Abstract
Introduction/Purpose
While AF is a disease of the elderly, it can occur earlier in the presence of risk factors such as obesity. Bariatric surgery patients are significantly younger and more obese than previously described populations with AF. Therefore, it remains to be determined whether current estimates of the prevalence and predictors for AF remain true in the bariatric surgery population.
Materials and Methods
We performed a cross-sectional analysis of 1341 consecutive patients who underwent bariatric surgery from 1/2008 to 10/2012. Baseline characteristics were compared between patients with and without AF. For additional comparison, 176 patients with AF and body mass index (BMI) >40 kg/m2 were identified from the Vanderbilt AF Registry. A multivariable logistic regression was performed to identify predictors of AF within the bariatric surgery cohort.
Results
The prevalence of AF in the bariatric surgery cohort was 1.9% (25/1341). Patients with AF were older (median 56 years (Interquartile range [52-64) vs.46 [38-56] years, p<0.001), were more often male (48% vs. 23%, p=0.004), had more comorbidities, but had no difference in BMI (50 kg/m2 [44-58] vs. 48 [43-54], p=0.4). In multivariable analysis, the odds of AF increased 2.2-fold by age per decade (95% CI: 1.4-3.5, p<0.001) and 2.4-fold by male gender (1.1-5.4, p=0.03) when adjusted for BMI. BMI was not independently associated with AF (OR 1.15 [95% CI: 0.98-1.41], p=0.09).
Conclusions
The prevalence of AF is 1.9% among patients undergoing bariatric surgery. Risk of AF was found to increase with age and male gender, but not with higher BMI.
Keywords: Atrial fibrillation, obesity, bariatric surgery, gastric bypass
INTRODUCTION/PURPOSE
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with increased risk of death, dementia, heart failure, and stroke. The risk of AF increases with age and the presence of acquired risk factors such as obesity (1-5). Epidemiologic studies have demonstrated an increased risk for incident AF in obese patients with body mass index (BMI) ≥30 kg/m2 (1); however, patients referred for bariatric surgery are more obese, and significantly younger than the previously described populations. Therefore, it remains to be determined if current estimates of the prevalence and risk factors for AF hold true in the bariatric surgery population. Given the public health interest to develop novel strategies for primary and secondary prevention of AF, surgical weight loss may be an unexplored therapy for obese patients with AF that warrants further evaluation. Accordingly, given the mounting interest in evaluating weight loss therapy for AF, the aims of this study were to define the prevalence and identify the clinical predictors for AF among a large cohort of patients undergoing bariatric surgery.
MATERIALS AND METHODS
We performed a cross-sectional analysis of 1341 consecutive patients who underwent de-novo bariatric surgery at Vanderbilt University Medical Center (VUMC) from January 2008 to October 2012. Given a major goal of the study was to establish the prevalence of AF; all patients were included in the analysis. All types of bariatric surgery were eligible: Roux-en-Y-gastric bypass (RYGB), gastric banding, and sleeve gastrectomy. The study protocol was approved by the Vanderbilt University Institutional Review Board.
To compare the characteristics of bariatric surgery patients with AF to a larger group of morbidly obese patients with AF, 176 patients with BMI ≥40 kg/m2 and no history of bariatric surgery were identified from the Vanderbilt AF Registry. The Vanderbilt AF Registry is a prospective clinical database and DNA bio-repository established in 2001(6).
Electronic medical records were manually reviewed for all patients (MBS, SG, DP) and data was entered into a central database (Research Electronic Data Capture [REDCap]) (7). In addition, to ensure a diagnosis of AF was not missed, natural language tools available within the VUMC electronic medical record were used to search the terms: “atrial fibrillation”, “afib”, and/or “AF” within any note of the bariatric surgery patients. Clinical notes mentioning AF-related terms were read to confirm the diagnosis was made. In addition, ECGs were standard prior to bariatric surgery, and reviewed for evidence of AF (MBS, SG). A goal of this study was to develop a model to predict AF from standard clinical variables. Only patients diagnosed with AF prior to bariatric surgery were included as AF cases for multivariable modeling (see Statistical Analysis below).
