Abstract
Objective
Despite a multitude of detection and treatment advances in the past two decades, prostate cancer remains the second leading cause of cancer death among men in the United States. Technological evolution and expanding knowledge of tumor biomarkers have invigorated exploration in prostate cancer therapeutics. Prostate-specific membrane antigen (PSMA) was one of the first prostate cancer biomarkers successfully cloned. Since that time, it has been characterized as the prototypical cell-surface marker for prostate cancer and has been the subject of intense clinical inquiry. We review the relevant research in PSMA on the 20th anniversary of its cloning.
Methods and materials
A PubMed® search using the keywords “prostate-specific membrane antigen” or “glutamate carboxypeptidase II” provided 1019 results. An additional 3 abstracts were included from scientific meetings. Articles were vetted by title and abstract with emphasis placed on those with clinically relevant findings.
Results
Sixty articles were selected for inclusion. PSMA was discovered and cloned in 1993. Its structure and function were further delineated in the ensuing decade. Consensus sites of expression in normal physiology are prostate, kidney, nervous system, and small intestine. PSMA has been implicated in the neovasculature of several tumors including urothelial and renal cell carcinomas. In prostate cancer, expression of PSMA is directly related to Gleason grade. PSMA has been tested both in imaging and therapeutics in a number of prostate cancer clinical trials. Several recent approaches to target PSMA include use of small molecule inhibitors, PSMA-based immunotherapy, RNA aptamer conjugates, and PSMA-targeted prodrug therapy. Future study of PSMA in prostate cancer might focus on its intracellular functions and possible role in tumor neurogenesis.
Conclusions
Twenty years from its discovery, PSMA represents a viable biomarker and treatment target in prostate cancer. Research to delineate its precise role in prostate carcinogenesis and within the therapeutic armamentarium for patients with prostate cancer remains encouraging.
Keywords: prostate cancer, prostate-specific membrane antigen, folate, cancer therapeutics, tumor markers
Objectives
Prostate cancer is the most prevalent non-cutaneous malignancy in US men and remains the second leading cause of cancer death in this population.1 Recently, as a result of well-publicized large randomized controlled trials,2,3 the use of prostate-specific antigen (PSA) as a screening tool has come under fire. This has culminated in the publication of guidelines aimed at reducing or in some cases eliminating the use of PSA as a screening tool for prostate cancer.4,5 The necessary consequence of this reduction in screening is a decrease in the number of cancers detected. As a result, some have expressed concern that the abandonment of PSA screening may harbinger a return to prostate cancer presenting as symptomatic local or metastatic disease.6 It is clear, then, that innovative biomarkers for the detection and treatment of prostate cancer are sorely needed. Prostate-specific membrane was one of the first prostate cancer biomarkers successfully cloned and has been the subject of intense clinical inquiry as an imaging and therapeutic agent since 1993.7 Herein, we review lessons learned from 20-years of research on prostate-specific membrane antigen with a focus on the current clinical implications in prostate cancer imaging and therapeutics. Further, potential novel avenues of research taking advantage of PSMA’s biological function are proposed.
Materials and Methods
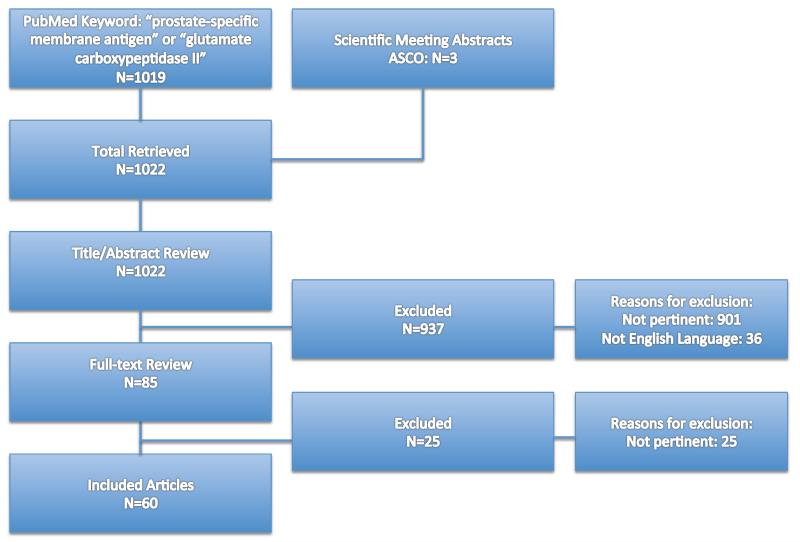
A PubMed® search using the keyword “prostate-specific membrane antigen” or “glutamate carboxypeptidase type II” yielded 1019 results. An additional 3 abstracts were included from scientific meetings. Articles were vetted by title and abstract with emphasis placed on those with clinically relevant findings. Articles not written in English were excluded. A total of 85 articles were selected based on abstract and the full-text was read in entirety. From this, 60 articles were selected for inclusion in the review (Figure 1).
