Abstract
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. With an increasing longevity and the absence of a cure, AD has become not only a major health problem but also a heavy social and economic burden worldwide. In addition to the presence of abundant intra- and extracellular neurotoxic amyloid β (Aβ) peptides, which form the amyloid plaques, and intracellular hyperphosphorylated tau protein, the main component of neurofibrillary tangles, consistent evidence indicate that the AD brain is characterized by extensive neuroinflammatory processes. The 5-Lipoxygenase (5LO) is a pro-inflammatory enzymatic pathway widely distributed within the central nervous system and is up-regulated in AD. In the last five years our group has been involved in unraveling the neurobiology of this protein and investigating its relationship with cellular and molecular events of functional importance in AD pathogenesis. By using a combination of in vitro and in vivo experimental tools and implementing genetic as well as pharmacological approaches today we know that 5LO is likely an endogenous regulator of Aβ formation via the modulation of the γ-secretase complex, and tau metabolism by modulating its phosphorylation state at specific epitopes via the cyclin-dependent kinase-5 (cdk-5). In addition, 5LO influences synaptic function and integrity and by doing so significantly affects learning and memory in the Tg2576 and 3×Tg AD transgenic mouse models. Taken together our data establish this protein as a pleiotropic contributor to the development of the full spectrum of the AD-like phenotype in these mouse models of the disease, making it a viable therapeutic target for the treatment of AD in humans.
Introduction
Alzheimer's disease (AD) is the most common cause of aging-associated neurodegenerative dementia, characterized by profound, irreversible memory impairment and global cognitive decline. The hallmark brain pathologies in AD are the deposition of extracellular amyloid plaques composed of amyloid-β (Aβ) protein as well as aggregation of intraneuronal neurofibrillary tangles consisting of hyperphosphorylated tau protein [1,2]. Although the prevalence of AD is currently thought to be in excess of 35 million people worldwide, due to the aging of the “baby-boomer” generation as well as increasing human longevity, this burden is thought to quadruple by 2050 [3]. Given this public health challenge, and that the current pharmacological armamentarium approved for AD is limited to symptomatic treatment (i.e., cholinesterase inhibitors and NMDA receptor antagonists), exploration of new molecular pathways as novel therapeutic targets in AD remains an attractive option for disease modifying drug development.
5-lipoxygenase (5LO)
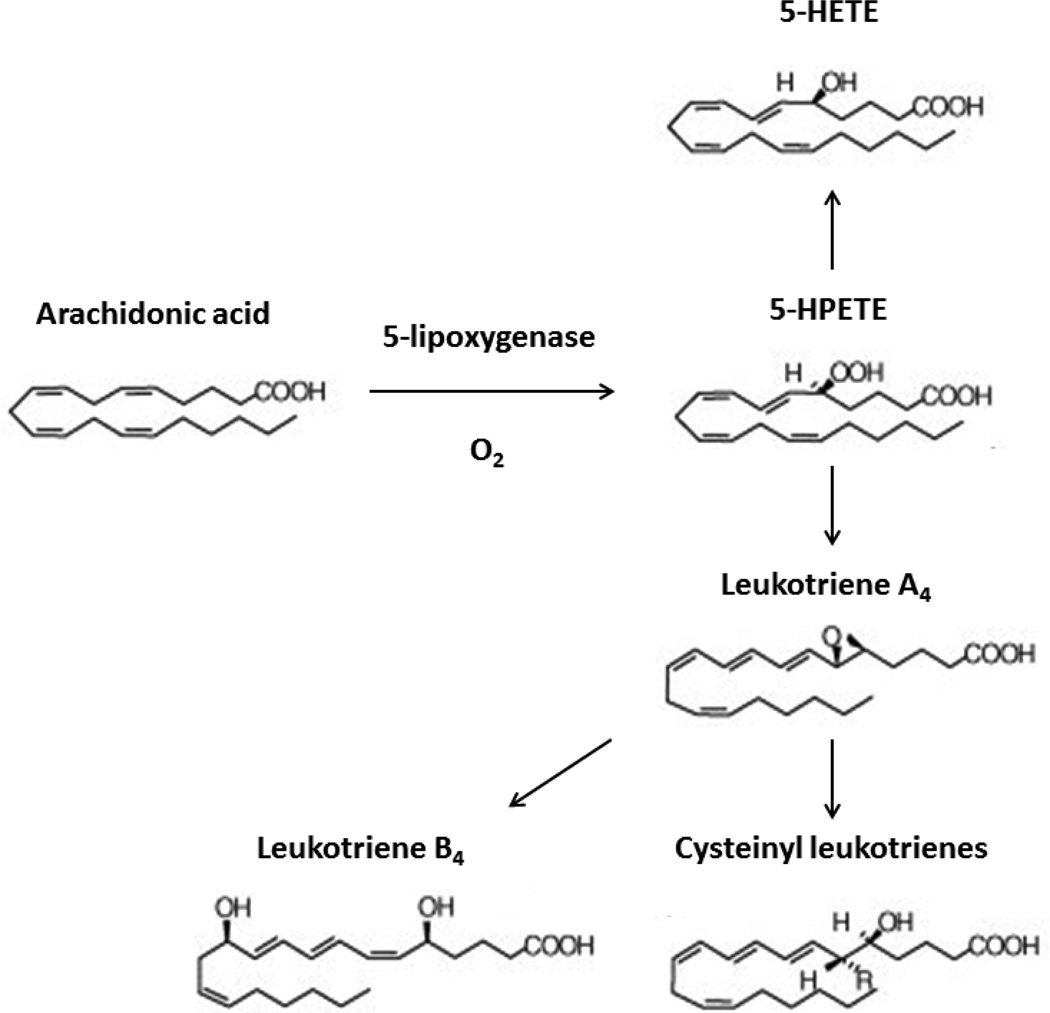