Demographics and patient characteristics were recorded. BMI was calculated as the weight in kilograms divided by the height in meters squared. Documentation of the comorbid conditions hypertension, type II diabetes, coronary artery disease, congestive heart failure, and obstructive sleep apnea were recorded. Renal function was expressed as the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) formula and the creatinine measurement from pre-operative evaluation. For patients with available echocardiogram data, left ventricular ejection fraction (LVEF), interventricular and left ventricular (LV) posterior wall thickness (millimeters [mm]), and left atrial (LA) diameter (mm) were recorded. Type of AF was recorded as paroxysmal if episodes terminated spontaneously within 7-days, or non-paroxysmal if episodes persisted ≥ 7 days and/or required electrical or pharmacologic cardioversion. A commonly used clinical thromboembolic risk prediction score for patients with AF, the CHADS-2 score, was recorded as 0, 1, or ≥2. Pharmacologic therapies with antiarrhythmic medications were recorded as Vaughn-Williams Class I, Class III (excluding amiodarone), amiodarone, or none. Amiodarone was considered separately given the overlap in mechanism between Vaughn-Williams Class I and III, and its unique adverse side-effect profile. Rate control therapy was recorded as use of beta-blockers, non-dihydropyridine (DHP) calcium channel blockers, digoxin, or none. Thromboembolic prophylaxis was recorded as warfarin, dabigitran, other anticoagulants, aspirin only, or none. AF ablation was defined as any catheter-based or surgical pulmonary vein isolation procedure, or surgical MAZE.
For the non-bariatric surgery AF cohort, demographics and baseline characteristics recorded at the time of enrollment in the Vanderbilt AF Registry were used for analysis in this study. Details of data collection have been previously described(6). Age at time of AF diagnosis was provided by patient report.
Categorical variables were expressed as frequency (percentage), and continuous variables were expressed as the median with interquartile range (IQR). Pearson’s Chi-square or Fisher’s exact tests were used to compare categorical variables, and Mann-Whitney U or Kruskal-Wallis tests for continuous variables. For comparisons of >2 groups, pair-wise comparisons were performed if the global test for significance was achieved using Pearson’s Chi-square or Kruskal-Wallis tests (P≤0.05). A multivariable logistic regression analysis was performed to determine the independent contribution of age (continuous), male gender (yes/no), and BMI (continuous) to predict AF (yes/no) among patients who underwent bariatric surgery. Multiplicative interaction terms were tested between age, gender, and BMI. To avoid over-fitting the statistical model, an 8:1 ratio of events per degree of freedom was used. The covariates age, gender, and BMI were pre-specified for inclusion in the model based on previously described association with AF (1,2). No records contained missing data. Overall, the multivariable model cost a total of 3 degrees of freedom. A pre-specified secondary analysis was performed using linear regression to examine the effect of age and BMI on LA size. Two-sided p-values <0.05 were considered statistically significant. Statistical analysis was performed in SPSS version 21 (IBM Corp., Armonk, NY).
RESULTS
The bariatric surgery cohort was comprised of 1341 patients with median age of 47 years (IQR 38-56), 24% male (N=318), and median BMI of 47 kg/m2 (43-54). Twenty-five patients were found to have AF prior to bariatric surgery for an overall prevalence of 1.9%. Seven additional patients without a diagnosis of AF prior to surgery were observed to develop peri-operative AF (< 3 months following bariatric surgery). Baseline characteristics of the bariatric surgery cohort stratified by AF status are presented in Table 1. Data was complete for all patients presented in Table 1. Patients with AF were significantly older (median age 56 years [IQR 52-64] vs. 46 [38-56], p<0.001), more often male (48% vs. 23%, p=0.004), and had a higher rate of obesity-related comorbid conditions including type II diabetes (60% vs. 38%, p=0.02), hypertension (96% vs. 67%, p=0.002), coronary artery disease (24% vs. 7%, p=0.002), congestive heart failure (32% vs. 3%, p<0.001), and obstructive sleep apnea (96% vs. 60%, p<0.001).