Figure 1.
Article selection for review
Results
Discovery, structure, and physiology of PSMA
PSMA was first cloned in 1993.7 Since then, it has been shown to be identical to both folate hydrolase 1 found at the jejunal brush border and N-acetyl-alpha-linked-acidic-dipeptidase (NAALADase) in the nervous system.8 The multiple names have led some to argue for standardization of nomenclature based on function, (e.g. glutamate carboxypeptidase type II), however, this has not been widely accepted. Regardless of name, PSMA is a type II transmembrane protein with an N-terminal cytoplasmic tail, a helical transmembrane structure, and an extracellular C-terminus. The extracellular portion, existing as a dimer, makes up the majority of the protein and includes a binding motif featuring two zinc ions. This extracellular binding domain has been shown to bind glutamate and glutamate-like structures, hence its natural substrates (N-acetyl-apartylglutamate, and folyl-poly-γ-glutamates) both have C-terminal glutamates. Internalization mechanisms for PSMA have been characterized and are believed to be mediated by cytoplasmic N-terminal tail interactions with calveolin-1 and clathrin-coated pits.9
As aforementioned, PSMA has been called many different names based largely on its location of discovery. Four consensus sites of expression in normal physiology exist: prostate (secretory-acinar epithelium), kidney (proximal tubules), nervous system glia (astrocytes and schwann cells), and the small bowel (jejunal brush border).10,11 Despite accepted expression in these tissues, the function is only well defined in nervous system glia and small bowel.
At the jejunal brush border, PSMA is called folate hydrolase 1. Here, it is responsible for assisting in folate absorption for transportation to the rest of the body. Dietary folates exist in the form of folyl-poly-γ-glutamates, but only monoglutamated folates can be absorbed by jejunal enterocytes.12 PSMA is responsible for removing the C-terminal glutamates from dietary folates to allow for enteral absorption in humans and pigs. Interestingly, although rats do not express PSMA at the brush border, a similar folate hydrolase is released in murine pancreatic secretions allowing for enteral absorption of dietary folates.13
In the nervous system, PSMA has been termed NAALADase, which is responsible for hydrolyzing N-acetyl aspartylglutamate (NAAG). NAAG is the most abundant peptide neurotransmitter in the mammalian nervous system.14 It is released from presynaptic vesicles via a calcium dependent mechanism and binds as an agonist to the metabotropic glutamate receptor 3 (mGluR3) at the presynaptic neurons and astrocytes.15 At presynaptic neurons and astrocytes, activation of mGluR3 inhibits release of glutamate and induces secretion of transforming growth factor β, respectively.16 Both of these processes are viewed as neuroprotective. PSMA at the neuronal synapse is responsible for fracturing NAAG into N-acetylaspartate and glutamate, effectively ending the agonist effects of NAAG on mGluR3.17 Armed with this understanding, it is not difficult to imagine how PSMA activity may result in neuronal glutamate-mediated excitotoxicity. In fact, predicated on the concept of decreased excitotoxicity, inhibition of PSMA has been shown to improve disease course in preclinical models of multiple sclerosis (MS), traumatic brain injury, stroke, ALS, schizophrenia, and neuropathic pain.18-20
The reason for the presence of PSMA in prostate epithelium and renal proximal tubules is currently undefined, but may be related to folate metabolism in these tissues: potentially the reuptake of folate in the kidneys and release of monoglutamated folates into the seminal fluid.
PSMA and genitourinary oncology
In addition to prostate cancer (discussed later), the presence of PSMA has been confirmed in kidney and bladder cancer, well as the neovasculature of numerous non-genitourinary cancers.21 Baccala and colleagues performed a microarray analysis on several kidney cancer subtypes and discovered PSMA expression in the tumor neovasculature of clear cell and chromophobe renal cell carcinoma.22 Interestingly, there was no expression noted in papillary renal cell carcinoma, a tumor derived from proximal renal tubular cells where PSMA is normally expressed. This finding was confirmed by Al-Ahmadie and associates, lending further credence to the biologic diversity of these tumors.23 Currently there is one registered clinical trial looking at PSMA-based vaccine therapy in treating patients with kidney cancer.