The lipoxygenase (LOs) proteins are a group of lipid-peroxidizing enzymes that insert molecular oxygen into esterified and free polyunsaturated fatty acids such as arachidonic acid, generating bioactive lipid moieties. The 5-lipoxygenase (5LO) in particular catalyzes the conversion of arachidonic acid to 5-hydroxy-peroxy-eicosatetrenoic acid (5-HPETE), which can subsequently be metabolized to 5-hydroxy-eicosatetrenoic acid (5-HETE) as well as different leukotrienes [4] (Figure 1). Since leukotrienes are potent pro-inflammatory molecules, much work has investigated the role that 5LO plays in vascular inflammation, atherosclerosis, allergy, and cancer biology [5]. Currently, clinical application of 5LO modulation is focused on control of asthma by the 5LO inhibitor, zileuton (Zyflo).
Figure 1. The 5-Lipoxygenase enzymatic pathway.
The 5-lipoxygenase (5LO) by inserting molecular oxygen into carbon 5 catalyzes the conversion of arachidonic acid to 5-hydroxyperoxy-eicosatetrenoic acid (5-HPETE), which can subsequently be metabolized to 5-hydroxyeicosatetrenoic acid (5-HETE) as well as different leukotrienes such as leukotrienes A4 and leurkotriene B4.
Despite this investigation in the periphery, 5LO’s role in the central nervous system has only recently received attention. The 5LO enzyme is widely expressed in the central nervous system, where it localizes mainly in neuronal cells [5–7]. In human and murine brains, 5LO is abundantly expressed in the hippocampus and cerebral cortex, with its levels and activity rising as a function of aging [8]. Since aging is an unavoidable risk factor in the development of sporadic AD, and 5LO is expressed in regions of the brain that are particularly affected by AD, we originally hypothesized that 5LO could play a function role in its progression. Although one limited study with 34 individuals had previously hinted that polymorphisms in the 5LO gene (ALOX5) may be associated with AD risk [9], ours was the first group to show that 5LO steady-state protein levels were significantly increased in the brains of patients with sporadic AD compared to age-matched controls [10]. The work was later confirmed by another group by using an immunohistochemistry approach [11]. Additionally, a more recent report showed a significant up-regulation in 5LO gene expression and enzyme activation, as measured by leukotriene B4 levels, in peripheral blood mononuclear cells of AD subjects compared to healthy controls [12]. Interestingly, we also found that compared to wild-types animals, 5LO levels were also elevated in a transgenic mouse model of AD-like brain amyloidosis (the Tg2576 mouse, see below), affording us the opportunity to experimentally study if and how 5LO could participate in the AD phenotype development. Below, we summarize the data so far available supporting a functional role for 5LO in AD-related Aβ metabolism, tau phosphorylation, synaptic integrity and function as well as behavior. Based on this knowledge, we suggest that the development of selective and specific 5LO inhibitors hold significant promise as novel disease-modifying agents. Considering that the 5LO inhibitor Zyflo has already been approved by the FDA for use in humans and isreadily commercially available, our work underscores the tremendous translational importance of developing 5LO-centered therapeutics for AD.