Table 1. Atrial Fibrillation in Patients Undergoing Bariatric Surgery (N=1341).
| Characteristics | Bariatric Surgery without AF N=1316 |
Bariatric Surgery with AF N=25 |
p-Value |
|---|---|---|---|
| Age | 46(38,56) | 56(52,64) | <0.001 |
| Gender-Male | 306(23%) | 12(48%) | 0.004 |
| Race-White | 1062(81%) | 16(64%) | 0.04 |
| Body Mass Index (kg/m2) | 48(43,54) | 50(44,58) | 0.4 |
| Type of Surgery | 0.3 | ||
| Roux-en-Y Gastric Bypass | 858(65%) | 17(68%) | |
| Sleeve Gastrectomy | 208(16%) | 6(24%) | |
| Gastric Banding | 250(19%) | 2(8%) | |
| Diabetes Mellitus | 494(38%) | 15(60%) | 0.02 |
| Hypertension | 878(67%) | 24(96%) | 0.002 |
| Coronary Artery Disease | 98(7%) | 6(24%) | 0.002 |
| Congestive Heart Failure | 42(3%) | 8(32%) | <0.001 |
| Obstructive Sleep Apnea | 795(60%) | 24(96%) | <0.001 |
| Renal Dysfunction (GFR<60) | 132(10%) | 4(16%) | 0.3 |
GFR=glomerular filtration rate
Given our limited sample size of bariatric surgery patients with AF, we sought to explore the generalizability of our findings to a larger non-surgical group of morbidly obese patients with AF (Table 2). The bariatric surgery cohort had a higher BMI (50 kg/m2 [IQR 44- 58] vs. 44 [42-48], p=0.002), but there was no detectable difference in age (56 years [IQR 52-64] vs. 58 [51-64], P=0.5), or proportion male (48% vs. 56%, p=0.4). Of note, among morbidly obese patients with AF, the median age of first diagnosis was 54 years of age (IQR 47-61). The prevalence between the bariatric surgery group and the non-bariatric surgery group in regard to diabetes (60% vs. 33%, p=0.009), hypertension (96% vs. 79%, p=0.04), and OSA (96% vs. 56%, p<0.001) were all significantly different. Differences between other comorbid conditions did not reach statistical significance. There was also a significant difference between racial characteristics in patients undergoing bariatric surgery compared to those who did not (64% white vs. 86%, p<0.007).
Table 2. Baseline Characteristics in Obese Patients with Atrial Fibrillation.
| Characteristics | Valid Cases |
Bariatric Surgery N=25 |
Non-Bariatric Surgery N=176 |
p-Value |
|---|---|---|---|---|
| Age | 25/175 | 56(52,64) | 58(51,64) | 0.5 |
| Age at First Diagnosis of AF | NA/172 | Not Reported |
54(47-61) | N/A |
| Gender-Male | 25/176 | 12(48%) | 99(56%) | 0.4 |
| Race-White | 25/176 | 16(64%) | 151(86%) | 0.007 |
| Body Mass Index (kg/m2) | 25/176 | 50(44,58) | 44(42,48) | 0.002 |
| Diabetes Mellitus | 25/175 | 15(60%) | 58(33%) | 0.009 |
| Hypertension | 25/175 | 24(96%) | 138(79%) | 0.04 |
| Coronary Artery Disease | 25/173 | 6(24%) | 38(22%) | 0.8 |
| Congestive Heart Failure | 25/173 | 8(32%) | 46(27%) | 0.6 |
| Obstructive Sleep Apnea | 25/169 | 24(96%) | 95(56%) | <0.001 |
| Renal Dysfunction (GFR<60) | 25/169 | 4(16%) | 52(31%) | 0.1 |
Echocardiographic parameters for the subset of patients who underwent echocardiogram are presented in Table 3. LA enlargement was present in the Bariatric Surgery with AF and Non-Surgical Obese AF Cohorts and significantly larger than the Bariatric Surgery without AF Cohort (45 mm [IQR 41-53] vs. 47 [41-50], vs. 40 [35-43], p<0.05).
Table 3. Echocardiographic Parameters of Obese Patients with AF.
| Parameter | Valid Cases |
Bariatric Surgery without AF |
Bariatric Surgery with AF |
Non-surgical Patients with AF |
p-Value |
|---|---|---|---|---|---|
| LV Ejection Fraction |
326/19/151 | 57 (55,63) | 55 (48,60) | 55(50,60) | <0.05† |
| Interventricular Septal thickness (mm) |
294/18/130 | 11(10,13) | 12(11,13) | 12(11,13) | <0.05† |
| LV Posterior Wall thickness (mm) |
304/17/133 | 11(10,12) | 11(10,13) | 12(10,13) | <0.05† |
| LA Diameter (mm) |
306/19/134 | 40(35,43) | 45(41,53) | 47(41,50) | <0.05*† |
Global test for signifcance using Kruskal-Wallis was p<0.05. Pair-wise comparisons used Mann-Whitney U test.