In bladder cancer, PSMA expression has been confirmed both in the cytoplasm of malignant cells and their associated tumor neovasculature. Samplaski et al. used tissue microarrays to assess the expression of PSMA in urothelial, squamous, adeno-, and small cell bladder carcinomas.24 PSMA was present in 3%, 12%, an 18% of urothelial, adeno-, and small cell carcinomas, respectively. No expression was demonstrated for squamous cell carcinoma. All bladder cancers expressed PSMA in the tumor neovasculature. Currently, there are no clinical trials examining the use of PSMA therapeutics in bladder carcinoma.
PSMA and prostate cancer
PSMA was first discovered in the prostate with use of the IgG1 monoclonal antibody termed 7E11-C5.3 derived from the cell membrane of the LNCaP prostate cancer cell line.25 Subsequently, PSMA expression has been shown to increase from benign prostatic hyperplasia to high-grade prostatic intraepithelial neoplasia to prostatic adenocarcinoma.26 Further, as malignant tumors increase in grade, stronger PSMA expression is observed.27 In vitro, PSMA expression is correlated with increased cellular folate content, which confers a proliferative advantage to expressing cells.28,29 In fact, high PSMA expression has been independently associated with androgen independence and progression.30,31 Taken together, these data support potential clinical uses for PSMA in prostate cancer patients. Thus far, major clinical applications have been in the domains of imaging and therapeutics.
PSMA and prostate cancer imaging (Table 1)
Table 1.
PSMA clinical applications in prostate cancer imaging
| Author | PSMA Compound | Class | Imaging modality |
Patient population |
Outcome |
|---|---|---|---|---|---|
| Rosenthal et al. [40] |
In-111 capromab | Antibody | Nuclear | High risk, evaluation LN metastases |
PPV: 62% NPV 72% Sens 62% Spec 72% *better than CT/MRI |
| Rosenthal et al. [40] |
In-111 capromab | Antibody | Nuclear | Prostatic fossa recurrence |
PPV 50% NPV 70% Sens 49% Spec 71% |
| Pandit- Taskar et al. [46] |
In-111 J591 | Antibody | Nuclear v. conventional CT/bone scan |
Metastatic CRPC |
Localized to 93.7% skeletal lesions detected by CT/bone scan *better than CT |
| Barrett et al. [52] |
I-123-MP-1072 I-123-MIP-1095 |
Small molecule PSMA inhibitor |
Nuclear SPECT/CT |
Metastatic CaP |
Lesions visualized in soft tissue, bone, an prostate at 0.5- 1h post injection |
In: Indium, LN: Iymph nodes, PPV: positive predictive value, NPV: negative predictive value, Sens: sensitivity, Spec: specificity, CRPC: castration-resistant prostate cancer, I: Iodine, CaP: prostate cancer
Prostate cancer imaging utilizing PSMA can be divided into two categories: antibody and low molecular weight compounds (i.e. small molecules). The first monoclonal antibody used in prostate cancer immunoscintigraphy, 7E11/CYT-356, was labeled with Indium-111 (111In) and referred to as 111In-capromab or ProstaScint®.32 In fact, 111In-capromab is the only current FDA-approved PSMA-based imaging agent for use in the context of “newly diagnosed patients with biopsy-proven prostate cancer thought to be clinically localized…who are at high risk for pelvic lymph node metastases” and “…in post-prostatectomy patients with a rising PSA and a negative or equivocal standard metastatic evaluation in whom there is a high clinical suspicion of occult metastatic disease.”33 111In-capromab stages primary prostate cancer with 68% positive predictive value, 72% negative predictive value, 62% sensitivity, and 72% specificity.34 Recent efforts have focused on improving the accuracy of 111In-capromab by correlating with other imaging modalities (e.g. diffusion weighted MRI).35 However, most favor moving away from 111In-capromab-based imaging in favor of other imaging modalities.36
One of the major drawbacks of 111In-capromab is that it recognizes an intracellular epitope and therefore does not have the capability to bind live cells.37 Second generation antibodies have been developed in an effort to circumvent these shortcomings. J591 is one such antibody that, unlike its predecessor, is specific for the extracellular domain of PSMA and can bind living cells. J591 is conjugated to 1,4,7,10-tetraazacyclododecane-1,4,7,10-tratraacetic acid (DOTA) and subsequently radiolabeled.38 111In-labeled J591 has been tested against conventional imaging (CT and bone scan) in the detection of osseous metastases. In this setting, antibody imaging localized to 93.7% of skeletal lesions detected by conventional imaging and 13/18 bony lesions detected only with antibody imaging were later confirmed to be true metastases.39 Other antibodies against PSMA have also been developed and show some promise in preclinical prostate cancer models.40
The widely-cited downside to antibody–based imaging is the long time required for the antibody to clear from non-target tissues, thus necessitating several days between antibody injection and imaging.33 RNA aptamers and small molecule PSMA inhibitors comprise a relatively novel group of compounds, which bypass this problem. RNA aptamer conjugation to chelators capable of coordinating PET-radionuclides has recently been achieved, however, it has yet to be applied in vivo.41 Phosphate- and urea-based PSMA inhibitors have been used in preclinical imaging models. Lapi and colleagues demonstrated 18F-labeled phosphorimidate accurately detected PSMA positive tumors in a mouse xenograft model.42 Pomper’s group has done extensive work with radiolabeled urea-based PSMA inhibitors and showed good imaging efficacy in mouse xenograft models and in patients with metastatic prostate cancer.43,44 A recently completed phase I clinical trial of an Iodine-131 urea-based PSMA inhibitor has demonstrated ability to detect prostate cancer in prostate, soft tissue, and bone with good pharmacokinetic and pharmacodynamics profiles.45
Therapeutic clinical trials of PSMA for prostate cancer (Table 2)
Table 2.