5LO and Aβ
The Aβ peptides are formed from sequential cleavages of the Aβ precursor protein (APP) by β-site secretase-1 (BACE-1), followed by the γ-secretase complex, which is composed at least of four different intramembrane proteins: presenilin 1 (PS1), presenilin-enhancer 2 (PEN2), anterior pharynx-defective 1 (APH1) and nicastrin (NCT), in a 1:1:1:1 ratio [13]. Once produced, Aβ peptides are prone to oligomerize and it is thought that aggregation of low-n oligomers eventually forms fibrils of amyloid, leading to the formation of the amyloid plaques [14]. Genetic analyses in families with early-onset AD have revealed multiple mutations in the synthetic pathway of Aβ, particularly in APP and presenilin which then predispose these individuals to greater production of Aβ [15]. Therefore, of the two pathologies in AD, Aβ has received significantly more attention in clinical trials attempting to alter AD progression. Since in our initial studies we found that 5LO expression increases with age and is expressed in regions of the brain in which Aβ plaques are also found, we asked whether 5LO modulation would change amyloid plaque burden in vivo. To address this question we utilized Tg2576 mice, a transgenic mouse model that expresses the K670N/M671L APP mutation found in a Swedish family with early-onset AD, which develop age-dependent brain amyloidosis similar to that seen in humans. We crossed the Tg2576 with mice genetically deficient for 5LO (i.e., homozygous knockout of 5LO) and compared them with regular Tg2576 animals to see how brain amyloidosis was affected when 5LO was not genetically available. We found that both soluble and insoluble Aβ peptides were significantly reduced in the brains of Tg2576 animals lacking 5LO, and that this reduction was even more apparent as the animals aged from middle- to late-life. On immunohistochemical analyses, this reduction in Aβ peptides translated to fewer amyloid β plaques and reduced total amyloid burden [10]. What was singular about this initial work was that Aβ reduction caused by 5LO knockout did not seem to change steady state levels of APP or increase several proteins thought to participate in Aβ clearance in the brain. To verify our knockout results, we also conducted studies of pharmacological inhibition of 5LO. Thus, we fed Tg2576 animals rodent chow supplemented with the 5LO inhibitor zileuton, from early adulthood, and found as with our knockout studies, that this pharmacologic approach also reduced brain Aβ peptide levels and amyloid plaques burden [16]. Interestingly, a recent paper showed that pharmacologic blockade of 5LO activating protein reduces amyloid angiopathy in TgCRND8 mice and this reduction was associated with a significant decrease in the steady state levels of nicastrin, one of the components of the γ-secretase complex [17].
Following these results, we searched for an explanation of how 5LO modulates Aβ production in vivo. We first considered the BACE-1 metabolic pathway: if steady-state levels of this secretase were to be decreased or its net activity reduced, this could lead to lower levels of Aβ without any change in APP. However, we found that neither knockout nor inhibition of 5LO with another 5LO inhibitor, AA-861, changed BACE-1 expression levels or activity. We then investigated the four proteins of the γ-secretase complex: PS1, PSEN2, APH1 and NCT. Both pharmacologic inhibition and knockout of 5LO significantly reduced steady state levels of the γ-secretase in mouse brain [10,16]. To further support our findings in vitro, we used neuronal cells that stably expressed the same APP Swedish transgene (N2aAPPswe) as in our mouse model, and found that dose-dependent inhibition and knockout of 5LO significantly reduced γ-secretase expression and Aβ levels as well [18]. Thus, we had strong evidence that 5LO exerted its effect on Aβ production by regulating γ-secretase protein complex levels.