Comparison of Bariatric Surgery without AF vs. Bariatric Surgery with AF.
Comparison of Bariatric Surgery without AF vs. Non-surgical Patients with AF. NS=non-significant (p>0.05)
The current management used for AF in obese patients is presented in Table 4. Within the Bariatric Surgery with AF Cohort, 25% of patients have undergone direct current cardioversion, 70% are at elevated risk of stroke (CHADS-2 ≥2), and 72% are on chronic anticoagulation. Among the Non-surgical Obese AF Cohort, 28% have undergone AF ablation, and 13% are on chronic amiodarone therapy.
Table 4. Atrial Fibrillation Characteristics in Obese Patients.
| Characteristics | Valid Cases |
Bariatric Surgery |
Non- Bariatric Surgery |
|---|---|---|---|
| Paroxysmal Atrial Fibrillation | 24/134 | 10(42%) | 41(31%) |
| History of Direct Current Cardioversion | 25/128 | 7(28%) | 67(52%) |
| CHADS-2 Score | 23/176 | ||
| 0 | 0(0%) | 49(28%) | |
| 1 | 7(30%) | 60(34%) | |
| ≥2 | 16(70%) | 67(38%) | |
| History of Stroke or Transient Ischemic Attack | 25/149 | 1(4%) | 13(9%) |
| Stroke Prophylaxis | 25/176 | ||
| Warfarin | 15(60%) | 113(64%) | |
| Dabigatran | 1(4%) | 6(3%) | |
| Other Anticoagulant | 2(8%) | 0(0%) | |
| Aspirin Only | 2(8%) | 36(21%) | |
| None | 5(20%) | 21(12%) | |
| Antiarrhythmic Drug Use | 25/134 | ||
| Class I | 2(8%) | 14(10%) | |
| Class III (excluding amiodarone) | 6(24%) | 26(19%) | |
| Amiodarone | 0(0%) | 18(13%) | |
| None | 17(68%) | 76(57%) | |
| History of Atrial Fibrillation Ablation | 25/134 | 1(4%) | 37(28%) |
| Rate Control* | 25/176 | ||
| Beta-blocker | 15(60%) | 74(42%) | |
| Non-DHP Calcium Channel Blocker | 4(16%) | 35(20%) | |
| Digoxin | 4(16%) | 33(19%) | |
| None | 7(28%) | 0(0%) |
DHP=dihydropyridine; NA=not applicable;
> 1 rate control medication may be used per patient.
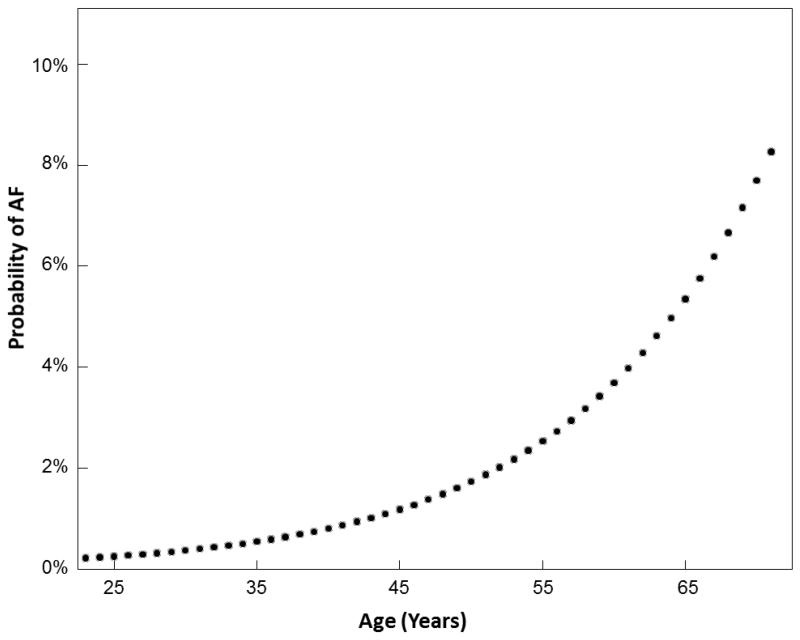
In the bariatric surgery cohort, the odds of AF increased 2.2 fold by age per decade (95% CI: 1.4-3.5, p<0.001) and 2.4-fold by male gender (1.1-5.4, p=0.03) when adjusted for BMI (Table 5). Among patients undergoing bariatric surgery, BMI was not a statistically significant predictor of AF (OR 1.15 [95% CI: 0.98-1.41], p=0.09). An interaction between age and gender was tested and non-significant (p=0.5). . A logistic regression model was fit using our data from patients undergoing bariatric surgery to predict the primary outcome of AF (yes or no) as a function of age in years (continuous variable). Using this model the probability of AF was plotted as a function of age. Based on this plot, the probability of AF in a patient undergoing bariatric surgery ranges from approximately 2% in a patient 50 years of age to 8% in a patient 65 years of age. In our secondary analysis using multivariable linear regression, age was found to increase LA size by 1.3 mm per decade (β=1.3±0.3; P<0.001), and BMI was found to increase LA size by 0.7 mm per 5 kg/m2 (β=0.7±0.2, p<0.001).
Table 5. Multivariable Model of AF in Patients Undergoing Bariatric Surgery (N=1341).