PSMA clinical applications in prostate cancer therapeutics
| Author | Clinical trial | PSMA compound | Class | Patient population | Outcome |
|---|---|---|---|---|---|
| Deb et al. [55] | Yes, Phase I | Y-90 capromab | Antibody Radioisotope |
Metastatic CRPC N=12 |
|
| Kahn et al. [56] |
Yes, Phase II | Y-90 capromab | Antibody Radioisotope |
Metastatic CaP N=8 |
|
| Bander et al. [57] |
Yes, Phase I |
Lu-177 J591 | Antibody Radioisotope |
Metastatic CRPC N=35 |
|
| Tagawa et al. [58] |
Yes, Phase II |
Lu-177 J591 | Antibody Radioisotope |
Metastatic CRPC N=47 |
|
| Tagawa et al. [59] |
Yes, Phase 1 |
Lu-177 J591 | Antibody Radioisotope |
Metastatic CRPC N=27 |
|
| Galsky et al. [60] |
Yes, Phase 1 |
MLN2704 (maytansinoid-1, antibody-drug conjugate) |
Antibody-drug conjugate (ADC) |
Metastatic CRPC N=23 |
|
| Jeske et al. [63] |
Yes, Phase II |
J591 Interleukin-2 |
Antibody Immunotherapy |
Recurrent CaP Metastatic CRPC |
|
Y: Yttrium, CRPC: castration-resistant prostate cancer, MTD: maximum tolerated dose, CaP: prostate cancer, Lu: Lutetium
Recently, there has been a flurry of new FDA-approved therapeutics aimed at improving survival in patients with castration-resistant prostate cancer (CRPC). Despite this great progress, such therapies confer only 4-5 months overall survival compared to placebo.46 Clearly, new agents are needed to improve care in this patient population. PSMA is an attractive target because the expression in normal cells is 100-1000 fold less than in prostate carcinoma cells.47 Thus far, antibody-based radiotherapy, antibody-drug conjugates, PSMA-targeted prodrug therapy, and PSMA-based immunotherapy have been explored.
The first antibody-based radiotherapeutic was Yttrium-90 capromab. In a phase 1 dose-escalation trial, 12 patients received 90Y capromab.48 Myelosuppression was the major dose-limiting toxicity and the maximum tolerated dose (MTD) was 9 mCi/m2. No patient had an objective PSA or radiologic response. A phase II study was stopped prematurely due to significant toxicity and lack of efficacy.49
The premier PSMA antibody-based radiotherapeutic is Lutetium-177 J591 (177Lu-J591). In a phase 1 clinical trial of 35 patients, 177Lu-J591 had an MTD of 70 mCi/m2 and demonstrated acceptable toxicity with excellent targeting of known metastatic sites.50 In a recently reported phase II clinical trial, 47 patients with metastatic CRPC were treated with 177Lu-J591. 51 Fifteen patients received 65mCi/m2 and 17 received 70 mCi/m2. A second cohort received 70 miC/m2 to verify response rate and assess biomarkers. Almost 60% of patients demonstrated a decline in PSA levels with 10.6% experiencing a >50% PSA decline. Treatment was associated with reversible myelosuppression in all patients. The phase 1 MTD (70mCi/m2) resulted in longer survival (21.8 v 11.9 months, p<0.03), but more grade 4 hematologic toxicity and platelet transfusions. A second phase 1 trial utilizing 177Lu-J591 in patients with CRPC demonstrated that fractioned dosing allowed for higher cumulative doses with less toxicity.52 A third phase 1 clinical trial in patients with CRPC receiving docetaxel, prednisone, and escalating doses of 177Lu-J591 is currently accruing patients.