However, while we had evidence suggesting 5LO modulated γ-secretase, we lacked a precise mechanistic explanation. Metabolites of 5LO exert many complex downstream signaling events, but we found particular interest in the cyclic AMP response element binding protein (CREB), as this transcription factor had been previously described to modulate the transcriptional activity of the PSEN2 gene in vivo as well as in vivo [18]. In N2aAPPswe cells we found that stimulation with 5HETE, the immediate downstream metabolite of 5LO action on arachidonic acid, elevated γ-secretase protein and mRNA levels, as well as the activation and nuclear translocation of the transcription factor CREB. When CREB pharmacological blockers or dominant negative vectors were used to prevent CREB activation, 5HETE failed to increase γ-secretase mRNA, protein or Aβ levels, suggesting that 5LO acted on CREB to increase γ-secretase at the transcriptional levels [16, 18].
With this new piece of evidence we went back to our samples and found that in the brains of Tg2576 mice in which 5LO was knocked out or was pharmacologically inhibited, CREB activation was also reduced compared to control animals. In later experiments, we overexpressed 5LO in neuronal cells as well as in Tg2576 animals using an adeno-associated viral vector strategy and found that the converse of our knockout and inhibition experiments were equally as valid: increased 5LO protein level resulted in a significant elevation of phosphorylated CREB, γ-secretase proteins, Aβ levels and amyloid plaques deposition [19]. Even though CREB modulation has been suggested to regulate BACE-1 expression, in our experiments we were unable to observe any modification of BACE-1 by either 5LO or CREB augmentation or disruption [20]. Further studies are warranted to investigate this important aspect of the neurobiology of CREB.
On a final note, γ-secretase inhibition in AD has been, and continues to be a highly desirable mode of reducing Aβ production. However, full blockade of γ-secretase has deleterious effect because this enzyme is also an active player in the proteolytic processing of other substrates besides APP such as Notch-1 and cadherins, both of which are important in transducing biologically relevant intracellular signals. Supporting this concept, during a recent trial testing the γ-secretase inhibitor semagacestat (LY 450139), AD patients actually experienced a functional decline compared to patients who took a placebo in addition to several other significant adverse effects [21]. Thus, development of compounds able to selectively inhibit Aβ production without affecting the cleavage of other substrates is the most desirable approach for AD therapy to minimize the toxic effects. Therefore, to see if Notch was also compromised in our system, we assayed whether its signaling pathway was affected in both our cellular and animal models. To our surprise, we found that 5LO-dependent modulation of γ-secretase is completely independent of Notch processing. While at the present time it is not known why a reduction in steady-state levels of γ-secretase induced by knockout or inhibition of 5LO reduces amyloid levels without altering Notch, our findings are in line with the novel concept of “modulatory action” of 5LO towards this secretase. Thus, recently a new class of drug has emerged as modulators and not direct inhibitors of the γ-secretase which would reduce Aβ formation without interfering with the processing of other biologically important substrates such as Nocth [22]. We speculate that 5LO activation may somehow alter membrane dynamics allowing greater access to APP than Nocth processing, although this area 5LO neurobiology biology remains completely unexplored.
Since newer generations of γ-secretase inhibitors are being screened specifically for Notch-sparing properties, this discovery places further importance on pharmacologic targeting of 5LO in AD.
