Wittes [83], the primary aim of a clinical trial is to maintain statistical power and clinical applicability, while maximizing internal validity. As such, midstream adjustments to the study protocol may be appropriate when they can be accomplished without jeopardizing the ability to infer treatment effects. In our study, the failure of randomization to control for an extraneous variable which may be causally related to the study’s primary endpoint called for such an adjustment, as it threatened our ability to interpret the study findings. Our decision to alter the randomization scheme came after careful weighing of the advantages and disadvantages of this and other approaches and consultation with outside experts in clinical trial design. As should be the case whenever such action is taken, this decision was made by investigators with no knowledge of outcome data. In contrast, we did not alter the schedule of the telephone interviews based on our suspicion that they inadvertently led to reduced pressure ulcer rates in the control group. Instead, we initiated the concomitant cohort study to answer this scientific question without jeopardizing the validity of the RCT.
Limitations
The challenges we experienced in conducting a large-scale RCT of an individualized, multicomponent, psychosocial intervention are most relevant to researchers embarking on similarly complex trials, and less germane to researchers evaluating relatively straightforward and structured treatment protocols. Similarly, the demographic characteristics of our study population introduced challenges in implementation that may not generalize to more advantaged populations, or those with less heterogeneous medical conditions.
Conclusion
Conducting rigorous appraisals of complex non-pharmacologic interventions among underserved diverse populations is a challenging undertaking, which requires flexibility in identifying potential adaptations within all major study phases (recruitment, intervention delivery, assessment, etc.). However, there is a pressing need to evaluate the efficacy of such preventive and rehabilitative interventions [1–5]. We have outlined strategies to circumvent problems encountered in the execution of one such study, with the aim of facilitating success among researchers carrying out this important work.
Acknowledgments
Funding
This work was supported by grant R01HD056267 from the National Center for Medical Rehabilitation Research (NCMRR), the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
The authors are indebted to Rancho Los Amigos National Rehabilitation Center for its support of this study. We thank Michael Weinrich from the NCMRR for his support of this project, and especially our program officer from the NCMRR, Louis Quatrano. In addition, the investigators thank those who served on the Data Safety and Monitoring Board for their ongoing guidance and support: Richard Salcido, MD (chair); Tom Belin, PhD; Joy Hammel, PhD, OTR; Forrest Pendleton; Denise Tate, PhD; and Louis Quatrano, PhD. Finally, the PUPS investigators acknowledge all trial participants, without whom the study would not be possible.
The Pressure Ulcer Prevention Study is registered at ClinicalTrials.gov (NCT01999816).
Appendix
In addition to the authors, members of the PUPS Study Group include:
Co-Investigators
Barbara Bates-Jensen, PhD, RN, CWOCN, FAAN (Wound Care Specialist)
Michael Scott, MD
Consultants
Marcus J. Fuhrer, PhD
Nancy Gibbs, MD
Interveners
Arameh Anvarizadeh, OTD, OTR/L
Mark Armstrong, RN
Jane Baumgarten, BS, OTR/L
Delphine O’Rourke Davidson, RN
Annee Deering-Fitzgerald, MS, RN
Celso Delgado, Jr., OTD, OTR/L
Ronald Jarvina, RN
Yvette Ngann, RN
Kelly Peck, MS, RN
Clarissa Saunders-Newton, PhD, OTR/L
Jenna Trammel, MS, RN
Lisbeth Vega, OTD, OTR/L
Ana Verran, MA, OTR/L
Project Staff
Daniella Florindez, BA
Samruddhi Ghaisas, OTD, OTR/L
Kiley Hanish, OTD, OTR/L
Cynthia Kushi, OTR/L
Jeremy Seip, OTD, OTR/L
Michael Tien, MPH
Ashwini Vaishampayan, OTD, OTR/L
Ulcer Data Reconciliation Team
Claire Jingwen Li, BS, OTR
Robert Maxwell, MSN, RN
Brooke Bianco, MSN, RN
Assessment Team
Mei Chan, MSN, RN
Jardine Cordero-Pagunsan, RN
Hilda Diaz, RN
Don Fogelberg, PhD, OTR/L
Tony Guillen, RN
Jinpei Hong, RN
LaTanya Jenkins, RN
Lisa Mizushima, RN
German Sanchez, RN
Alison Stoneham, RN
RLANRC Site Support
Laura Matrecito
Deandra Pedroza
Angie Rivera
Fiscal Administration
Patricia Gutierrez
Janis Wise
Manuscript Preparation
Sarah Gleason, BA
Stephanie Mielke, OTD, OTR/L
Daniel Park, OTD, OTR/L
References
- 1.Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000 Sep 16;321:694–6. doi: 10.1136/bmj.321.7262.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte J, Hart T. It’s more than a black box; it’s a Russian doll: Defining rehabilitation treatments. Am J Phys Med Rehabil. 2003;82(8):639–52. doi: 10.1097/01.PHM.0000078200.61840.2D. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Medical Rehabilitation Research (NCMRR) [accessed 2012 Sept 4];Report to the NACHHD Council. 2006 Jan; Available from: http://www.nichd.nih.gov/publications/pubs/Documents/ncmrr_report_online_2006.pdf.