Antibody-drug conjugates (ADC) utilizing J591 have also been generated. MLN2704 is maytansinoid 1 (DM1, an antimicrotubule agent) conjugated to J591. Phase 1 studies in 23 patients demonstrated >50% PSA decline in 2 patients with grade 3 toxicities occurring in 3 patients.53 A multicenter phase I/II study in 62 men with metastatic CRPC tested four regimens. PSA declines were most frequently noted at 330mg/m2 (4/6 had PSA stabilization or decline >50%), however treatment was limited by toxicity.54 Thus, ADC therapy appears promising and new compounds are being designed to decrease associated toxicity.
Recently, a tumor endothelial cell prodrug for prostate cancer therapy has been engineered.55 Denmeade and colleagues coupled a PSMA-specific peptide to an inhibitor (thapsigargin, G202) of the sarcoplasmic/endoplasmic reticulum calcium adenosine triphosphate (SERCA) pump. SERCA is a critical intracellular protein whose function is required by all cell types for viability. The conjugate is inactive until the PSMA-specific peptide is cleaved, thereby initiating local SERCA inhibition. In preclinical xenograft models, G202 produced substantial prostate cancer tumor regression at doses that were minimally toxic to the host. As a result of preclinical success, an open label dose-escalation trial has been initiated (NCT01056029).
A final category of PSMA-based therapeutics currently in its infancy is immunotherapy or so-called “vaccine therapy.” Based on the theory that IL-2 stimulates NK cells and enhances antibody-dependent cellular cytotoxicity, Bander’s group performed a phase II trial in patients with recurrent prostate cancer.56 Nine of 16 patients had stable PSA with PSA declines up to 34%. Toxicity was mild. The authors concluded repetitive dosed mAb J591 is a viable strategy for use in combination with immune modulatory therapy in prostate cancer. Slovin and colleagues have gone a step further, generating a viral replicon system encoding PSMA (PSMA-VRP), which is currently under phase I evaluation in patients with CRPC.57 Of 12 who received PSMA-VRP, a PSMA-specific cellular response was noted in 3 patients. There did not appear to be a clinical benefit, however the authors noted the presence neutralizing antibodies suggesting suboptimal dosing. Investigation in the area of PSMA-based immunotherapeutics is ongoing.
A review of the most recent clinical trials database (clinicaltrials.gov) queried for “prostate-specific membrane antigen” and “prostate cancer” reveals 14 current clinical trials assessing the role of PSMA in prostate cancer. This marked interest lends credence to its potential as the prototypical cell-surface biomarker in prostate cancer.
Avenues for future study
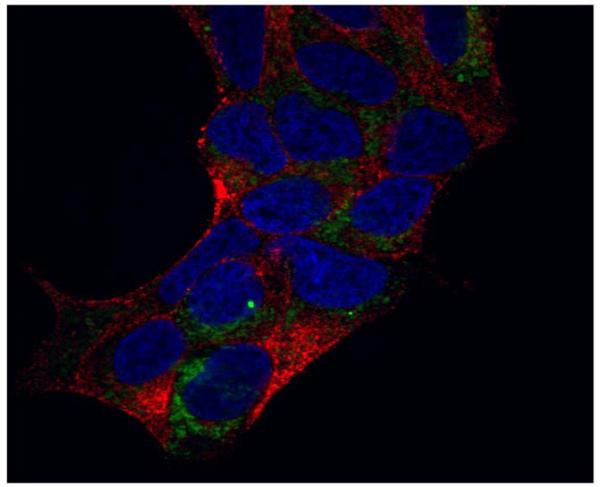
Future directions in PSMA-related prostate cancer tumor biology may be related to its intracellular association. In 1997, Troyer and colleagues demonstrated localization of PSMA at the inner face of the plasma membrane and in association with the mitochondria in the LNCaP cell line.58 Preliminary data from our lab confirm an intracellular association of PSMA through immunohistochemistry and confocal microscopy (Figure 2, data not yet published). As this biology is further delineated, new pathways for prostate cancer therapeutics may be accessed.
Figure 2.
Confocal microscopy after immunohistochemical staining for PSMA on LNCaP cells with GFP-marked mitochondria.