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| Age (per 10 years) | 2.2(1.4-3.5) | <0.001 |
| Gender-Male | 2.4(1.1-5.4) | 0.03 |
| BMI (per 5 kg/m2) | 1.15(0.98-1.41) | 0.09 |
DISCUSSION
The overall prevalence of AF in patients who underwent bariatric surgery was 1.9% (25/1341). Consistent with prior studies, age and male gender were significant predictors of AF, but BMI was not (2-5). These finding suggest that among patients undergoing bariatric surgery, which represent the extreme end of the obesity spectrum, the probability of AF is more strongly associated with advancing age rather than further elevation of BMI. Our findings indicate that the probability of AF among patients undergoing bariatric surgery is higher than in the general population, and can be best predicted by patient age.
Data from 1.89 million health maintenance organization participants estimated the prevalence of AF to be <0.1% in patients <55 years of age, and an overall prevalence of 0.95% (8). Given the relatively young age of bariatric surgery cohorts, including ours (median age 47 years, IQR 38-56), the prevalence of AF would be predicted to be low (9,10). Nonetheless, our observed prevalence was 1.9% overall, and exponentially increased with age to over 10% in patients 70 years of age (Figure 1). This demonstrates the substantial contribution of obesity and obesity-related comorbidities towards the multifactorial development of AF.
FIGURE 1. Probability of AF According to Age in Patients Undergoing Bariatric Surgery.
Previous data from the Framingham Heart Study Cohort established obesity as a significant risk factor for incident AF independent of age and other clinical predictors, and demonstrated an independent association between BMI and LA size. When adjusted for age and gender, the association between BMI and AF did not reach statistical significance in our study; however, subgroup analysis in patients with available echocardiogram data demonstrated an independent association between age and BMI on LA size. Given LA size is considered an intermediate phenotype for AF; this suggests an association between BMI and AF may exist among bariatric surgery patients that our study was underpowered to detect.
Health care costs directly associated with the management of AF exceed $6.5 billion dollars per year in the U.S. (11). Contributing to this problem is the poor efficacy of existing therapies and untoward side-effects of antiarrhythmic drugs and anticoagulant use. Our study demonstrates that AF is a clinically significant problem in morbidly obese patients with most patients (68%) having persistent AF, over 50% needing direct current cardioversion, 9% have suffered a stroke or transient ischemic attack, and 13% requiring chronic amiodarone therapy. Furthermore, 27% of patients underwent AF ablation despite reduced efficacy in obese patients and an increased risk of procedural complications(12-14).