5LO and tau
Aside from Aβ, AD brain pathology consists also of intracellular neurofibrillary tangles composed of the microtubule-associated tau protein. Tau protein is normally found in neuronal axons where it promotes microtubule assembly and stabilization but in post-mortem brains of AD patients, tau is hyperphosphorylated, a pathological step that is believed to result in the formation of insoluble tau oligomers and paired filaments, which have a high tendency to form insoluble aggregates and finally neurofibrillary tangles The phosphorylation of tau is thought to be part of a post-transcriptional regulation of the protein and the final result of the opposing actions of tau-associated kinases, which add phosphate, and phosphatases, which remove phosphate. The tau-associated kinases include among others: cyclin-dependent kinase 5 (cdk5), as well as glycogen synthase kinase 3-beta (GSK3-β), both of which have been found to be altered in the brains of AD patients [23–25]. Since we found that pharmacologic inhibition of 5LO resulted in reduced Aβ burden in vivo, we asked whether tau phosphorylation would also be affected. To that end, we assessed brains of Tg2576 mice previously administered with the 5LO inhibitor zileuton for endogenous mouse tau levels and its phosphorylated isoforms by using immunoblotting and immunohistochemistry analyses. First, compared with controls, we did not find that 5LO inhibition changed total levels of mouse tau in any brain region analyzed. However, we found that inhibition of 5LO resulted in reduced levels of phosphorylation at specific epitopes of tau, some of which are considered major player in the formation of the neurofibrillary tangles. To determine the mechanism whereby 5LO inhibition influenced thisbiological event, we assayed for total levels of cdk5 and total GSK-3β and phosphorylated GSK3-β (the active form of this kinase). It has been previously reported that cdk5 affects tau hyperphosphorylation at Ser 202 and Ser396/S404 sites via the regulation of GSK3 activity [24]. While total levels of these two proteins along with protein phosphatase 2a remained unchanged, 5LO inhibition specifically reduced only the level of the cdk5 co-activators (i.e., p35 and p25) [26]. To confirm these observations, we used a kinetic activity assay using mouse brain extracts and found that indeed cdk5 kinase ex vivo activity was reduced upon 5LO inhibition or knockout. By contrast, overexpression of 5LO in Tg2576 mice similarly increased the same phosphorylated tau epitopes, with a concomitant increase in cdk5 activity [27]. In vitro, inhibition or knockdown of cdk5 activity prevented tau phosphorylation specifically at epitopes Ser 396 and Ser396/S404 in neuronal cells overexpressing 5LO. Collectively, these data indicated that 5LO plays a functional role in tau metabolism whereby modulating the activity of the cdk5 kinase pathway.
Despite these encouraging results, Tg2576 animals are unable to develop full blown neurofibrillary tangles type of lesions like the ones found in human AD brains, which precludes a complete investigation into the insoluble tau dynamics. For this reason, we decided to employ the triple transgenic mouse model of AD, 3×Tg, in our 5LO studies. The 3×Tg animals not only contain the Swedish APP transgene found in Tg2576 animals, but also PS1 and a human mutant tau transgene, and as a result they develop both high levels of Aβ and amyloid plaques, as well as intracellular neurofibrillary tangles making it an appropriate model for better studying the role of 5LO in advanced AD tau metabolism and related neuropathology [28].
Using the 3×Tg model, we employed 5LO inhibition, knockdown and overexpression studies as with the Tg2576 model. As with those animals, we found that genetic absence or pharmacological inhibition of 5LO significantly reduced Aβ formation and deposition, while high expression levels of 5LO resulted in elevated Aβ levels and plaque formation, both of which occurred through CREB-mediated transcriptional modulation of the γ-secretase complex. Additionally, these mice had a significant elevation in the phosphorylation levels of tau at specific epitopes Ser 202, Ser 396 and Ser396/S404 depending on the high levels or absence of 5LO, which associated with a significant increase in the insoluble fraction of tau, and were mediated by the involvement of the cdk5 pathway [29–31].