Green=mitochondria, Red=PSMA, Blue=nuclei
Another potential for future research involves the relatively new concept of prostate cancer axonogenesis and neurogenesis. As aforementioned, PSMA plays a significant role in the nervous system and is already being explored in a clinical context to treat numerous neurological disorders. Ayala and colleagues have demonstrated increased nerve density in cancerous areas of human prostate compared to preneoplastic controls.59 Moreover, patients with prostate cancer have increased numbers of neurons in prostatic ganglia compared to controls. In vitro models confirmed that prostate cancer cells interacting with nerves in perineural invasion induced neurite outgrowth. Glutamate receptors in the brain have been shown to play a role in neurogenesis.60 Thus, PSMA may catabolize NAAG to N-acetylaspartate and glutamate, resulting in higher levels of glutamate at the synapse, leading to prostate cancer neurogenesis, a subject of current inquiry in our lab.
Conclusions
PSMA remains an attractive target for the detection, diagnosis, and treatment of prostate cancer 20 years from the date of its cloning. Therapeutics based on it biological role in the nervous system where it functions as a NAALADase are promising in preclinical models. There has yet to be a clinically important impact on survival in as a result of PSMA-based therapeutics in prostate cancer. However, several recent new approaches to target PSMA including the use of small molecule inhibitors, improved radioactive antibodies/antibody-drug conjugates, PSMA-based immunotherapy, RNA aptamer conjugates, and PSMA-targeted prodrug therapy have reinvigorated research in the field. Future study in PSMA and prostate cancer might focus on its intracellular functions and its possible role in tumor neurogenesis. As our understanding of tumor biology advances and molecular techniques become more refined, PSMA-based therapeutics will likely play a role in the developing armamentarium of treatment for CRPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Amico AV, Whittington R, Malkowicz B, et al. Endorectal magnetic resonance imaging as a predictor of biochemical outcome after radical prostatectomy in men with clinically localized prostate cancer. The Journal of urology. 2000 Sep;164(3 Pt 1):759–763. doi: 10.1097/00005392-200009010-00032. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. The New England journal of medicine. 2009 Mar 26;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Miles BJ, Shalev M, Aguilar-Cordova E, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Human gene therapy. 2001 Nov 1;12(16):1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 4.Carter HB, Albertsen PC, Barry MJ, et al. Early Detection of Prostate Cancer: AUA Guideline. The Journal of urology. 2013 May 6; doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA, Force USPST Screening for prostate cancer: U. S. 2012 Preventive;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 6.Makovey I, Stephenson AJ, Haywood S. Response to the U.S. Preventative Services Task Force Decision on Prostate Cancer Screening. Current urology reports. 2013 Apr 9; doi: 10.1007/s11934-013-0318-9. [DOI] [PubMed] [Google Scholar]
- 7.Israeli RS, Powell CT, Fair WR, Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer research. 1993 Jan 15;53(2):227–230. [PubMed] [Google Scholar]
- 8.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proceedings of the National Academy of Sciences of the United States of America. 1996 Jan 23;93(2):749–753. doi: 10.1073/pnas.93.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barinka C, Rojas C, Slusher B, Pomper M. Glutamate carboxypeptidase II in diagnosis and treatment of neurologic disorders and prostate cancer. Current medicinal chemistry. 2012;19(6):856–870. doi: 10.2174/092986712799034888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mhawech-Fauceglia P, Zhang S, Terracciano L, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007 Mar;50(4):472–483. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- 11.Sacha P, Zamecnik J, Barinka C, et al. Expression of glutamate carboxypeptidase II in human brain. Neuroscience. 2007 Feb 23;144(4):1361–1372. doi: 10.1016/j.neuroscience.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert reviews in molecular medicine. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shafizadeh TB, Halsted CH. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. The Journal of nutrition. 2007 May;137(5):1149–1153. doi: 10.1093/jn/137.5.1149. [DOI] [PubMed] [Google Scholar]
- 14.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. Journal of neurochemistry. 2000 Aug;75(2):443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 15.Zollinger M, Amsler U, Do KQ, Streit P, Cuenod M. Release of N-acetylaspartylglutamate on depolarization of rat brain slices. Journal of neurochemistry. 1988 Dec;51(6):1919–1923. doi: 10.1111/j.1471-4159.1988.tb01178.x. [DOI] [PubMed] [Google Scholar]