- 4.Medical Research Council. A framework for the development and evaluation of RCTs for complex interventions to improve health. London: MRC; 2000. [Google Scholar]
- 5.Hawe P, Shiell A, Riley T. Complex interventions: How “out of control” can a randomised controlled trial be? BMJ. 2004;328:1561–1563. doi: 10.1136/bmj.328.7455.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakley A, Strange V, Bonell C, et al. Process evaluation in randomised controlled trials of complex interventions. BMJ. 2006;332:413–416. doi: 10.1136/bmj.332.7538.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allore HG, Tinetti ME, Gill TM, Peduzzi PN. Experimental designs for multicomponent interventions among persons with multifactorial geriatric syndromes. Clin Trials. 2005;2(1):13–21. doi: 10.1191/1740774505cn067oa. [DOI] [PubMed] [Google Scholar]
- 8.Gitlin LN, Burgh DM, Dodson C, et al. Strategies to recruit older adults for participation in rehabilitation research. J Am Geriatr Soc. 1995;11:10–19. [Google Scholar]
- 9.Pyatak EA, Blanche EI, Garber SL, et al. Conducting intervention research among underserved populations: Lessons learned and recommendations for researchers. Arch Phys Med Rehabil. 2013;94:1190–98. doi: 10.1016/j.apmr.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson J, Kennedy BL, Mandel D, et al. Derivation and pilot assessment of a health promotion program for Mandarin-speaking Chinese older adults. Int J Aging Hum Dev. 2000;50:127–149. doi: 10.2190/9V9H-E4L7-BTJP-9WMJ. [DOI] [PubMed] [Google Scholar]
- 11.Keller C, Records K, Coe K, et al. Promotoras’ roles in integrative validity and treatment fidelity efforts in randomized controlled trials. Fam Community Health. 2012;35:120–129. doi: 10.1097/FCH.0b013e31824650a6. [DOI] [PubMed] [Google Scholar]
- 12.Consortium for Spinal Cord Medicine. Pressure ulcer prevention and treatment following spinal cord injury: A clinical practice guideline for health-care professionals. Waldorf, MD: Paralyzed Veterans of America; 2000. [DOI] [PubMed] [Google Scholar]
- 13.Garber SL, Rintala DH, Holmes SA, et al. A structured educational model to improve pressure ulcer prevention knowledge in veterans with spinal cord dysfunction. J Rehabil Res Dev. 2002;39:575–588. [PubMed] [Google Scholar]
- 14.Gelis A, Dupeyron A, Legros P, et al. Pressure ulcer risk factors in persons with spinal cord injury part 2: The chronic stage. Spinal Cord. 2009;47:651–661. doi: 10.1038/sc.2009.32. [DOI] [PubMed] [Google Scholar]
- 15.Guihan M, Bombardier CH. Potentially modifiable risk factors among veterans with spinal cord injury hospitalized for severe pressure ulcers: A descriptive study. J Spinal Cord Med. 2012;35:240–250. doi: 10.1179/2045772312Y.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guihan M, Garber SL, Bombardier CH, et al. Lessons learned while conducting research on prevention of pressure ulcers in veterans with spinal cord injury. Arch Phys Med Rehabil. 2007;88:858–861. doi: 10.1016/j.apmr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 17.National Spinal Cord Injury Statistical Center. [accessed 2012 Sept 25];The 2006 annual statistical report for the Model Spinal Cord Injury Care Systems. 2006 Available from: https://www.nscisc.uab.edu/PublicDocuments/reports/pdf/NSCIC%20Annual%2006.pdf.