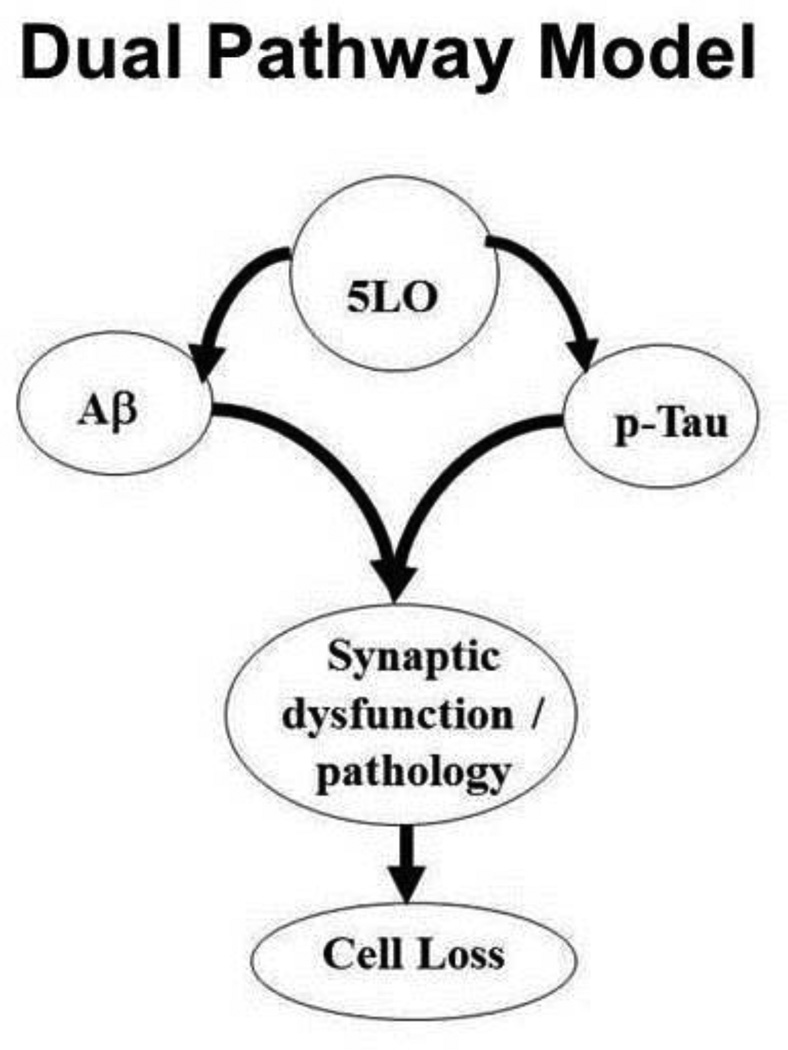
Since 5LO can act as an endogenous modulator of Aβ, and experimental evidence suggests that tau phosphorylation can occur secondary to Aβ, we were also curious to understand whether the observed 5LO-mediated modulation of tau phosphorylation state was dependent on the effect on Aβ. Using an in vitro approach, we overexpressed 5LO while at the same time inhibiting Aβ production by using the specific γ-secretase inhibitor L685,485. Under this experimental condition, γ -secretase inhibition drastically reduced Aβ levels, but was not able to alter 5LOinduced tau hyper-phosphorylation. Taken together, these data support the hypothesis that 5LO independently modulates both Aβ as well as tau metabolism in AD. They are in conflict with the “serial model” of AD pathogenesis in which Aβ is on top of the cascade and responsible for all the downstream pathological events, but provide a strong biological basis for the “dual pathway” model, in which the two players (Aβand tau) even though can be regulated by a common modulator act independently from each other (Figure 2).
Figure 2. The dual pathway model of AD pathogenesis.
The 5-lipoxygenase enzymatic pathway influences both Aβ processing and tau metabolism independently from each other. Aberration of Aβproduction and tau phosphorylation result in synaptic dysfunction and altered integrity (synaptic pathology), which underlines the development of learning and memory deficits, and ultimately culminate in cell loss.
5LO and AD synaptic function and memory impairment
Data from transgenic mouse models of the disease as well as brains of AD patients have strongly suggested that the Aβ and tau neuropathologies compromise synaptic function, with such impairment occurring well before symptoms of the disease manifest [32]. This dysfunction is characterized by alteration in markers of presynaptic integrity, such as synaptophysin, as well as markers of post-synaptic integrity, such as post-synaptic density protein 95 (PSD-95), eventually leading to impairments in long-term potentiation (LTP), which is considered a marker of synaptic function. Presumably, if 5LO modulates Aβ and tau pathology, it would also alter synaptic integrity and LTP in AD animals. To see if this was the case, we continued our investigation of 5LO in the same AD animal models, focusing on these important aspects of the neurobiology of AD.