- 16.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annual review of pharmacology and toxicology. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. The Journal of biological chemistry. 1987 Oct 25;262(30):14498–14506. [PubMed] [Google Scholar]
- 18.Feng JF, Gurkoff GG, Van KC, et al. NAAG peptidase inhibitor reduces cellular damage in a model of TBI with secondary hypoxia. Brain research. 2012 Aug 21;1469:144–152. doi: 10.1016/j.brainres.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas AG, Wozniak KM, Tsukamoto T, et al. Glutamate carboxypeptidase II (NAALADase) inhibition as a novel therapeutic strategy. Advances in experimental medicine and biology. 2006;576:327–337. doi: 10.1007/0-387-30172-0_24. discussion 361-323. [DOI] [PubMed] [Google Scholar]
- 20.Zuo D, Bzdega T, Olszewski RT, Moffett JR, Neale JH. Effects of N-acetylaspartylglutamate (NAAG) peptidase inhibition on release of glutamate and dopamine in prefrontal cortex and nucleus accumbens in phencyclidine model of schizophrenia. The Journal of biological chemistry. 2012 Jun 22;287(26):21773–21782. doi: 10.1074/jbc.M112.363226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Human pathology. 2009 Dec;40(12):1754–1761. doi: 10.1016/j.humpath.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Baccala A, Sercia L, Li J, Heston W, Zhou M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007 Aug;70(2):385–390. doi: 10.1016/j.urology.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Al-Ahmadie HA, Olgac S, Gregor PD, et al. Expression of prostate-specific membrane antigen in renal cortical tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008 Jun;21(6):727–732. doi: 10.1038/modpathol.2008.42. [DOI] [PubMed] [Google Scholar]
- 24.Samplaski MK, Heston W, Elson P, Magi-Galluzzi C, Hansel DE. Folate hydrolase (prostate-specific membrane [corrected] antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011 Nov;24(11):1521–1529. doi: 10.1038/modpathol.2011.112. [DOI] [PubMed] [Google Scholar]
- 25.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer research. 1987 Sep-Oct;7(5B):927–935. [PubMed] [Google Scholar]
- 26.Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998 Jun 1;82(11):2256–2261. doi: 10.1002/(sici)1097-0142(19980601)82:11<2256::aid-cncr22>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Wright GL, Jr., Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urologic oncology. 1995 Jan-Feb;1(1):18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 28.Yao V, Bacich DJ. Prostate specific membrane antigen (PSMA) expression gives prostate cancer cells a growth advantage in a physiologically relevant folate environment in vitro. The Prostate. 2006 Jun 1;66(8):867–875. doi: 10.1002/pros.20361. [DOI] [PubMed] [Google Scholar]
- 29.Yao V, Berkman CE, Choi JK, O’Keefe DS, Bacich DJ. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. The Prostate. 2010 Feb 15;70(3):305–316. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 30.Wright GL, Jr., Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996 Aug;48(2):326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 31.Perner S, Hofer MD, Kim R, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Human pathology. 2007 May;38(5):696–701. doi: 10.1016/j.humpath.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Murphy GP, Maguire RT, Rogers B, et al. Comparison of serum PSMA, PSA levels with results of Cytogen-356 ProstaScint scanning in prostatic cancer patients. The Prostate. 1997 Dec 1;33(4):281–285. doi: 10.1002/(sici)1097-0045(19971201)33:4<281::aid-pros9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Foss CA, Mease RC, Cho SY, Kim HJ, Pomper MG. GCPII imaging and cancer. Current medicinal chemistry. 2012;19(9):1346–1359. doi: 10.2174/092986712799462612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenthal SA, Haseman MK, Polascik TJ. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. Techniques in urology. 2001 Mar;7(1):27–37. [PubMed] [Google Scholar]
- 35.Hardie AD, Rieter WJ, Bradshaw ML, Gordon LL, Young MA, Keane TE. Improved performance of SPECT-CT In-111 capromab pendetide by correlation with diffusion-weighted magnetic resonance imaging for identifying metastatic pelvic lymphadenopathy in prostate cancer. World journal of urology. 2013 Apr 18; doi: 10.1007/s00345-013-1079-2. [DOI] [PubMed] [Google Scholar]
- 36.Akin O, Hricak H. Imaging of prostate cancer. Radiologic clinics of North America. 2007 Jan;45(1):207–222. doi: 10.1016/j.rcl.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Troyer JK, Feng Q, Beckett ML, Wright GL., Jr. Biochemical characterization and mapping of the 7E11-C5.3 epitope of the prostate-specific membrane antigen. Urologic oncology. 1995 Jan-Feb;1(1):29–37. doi: 10.1016/1078-1439(95)00004-2. [DOI] [PubMed] [Google Scholar]
- 38.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer research. 2000 Sep 15;60(18):5237–5243. [PubMed] [Google Scholar]