- 18.McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: A regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- 19.Salcido R, Popescu A, Potter PJ, et al. [accessed 2012 Nov 18];Pressure ulcers and wound care. c2012 Available from: http://emedicine.medscape.com/article/319284-overview.
- 20.Baranoski S. Raising awareness of pressure ulcer prevention and treatment. Adv Skin Wound Care. 2006;19:398–405. [PubMed] [Google Scholar]
- 21.Cutajar R, Roberts A. The relationship between engagement in occupations and pressure sore development in Saudi men with paraplegia. Br J Occup Ther. 2005;68:307–314. [Google Scholar]
- 22.Gorecki C, Brown JM, Nelson EA, et al. Impact of pressure ulcers on quality of life in older patients: A systematic review. J Am Geriatr Soc. 2009;57:1175–1183. doi: 10.1111/j.1532-5415.2009.02307.x. [DOI] [PubMed] [Google Scholar]
- 23.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 24.National Spinal Cord Injury Statistical Center. [accessed 2012 Sept 25];Spinal cord injury facts and figures at a glance. 2012 Available from: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts 2012 Feb Final.pdf.
- 25.Dorsett P, Geraghty T. Health-related outcomes of people with spinal cord injury--a 10 year longitudinal study. Spinal Cord. 2008;46:386–391. doi: 10.1038/sj.sc.3102159. [DOI] [PubMed] [Google Scholar]
- 26.Garber SL, Rintala DH, Rossi CD, et al. Reported pressure ulcer prevention and management techniques by persons with spinal cord injury. Arch Phys Med Rehabil. 1996;77:744–749. doi: 10.1016/s0003-9993(96)90251-8. [DOI] [PubMed] [Google Scholar]
- 27.Schubart JR, Hilgart M, Lyder C. Pressure ulcer prevention and management in spinal cord-injured adults: Analysis of educational needs. Adv Skin Wound Care. 2008;21:322–329. doi: 10.1097/01.ASW.0000323521.93058.47. [DOI] [PubMed] [Google Scholar]
- 28.Clark F, Azen SP, Zemke R, et al. Occupational therapy for independent-living older adults: A randomized controlled trial. JAMA. 1997;278:1321–1326. [PubMed] [Google Scholar]
- 29.Clark F, Azen SP, Carlson M, et al. Embedding health-promoting changes into the daily lives of independent-living older adults: Long-term follow-up of occupational therapy intervention. J Gerontol B Psychol Sci Soc Sci. 2001;56:60–63. doi: 10.1093/geronb/56.1.p60. [DOI] [PubMed] [Google Scholar]
- 30.Hay J, Labree L, Luo R, et al. Cost-effectiveness of preventive occupational therapy for independent-living older adults. J Am Geriatr Soc. 2002;50:1381–1388. doi: 10.1046/j.1532-5415.2002.50359.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark F, Jackson J, Carlson M, et al. Effectiveness of a lifestyle intervention in promoting the well-being of independently living older people: Results of the Well Elderly 2 Randomised Controlled Trial. J Epidemiol Community Health. 2012;66:782–790. doi: 10.1136/jech.2009.099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson J, Carlson M, Mandel D, et al. Occupation in lifestyle redesign: The Well Elderly Study occupational therapy program. Am J Occup Ther. 1998;52:326–336. doi: 10.5014/ajot.52.5.326. [DOI] [PubMed] [Google Scholar]
- 33.Mandel D, Jackson J, Zemke R, et al., editors. Lifestyle Redesign: Implementing the Well Elderly Program. Bethesda, MD: AOTA Press; 1999. [Google Scholar]
- 34.Guihan M, Garber SL, Bombardier CH, et al. Predictors of pressure ulcer recurrence in veterans with spinal cord injury. J Spinal Cord Med. 2008;31:551–559. doi: 10.1080/10790268.2008.11754570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark F, Rubayi S, Scott M, et al. Lifestyle redesign for pressure ulcer prevention in SCI. Los Angeles (CA): University of Southern California and Rancho Los Amigos National Rehabilitation Center; NIH Grant Proposal. 1 R01 HD056267–01. [Google Scholar]
- 36.DeNavas-Walt C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2011. U.S. Census Bureau, Current Population Reports. Washington, D.C: U.S. Government Printing Office; 2012. Sep, Report no. P60–243. [Google Scholar]
- 37.Erickson W, Lee C, von Schrader S. Disability Statistics from the 2011. American Community Survey (ACS); Ithaca, NY: 2013. [Google Scholar]