First, we observed that overexpression of 5LO resulted in reduced synaptophysin and PSD-95, while inhibition and knockout of 5LO increased levels of these proteins in the brains of Tg2576 as well as 3×Tg animals. To investigate whether this had functional relevance, we utilized an electrophysiological approach to see whether 5LO affected hippocampal LTP. While we confirmed previous reports showing that 3×Tg animals have significant impairment in LTP when compared to wild-type control mice, next we showed that genetic absence of 5LO or its pharmacological blockade with zileuton both were able to rescue this dysfunction, restoring LTP to the same levels of the wild-type mice [32].
However, for a molecular target to be useful in AD, it must rescue behavioral as well as biochemical insults. In humans, AD memory impairments typically declare themselves initially as disruption of episodic memory and consolidation of new memories, eventually progressing to global cognitive decline. Methods to assess analogous domains in rodent models are numerous, but to assess whether 5LO rescued the altered behavioral phenotype in AD mouse models, we selected Y-maze exploratory behavior, and 24-hour fear-conditioned memory recall. Aberration in Y-maze exploratory behavior represents impairment in short-term working memory while fear-conditioned memory impairment assesses hippocampal-dependent and hippocampal-independent memory building processes. In line with our data on synaptic integrity and LTP, 5LO inhibition and knockout restored learning and memory impairments in the transgenic mice as assessed by these paradigms to a level indistinguishable from their wild-type controls [15, 17, 32]. Therefore, given this collective data, we have established that 5LO plays a role in improving the behavioral phenotype of AD animal models as well as its biochemical and electrophysiological dysfunctions.
Conclusion: Implications for 5LO-based therapy in AD
AD is an irreversible age-associated neurodegenerative condition characterized by progressive memory loss and cognitive decline. In its most common form, sporadic AD risk is considered to stem from interactions between multiple environmental risk factors and different genetic vulnerabilities making intervention rather challenging. As result, recent research in AD has shifted towards prevention and away from symptomatic treatment, leaving at least five million AD patients in the US and 30 million worldwide with little hope for new, effective drugs. We have discovered that the 5LO pathway likely plays a crucial role in the development of the full pathologic phenotype of AD which includes aberrant Aβproduction and deposition, altered tau phosphorylation, synaptic pathology and dysfunction, and ultimately behavioral impairments. Therefore, 5LO-targeted therapeutics have the potential to bridge the current AD treatment gap by being both therapeutic as well as preventative. While further characterization of 5LO inhibition is required before clinical trials are initiated in AD, in general this approach has a favorable, and well characterized, adverse effect profile that may obviate concerns that other AD-directed therapeutics may have. Based on the knowledge accumulated so far and these considerations, we believe that 5LO inhibitors hold significant promise as attractive disease-modifying agents in AD.
Acknowledgements
The work from the author’s lab described in this article was supported by grants from the National Institute of Health (AG033568; NS071096), the Alzheimer’s Association and the Alzheimer’s Art Quilt Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong CW, Quaranta V, Glenner GG. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci USA. 1984;82:8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thies W, Bleiler L Alzheimer’ Association. 2013 Alzheimer’ disease facts and figures. Alzheimers and Dementia. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 5.Pergola C, Werz O. 5-lipoxygenase inhibitors: a review of recent developments and patents. Expert Opin Ther Pat. 2010:355–375. doi: 10.1517/13543771003602012. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto T, Lindgren JA, Hokfelt T, Samuelsson B. Regional distribution of leukotriene and monohydroxyeicosatetraenoic acid production in the rat brain. Highest leukotriene C4 formation in the hypothalamus. FEBS Lett. 1987;216:123–127. doi: 10.1016/0014-5793(87)80769-x. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren JA, Hokfelt T, Dahlen SE, Patrono C, Samuelsson B. Leukotrienes in the rat central nervous system. Proc Natl Acad Sci USA. 1984;81:6212–6216. doi: 10.1073/pnas.81.19.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–1462. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Qu T, Manev R, Manev H. 5-lipoxygenase (5-LOX) promotor polymorphism in patients with early-onset and late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2001;13:304–305. doi: 10.1176/jnp.13.2.304. [DOI] [PubMed] [Google Scholar]