- 39.Pandit-Taskar N, O’Donoghue JA, Morris MJ, et al. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008 Jul;49(7):1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alt K, Wiehr S, Ehrlichmann W, et al. High-resolution animal PET imaging of prostate cancer xenografts with three different 64Cu-labeled antibodies against native cell-adherent PSMA. The Prostate. 2010 Sep 15;70(13):1413–1421. doi: 10.1002/pros.21176. [DOI] [PubMed] [Google Scholar]
- 41.Rockey WM, Huang L, Kloepping KC, Baumhover NJ, Giangrande PH, Schultz MK. Synthesis and radiolabeling of chelator-RNA aptamer bioconjugates with copper-64 for targeted molecular imaging. Bioorganic & medicinal chemistry. 2011 Jul 1;19(13):4080–4090. doi: 10.1016/j.bmc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lapi SE, Wahnishe H, Pham D, et al. Assessment of an 18F-labeled phosphoramidate peptidomimetic as a new prostate-specific membrane antigen-targeted imaging agent for prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009 Dec;50(12):2042–2048. doi: 10.2967/jnumed.109.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Pullambhatla M, Banerjee SR, et al. Synthesis and biological evaluation of low molecular weight fluorescent imaging agents for the prostate-specific membrane antigen. Bioconjugate chemistry. 2012 Dec 19;23(12):2377–2385. doi: 10.1021/bc3003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012 Dec;53(12):1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrett JA, Coleman RE, Goldsmith SJ, et al. First-in-Man Evaluation of Two High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2013 Jan 9; doi: 10.2967/jnumed.112.111203. [DOI] [PubMed] [Google Scholar]
- 46.Cookson MS, Roth BJ, Dahm P, et al. Castration-Resistant Prostate Cancer: AUA Guideline. The Journal of urology. 2013 May 9; doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: levels in tissues, seminal fluid and urine. The Prostate. 2000 May 1;43(2):150–157. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Deb N, Goris M, Trisler K, et al. Treatment of hormone-refractory prostate cancer with 90Y CYT-356 monoclonal antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996 Aug;2(8):1289–1297. [PubMed] [Google Scholar]
- 49.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer biotherapy & radiopharmaceuticals. 1999 Apr;14(2):99–111. doi: 10.1089/cbr.1999.14.99. [DOI] [PubMed] [Google Scholar]
- 50.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Jul 20;23(21):4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 51.Tagawa ST, Milowsky MI, Morris MJ, et al. Phase II study of lutetium-177 labeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 May 28; doi: 10.1158/1078-0432.CCR-13-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tagawa ST, Milowski MI, Morris M. Phase II trial of 177 Lutetium radiolabeled anti-prostate specific membrane antigen (PSMA) monoclonal antibody J591 (177Lu-J591) in patients (pts) with metastatic castrate resistant prostate cancer (metCRPC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;28(15, supplement) [Google Scholar]
- 53.Galsky MD, Eisenberger M, Moore-Cooper S, et al. Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 May 1;26(13):2147–2154. doi: 10.1200/JCO.2007.15.0532. [DOI] [PubMed] [Google Scholar]
- 54.Akhtar NH, Pail O, Saran A, Tyrell L, Tagawa ST. Prostate-specific membrane antigen-based therapeutics. Advances in urology. 2012;2012:973820. doi: 10.1155/2012/973820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denmeade SR, Mhaka AM, Rosen DM, et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Science translational medicine. 2012 Jun 27;4(140):140ra186. doi: 10.1126/scitranslmed.3003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SJ J, Milowski MI, CR S, KA S, Bander N, Nanus DM. Phase II trial of the anti-prostate specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 plus low-dose interleukin-2 (IL-2) in patients with recurrent prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(18S) Abstract 15558. [Google Scholar]
- 57.Slovin SF, Kehoe M, Durso R, et al. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine. 2013 Jan 30;31(6):943–949. doi: 10.1016/j.vaccine.2012.11.096. [DOI] [PubMed] [Google Scholar]
- 58.Troyer JK, Beckett ML, Wright GL., Jr. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. The Prostate. 1997 Mar 1;30(4):232–242. doi: 10.1002/(sici)1097-0045(19970301)30:4<232::aid-pros2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 59.Ayala GE, Dai H, Powell M, et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008 Dec 1;14(23):7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- 60.Xiao XL, Ma DL, Wu J, Tang FR. Metabotropic glutamate receptor 5 (mGluR5) regulates proliferation and differentiation of neuronal progenitors in the developmental hippocampus. Brain research. 2013 Feb 1;1493:1–12. doi: 10.1016/j.brainres.2012.11.015. [DOI] [PubMed] [Google Scholar]