While the link between obesity and AF is incompletely understood, it is recognized that increased adiposity promotes a pro-fibrillatory substrate through mechanisms including left atrial (LA) enlargement, LA pressure elevation, shortening of the LA and pulmonary vein effective refractory periods, left ventricular (LV) diastolic dysfunction, and through the pathophysiological changes accompanying type II diabetes mellitus and obstructive sleep apnea (15-19). Bariatric surgery is an effective therapy for weight loss, and has been found to reverse the structural cardiac changes associated with AF such as LA dilation, as well as cure type II diabetes and obstructive sleep apnea (9,20,21). Given that current therapies for AF are less effective in obese patients (12,22); bariatric surgery may represent a novel therapeutic option that warrants further evaluation.
This study has several limitations. Due to the frequency of asymptomatic or minimally symptomatic episodes of AF, cases of AF may have gone undiagnosed causing our results to underestimate the true prevalence of AF among bariatric surgery patients. AF is not considered a contraindication for bariatric surgery; therefore patients with AF were not selectively excluded from bariatric surgery during the pre-operative evaluation. The number of AF cases among our bariatric surgery cohort was small and limited the number of clinical predictors we could explore in multivariable analysis., our results represent a single-center experience which might limit the generalizability of our findings to other bariatric surgery populations. Future studies collecting a larger, multicenter cohort of bariatric surgery patients could better define the prevalence and predictors of AF in this population.
CONCLUSION
Despite the relatively young age of bariatric surgery patients, AF is common with an overall prevalence of 1.9%. Among bariatric surgery patients, which represent the extreme end of the obesity spectrum, age and male-gender are better predictors of development of AF compared to further increase in BMI.
Acknowledgments
Financial Support: American Heart Association Established Investigator (0940116N) and Clinical Research Program (11CRP7420009) Awards, and National Institutes of Health grants U19 HL65962, HL092217 and UL1 RR024975. This project was also supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Relationships with Industry: None
Footnotes
CONFLICT OF INTEREST DISCLOSURE STATEMENT
MBS: None
SG: None
DCP: None
RAT: None
ESG: None
DBW: None
RHC: None
DD: None
REFERENCES
- 1.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA : the journal of the American Medical Association. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA : the journal of the American Medical Association. 1994;271:840–4. [PubMed] [Google Scholar]
- 3.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. The American journal of medicine. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 4.Ruigomez A, Johansson S, Wallander MA, Rodriguez LA. Incidence of chronic atrial fibrillation in general practice and its treatment pattern. Journal of clinical epidemiology. 2002;55:358–63. doi: 10.1016/s0895-4356(01)00478-4. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. Journal of internal medicine. 2001;250:382–9. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 6.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart rhythm : the official journal of the Heart Rhythm Society. 2007;4:743–9. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA : the journal of the American Medical Association. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. The New England journal of medicine. 2012;367:695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 11.Wolowacz SE, Samuel M, Brennan VK, Jasso-Mosqueda JG, Van Gelder IC. The cost of illness of atrial fibrillation: a systematic review of the recent literature. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2011;13:1375–85. doi: 10.1093/europace/eur194. [DOI] [PubMed] [Google Scholar]
- 12.Chilukuri K, Dalal D, Gadrey S, et al. A prospective study evaluating the role of obesity and obstructive sleep apnea for outcomes after catheter ablation of atrial fibrillation. Journal of cardiovascular electrophysiology. 2010;21:521–5. doi: 10.1111/j.1540-8167.2009.01653.x. [DOI] [PubMed] [Google Scholar]
- 13.Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. American heart journal. 2008;155:310–5. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Shoemaker MB, Muhammad R, Farrell M, et al. Relation of morbid obesity and female gender to risk of procedural complications in patients undergoing atrial fibrillation ablation. The American journal of cardiology. 2013;111:368–73. doi: 10.1016/j.amjcard.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munger TM, Dong YX, Masaki M, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. 2012;60:851–60. doi: 10.1016/j.jacc.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki YK, Shi Y, Benito B, et al. Determinants of atrial fibrillation in an animal model of obesity and acute obstructive sleep apnea. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:1409–16 e1. doi: 10.1016/j.hrthm.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Linz D, Schotten U, Neuberger HR, Bohm M, Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8:1436–43. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 18.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54:1797–804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 20.Gaborit B, Jacquier A, Kober F, et al. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol. 2012;60:1381–9. doi: 10.1016/j.jacc.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. The New England journal of medicine. 2004;351:2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 22.Jongnarangsin K, Chugh A, Good E, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. Journal of cardiovascular electrophysiology. 2008;19:668–72. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]