- 10.Firuzi O, Zhou J, Chinnici CM, Wisniewski T, Praticò D. 5-lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikonomovic MD, Abrahamson EE, Uz T, Manev H, Dekovsky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J Histochem Cytochem. 2008;56:1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Francesco A, Arosio B, Gussago C, Dainese E, Mari D, D’Addario C, Maccarrone M. Involvement of 5-Lipoxygenase in Alzheimer’s disease: a role for DNA methylation. J. Alz Dis. 2013;37:3–8. doi: 10.3233/JAD-130506. [DOI] [PubMed] [Google Scholar]
- 13.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harbor Prespect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Chu J, Praticò D. Pharmacologic blockade of 5-lipoxygenase improves the amyloidotic phenotype of an Alzheimer’s disease transgenic mouse model: involvement of gamma-secretase. Am J Pathol. 2011;178:1762–1769. doi: 10.1016/j.ajpath.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Hawkes CA, Shaw JE, Brown M, Sampson AP, McLaurin J, Carare RO. Mk886 reduces cerebral amyloid angiopathy severity in TgCRND8 mice. Neurodegener Dis. 2013 Sep 6; doi: 10.1159/000351096. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Chu J, Praticò D. 5-lipoxygenase as an endogenous modulator of amyloid beta in vivo. Ann Neurol. 2011;69:34–46. doi: 10.1002/ana.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu J, Giannopoulos PF, Ceballos-Diaz C, Gold TE, Praticò D. Adeno-associated virus-mediated brain delivery of 5-lipoxygenase modulates the AD-like phenotype of APP mice. Mol Neurodegener. 2012;7 doi: 10.1186/1750-1326-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambamurti K, Kinsey R, Maloney B, Ge YW, Lahiri DK. Gene structure and organization of the human beta-secretase (BACE) promotor. FASEB J. 2004;18(9):1034–1036. doi: 10.1096/fj.03-1378fje. [DOI] [PubMed] [Google Scholar]
- 21.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, He F, Sun X, Thomas RG, Aisen PS, Siemers E, Sethuraman G, Mohs R Alzheimer’s Disease Cooperative Study Steering Committee. Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. New Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe MS. Inhibition and modulation of gamma-secretase for Alzheimer’s disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F. Tau protein kinases: involvement in Alzheimer’s disease. Ageing Res Rev. 2013;12:289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Yardin C, Terro F. Tau protein phosphatases in Alzheimer’s disease: the leading role of PP2A. Ageing Res Rev. 2013;12:39–49. doi: 10.1016/j.arr.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Plattner F, Angelo M, Giese KP. The Roles of Cyclin-dependent Kinase 5 and Glycogen Synthase Kinase 3 in Tau Hyperphosphorylation. J Biol Chem. 2006;281(35):25457–25465. doi: 10.1074/jbc.M603469200. [DOI] [PubMed] [Google Scholar]
- 26.Chu J, Praticò D. 5-lipoxygenase pharmacological blockade decreases tau phosphorylation in vivo: involvement of the cyclin-dependent kinase-5. Neurobiol Aging. 2013;34:1549–1554. doi: 10.1016/j.neurobiolaging.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu J, Li G, Ceballos-Diaz C, Golde T, Praticò D. The influence of 5-lipoxygenase on Alzheimer’s disease-related tau pathology: in vivo and in vitro evidence. Biol Psych. 2013;74(5):321–328. doi: 10.1016/j.biopsych.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Gold TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 29.Giannopoulos PF, Chu J, Joshi YB, Sperow M, Li G, Kirby LG, Praticò D. Gene knockout of 5-lipoxygenase rescues synaptic dysfunction and improves memory in the triple-transgenic model of Alzheimer’s disease. Mol Psychiatry. 2013;12 doi: 10.1038/mp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Chu J, Li JG, Praticò D. Zileuton improves memory deficits, amyloid and tau pathology in a mouse model of Alzheimer’s disease with plaques and tangles. PLoS One. 2013;8:e70991. doi: 10.1371/journal.pone.0070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu J, Giannopoulos PF, Ceballos-Diaz C, Golde T, Praticò D. 5-lipoxygenase gene transfer worsens memory, amyloid and tau brain pathologies in a mouse model of Alzheimer’s disease. Ann Neurol. 2012;72:442–454. doi: 10.1002/ana.23642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]