- 38.Chen Y, Devivo MJ, Jackson AB. Pressure ulcer prevalence in people with spinal cord injury: age-period-duration effects. Arch Phys Med Rehabil. 2005;86(6):1208–1213. doi: 10.1016/j.apmr.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Clark FA, Jackson JM, Scott MD, Carlson ME, Atkins MS, Uhles-Tanaka D, et al. Databased models of how pressure ulcers develop in daily-living contexts of adults with spinal cord injury. Arch Phys Med Rehabil. 2006;87(11):1516–1525. doi: 10.1016/j.apmr.2006.08.329. [DOI] [PubMed] [Google Scholar]
- 40.Jackson J, Carlson M, Rubayi S, et al. Qualitative study of principles pertaining to lifestyle and pressure ulcer risk in adults with spinal cord injury. Disabil Rehabil. 2010;32:567–578. doi: 10.3109/09638280903183829. [DOI] [PubMed] [Google Scholar]
- 41.Vaishampayan A, Clark F, Carlson M, et al. Preventing pressure ulcers in people with spinal cord injury: Targeting risky life circumstances through community-based interventions. Adv Skin Wound Care. 2011;24:275–84. doi: 10.1097/01.ASW.0000398663.66530.46. quiz 285–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanche EI, Fogelberg D, Diaz J, et al. Manualization of occupational therapy interventions: Illustrations from the Pressure Ulcer Prevention Research Program. Am J Occup Ther. 2011;65:711–719. doi: 10.5014/ajot.2011.001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton KD, Lydon JE, D’Alessandro DU, et al. The differential effects of intrinsic and identified motivation on well-being and performance: Prospective, experimental, and implicit approaches to self-determination theory. J Pers Soc Psychol. 2006;91:750–762. doi: 10.1037/0022-3514.91.4.750. [DOI] [PubMed] [Google Scholar]
- 44.Clark F, Carlson M, Zemke R, et al. Life domains and adaptive strategies of a group of low-income, well older adults. Am J Occup Ther. 1996;50:99–108. doi: 10.5014/ajot.50.2.99. [DOI] [PubMed] [Google Scholar]
- 45.Ward MM. Sense of control and self-reported health in a population-based sample of older Americans: Assessment of potential confounding by affect, personality, and social support. Int J Behav Med. doi: 10.1007/s12529-011-9218-x. Epub ahead of print 27 January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammell KW. Experience of rehabilitation following spinal cord injury: A meta-synthesis of qualitative findings. Spinal Cord. 2007;45:260–274. doi: 10.1038/sj.sc.3102034. [DOI] [PubMed] [Google Scholar]
- 47.Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: Polysemy or cacophony? Patient Educ Couns. 2007;66:13–20. doi: 10.1016/j.pec.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Lauver DR, Ward SE, Heidrich SM, et al. Patient-centered interventions. Res Nurs Health. 2002;25:246–255. doi: 10.1002/nur.10044. [DOI] [PubMed] [Google Scholar]
- 49.Richards KC, Enderlin CA, Beck C, et al. Tailored biobehavioral interventions: A literature review and synthesis. Res Theory Nurs Pract. 2007;21:271–285. doi: 10.1891/088971807782428029. [DOI] [PubMed] [Google Scholar]
- 50.Miller WR, Rollnick S. Motivational interviewing: preparing people to change addictive behavior. New York: Guilford Press; 1991. [Google Scholar]
- 51.Pendleton HM, Schultz-Krohn W, editors. Pedretti’s occupational therapy: Practice skills for physical dysfunction. 7. St. Louis, Mo: Elsevier; 2013. [Google Scholar]
- 52.Crepeau EB, Cohn ES, Schell BAB, editors. Willard & Spackman’s occupational therapy. 11. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 53.Radomski MV, Latham CAT. Occupational therapy for physical dysfunction. 6. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 54.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 55.Breitenstein SM, Gross D, Garvey CA, et al. Implementation fidelity in community-based interventions. Res Nurs Health. 2010;33:164–173. doi: 10.1002/nur.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parham LD, Cohn ES, Spitzer S, et al. Fidelity in sensory integration intervention research. Am J Occup Ther. 2007;61:216–227. doi: 10.5014/ajot.61.2.216. [DOI] [PubMed] [Google Scholar]
- 57.Horner SD. Best practices for improving intervention fidelity that every nurse should know. J Spec Pediatr Nurs. 2012;17:171–174. doi: 10.1111/j.1744-6155.2012.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bates-Jensen BM, Vredevoe DL, Brecht ML. Validity and reliability of the Pressure Sore Status Tool. Decubitus. 1992;5:20–28. [PubMed] [Google Scholar]
- 59.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 60.Marcus BH, Selby VC, Niaura RS, et al. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 61.Cohen S, Mermelstein R, Kamarck T, et al. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research, and applications, NATO ASI series, series D: Behavioral and social science - no. 24. Norwell, MA: Martinus-Nihjoff Pubs.(NE), Kluwer Academic; 1985. pp. 73–94. [Google Scholar]
