The presence of an ovarian endometrioma is associated with diminished fertility potential. We however do not know the underlying mechanism. The incidence of ovarian endometrioma has likely been on the rise possibly because more and more women across the world go through repeated cycles of ovulatory menstruation for prolonged periods during their lifetime. During these ovulatory cycles, retrograde seeding of menstrual tissue into a cystic corpus luteum, leading to its transformation to an endometrium-lined cyst, is the most plausible mechanism for endometrioma formation (1). The lining of an endometrioma cyst eventually becomes organized similar to the eutopic endometrium and grows and menstruates into the cysts cavity in response to estradiol or progesterone stimulation followed by their withdrawal leading to the clinically recognized “chocolate cyst”. It can be envisioned that the unfavorable ovarian environment, involving increased mechanical stress, rigidity, distorted anatomy leading to impaired blood supply and innervation, inflammation, monocytic cell infiltration and excessive cytokine and prostaglandin production, negatively alters oocyte development and quality. Management of ovarian endometrioma is one of the most challenging tasks for the reproductive endocrinologist. To surgically remove or not to remove an endometrioma for the best fertility outcome is a key question without a clear answer.
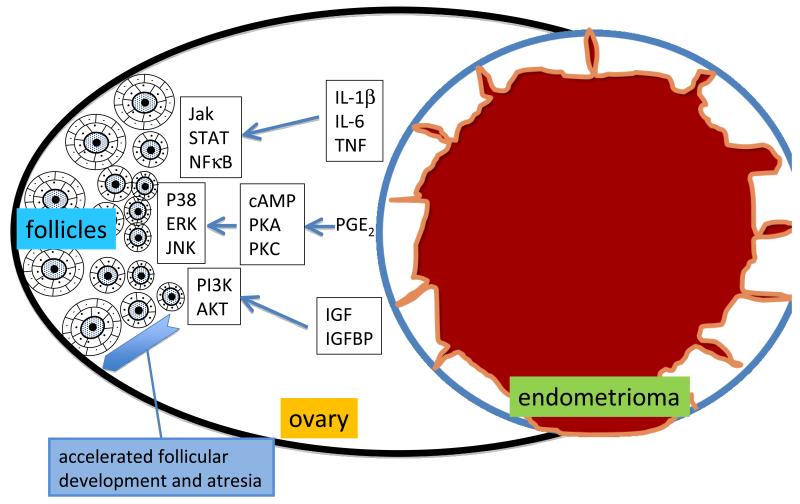
In this issue of Fertility and Sterility, Kitajima and coauthors report important findings including early follicular activation, enhanced oocyte apoptosis and accelerated depletion of follicles in an ovary bearing an endometrioma (2). These observations suggest that premature activation of follicular granulosa cells, which seems to be the primary event, causes untimely oocyte maturation leading to early death of the oocytes. This is a critical observation that can explain how menstruating endometrium-like tissue within an ovary may activate pathways that induce premature activation of early follicular development. Before getting into the discussion of the likely mechanisms responsible for this phenomenon, two physical consequences of an endometrioma are worth mentioning. 1-A large endometrioma can simply compromise the physiologic innervation and blood supply of the entire ovarian cortex; this in turn likely affects follicular development and oocyte fate via a number of mechanisms. 2-The mass effect and fibrosis induced by an endometrioma alter the mechanical force, which in turn may trigger distinct signaling pathways in the follicular cells residing in the ovarian cortex. In terms of distinct signal transduction pathways, I choose to consider three categories of well-studied molecular products of endometriosis below (Fig 1).
Fig 1.
The endometrioma transforms the macro- and micro-environment in the ovary to a highly inflammatory one. The endometriotic tissue may secrete a number of products including cytokines, chemokines and growth factors. These substances may activate specific signaling pathways in the follicular cells leading to premature follicular development and accelerated atresia. (Refer to the article for the abbreviations in the figure.)
First, endometriotic cells secrete remarkably high levels of cytokines and chemokines. Cytokines such as interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF) and chemokines such as IL-8, monocyte chemoattractant protein (MCP)-1 and “regulated on activation normal T-cell expressed and secreted” (RANTES) may trigger granulosa cell activation and premature follicular development (3). Via direct or indirect mechanisms, these substances can influence key signaling cascades in follicular cells including cyclic adenosine monophosphate/protein kinase-A (cAMP/PKA), Janus kinase/signal transducer and activator of transcription (JAK/STAT), nuclear factor kappa B (NFKB), phosphatidylinositol 3-kinase (PI3K) and intracellular calcium mobilization pathways among others in ovarian follicular cells, leading to premature follicular activation.
Second, prostaglandins, in particular PGE2, are produced in large amounts in endometriotic tissue (4). PGE2, via cAMP/PKA or PKC, activates the terminal mitogen activated protein kinases (MAPKs), protein 38 (p38), extracellular signal regulated protein kinase (ERK), Jun NH2-terminal kinase (JNK) in a number cell types including the follicular theca and granulosa cells. It is conceivable that PGE2 and other eicosanoids, generated in the inflammatory environment surrounding an endometrioma, may influence the rate of follicular activation and atresia.
Finally, the insulin-like growth factor (IGF)/IGF-binding protein (IGFBP) system that regulates a number of signaling pathways including PI3K/AKT was reported to be altered in endometriotic tissues. Since the IGF pathway interacts with the follicle stimulating hormone (FSH) pathway to regulate follicular theca-granulosa cell activation, it is tempting to hypothesize that an endometrioma may alter IGF availability and regulation of its downstream signaling pathways in the neighboring follicular cells (5). This in turn may lead to premature activation of ovarian follicular cells and premature oocyte development.
All of these potential pathologic mechanisms may impair the delicate physiologic systems involving FSH-independent or dependent ovarian follicular development. Thus, there is ample reason for inappropriate follicular activation caused by the presence of endometrioma in an ovary. A clinically relevant question is whether its surgical removal would be beneficial to a woman who is undergoing fertility treatment. This would depend on many factors including the surgeon’s level of knowledge and skill, the extent of collateral damage on the normal ovarian tissue harboring follicles, whether the accelerated rate follicular atresia significantly decreases after endometrioma removal and whether this would actually improve the take home baby rate. Well-designed basic and clinical studies are needed to find clear answers to these critical questions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vercellini P, Somigliana E, Vigano P, Abbiati A, Barbara G, Fedele L. ‘Blood On The Tracks’ from corpora lutea to endometriomas. BJOG. 2009;116:366–71. doi: 10.1111/j.1471-0528.2008.02055.x. [DOI] [PubMed] [Google Scholar]
- 2.Kitajima M, Dolmans M-M, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril. 2014 doi: 10.1016/j.fertnstert.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 3.Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, Taylor RN. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–22. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Banu SK, Burghardt RC, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits adhesion of human endometriotic epithelial and stromal cells through suppression of integrin-mediated mechanisms. Biol Reprod. 2013;88:1–11. doi: 10.1095/biolreprod.112.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adashi EY, Resnick CE, Hernandez ER, Hurwitz A, Roberts CT, Leroith D, Rosenfeld R. Insulin-like growth factor I as an intraovarian regulator: basic and clinical implications. Ann N Y Acad Sci. 1991;626:161–8. doi: 10.1111/j.1749-6632.1991.tb37910.x. [DOI] [PubMed] [Google Scholar]