- 62.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 63.Diener E, Emmons RA, Larsen RJ, et al. The Satisfaction With Life Scale. J Pers Assess. 1985;49:71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- 64.Middleton JW, Tate RL, Geraghty TJ. Self-efficacy and spinal cord injury: Psychometric properties of a new scale. Rehabil Psychol. 2003;48:281–288. [Google Scholar]
- 65.Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 66.Yang F, Jones RN. A comparison between PROMIS Depression items to the Patient Health Questionnaire (PHQ)-9, Geriatric Depression Scale (GDS), and the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV, SCID) J Ment Health Policy Econ. in press. [Google Scholar]
- 67.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 68.Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18:S68–80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 69.DeVivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50(5):365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 70.Carroll KM, Nuro KF. One Size Cannot Fit All: A Stage Model for Psychotherapy Manual Development. Clinical Psychology: Science and Practice. 2002;9(4):396–406. [Google Scholar]
- 71.Jackson J, Blanche E, Mandel D. A cultural adaptation of the well elderly intervention for spanish-speaking older adults. Research platform presented at: 91st Annual American Occupational Therapy Association Conference; Philadelphia, PA. 14–17 April 2011.. [Google Scholar]
- 72.Salzberg C, Byrne D, Cayten C, van Niewerburgh P, Murphy J, Viehbeck M. A new pressure ulcer risk assessment scale for individuals with spinal cord injury. Am J Phys Med Rehabil. 1996;75(2):96–104. doi: 10.1097/00002060-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 73.Blair E. Discussion: Gold is not always good enough: the shortcomings of randomization when evaluating interventions in small heterogeneous samples. J Clin Epid. 2004;57(12):1219–1222. doi: 10.1016/j.jclinepi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Kendall JM. Designing a research project: randomised controlled trials and their principles. Emer Med J. 2003;20(2):164–168. doi: 10.1136/emj.20.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eakin EG, Lawler SP, Vandelanotte C, et al. Telephone interventions for physical activity and dietary behavior change: A systematic review. Am J Prev Med. 2007;32:419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Burgio L, Corcoran M, Lichstein KL, Nichols L, Czaja S, Gallagher-Thompson D, et al. Judging outcomes in psychosocial interventions for dementia caregivers: The problem of treatment implementation. Gerontologist. 2001;41(4):481–489. doi: 10.1093/geront/41.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Institute of Medicine. Unequal treatment: confronting racial and ethnic disparities in healthcare. United States: National Academies Press; 2003. [PubMed] [Google Scholar]
- 78.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 79.Uybico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: A systematic review of recruitment interventions. JGIM. 2007;22:852–863. doi: 10.1007/s11606-007-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paskett ED, Reeves KW, McLaughlin JM, Katz ML, McAlearney AS, Ruffin MT, et al. Recruitment of minority and underserved populations in the United States: The centers for population health and health disparities. Contemp Clin Trials. 2008;29(6):847–861. doi: 10.1016/j.cct.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janson SL, Alioto ME, Boushey HA. Attrition and retention of ethnically diverse subject in multicenter randomized controlled research trial. Con Clin Trials. 2001;22:236S–243S. doi: 10.1016/s0197-2456(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 82.Carlson M, Jackson J, Mandel D, et al. Predictors of retention among African American and Hispanic older adult research participants in the Well Elderly 2 randomized controlled trial. J App Ger. 2012 Oct 26; doi: 10.1177/0733464812471444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wittes J. On changing a long-term clinical trial midstream. Stat Med. 2002;21(19):2789–2795. doi: 10.1002/sim.1282. [DOI] [PubMed] [Google Scholar]


