Abstract
Objective
To examine the role of physical activity in menopausal hot flashes. Competing models conceptualize physical activity as a risk or protective factor for hot flashes. Few studies have examined this relationship prospectively using physiologic measures of hot flashes and physical activity.
Design
Over two 48 hour-periods, 51 participants wore a physiologic hot flash monitor and activity monitor, and reported their hot flashes in an electronic diary. Physiologic hot flashes, reported hot flashes and reported hot flashes without physiological corroboration were related to activity changes using hierarchical generalized linear modeling, adjusting for potential confounders.
Setting
Community.
Patients
Midlife women.
Interventions
None.
Main Outcome Measures
Physiologically-detected hot flashes and reported hot flashes with and without physiologic corroboration.
Results
Hot flash reports without physiologic corroboration were more likely after activity increases (OR 1.04, 95% CI: 1.00-1.10, p=.01), particularly among women with higher levels of depressive symptoms (interaction p=.02). No other types of hot flashes were related to physical activity.
Conclusion
Acute increases in physical activity were associated with increased reporting of hot flashes lacking physiologic corroboration, particularly among women with depressive symptoms. Clinicians should consider the role of symptom perception and reporting in relations between physical activity and hot flashes.
Keywords: Menopause, hot flashes, physical activity
Introduction
An estimated 70 to 80% of women report hot flashes during the menopausal transition (1). Despite the common occurrence of these symptoms, the physiology of hot flashes is only partially understood. The role of physical activity as an acute trigger of hot flashes has been anecdotally reported, but there has been little rigorous investigation of this relationship. The current literature is mixed, with findings alternately suggesting positive, negative, and null relationships between physical activity and hot flashes (2). Differences in results may stem in part from varied assessments of both physical activity and hot flashes.
Most studies of physical activity and hot flashes have examined self-reported hot flashes in relation to habitual physical activity, with the hypothesis that higher levels of habitual physical activity and cardiorespiratory fitness may be protective against hot flashes (3). While some studies have found that women who report being physically active (4,5) or increase their habitual physical activity levels through exercise interventions (6,7), are less likely to report severe hot flashes than their more sedentary counterparts, most have found no association between reported physical activity levels (8–14) or increasing habitual physical activity over time (15–18) and self-reported hot flashes. Further research using objective measures of activity and fitness is needed to clarify the potentially protective role of habitual physical activity and fitness against hot flashes.
While habitual physical activity is posited to be protective, acute bouts of physical activity are anecdotally reported as a trigger of hot flashes. This hypothesized relationship aligns with theories of hot flash physiology that frame hot flashes as an acute heat dissipation event occurring in the context of a narrowed thermoneutral zone (19). According to this model, acute physical activity would trigger hot flashes by increasing core body temperature, leading to the heat dissipation event of a hot flash. However, few studies have rigorously examined the acute effects of physical activity on hot flash occurrence. Freedman (19) found that hot flashes physiologically-detected by a skin conductance monitor were more likely to occur following a laboratory-based exercise protocol, and Thurston et al. (20) found an increased likelihood of physiologically-detected hot flashes following diary-reported physical exertion. In contrast, other studies found that increases in aerobic exercise were associated with a decrease in reported hot flashes over the subsequent minutes or day (3,8). Some of this work suggests that relationships between hot flashes and physical activity differ by method of hot flash assessment, and that weight status and fitness level may modify these associations (3). Thus, the role of acute physical activity as a trigger for hot flashes remains to be clarified, and may vary by factors such as participant characteristics and the method of hot flash assessment utilized.
Differences in methods of hot flash assessment contribute to the challenge of interpreting this limited literature. Most studies of hot flashes utilize self-report measures, which may be subject to error, retrospective reporting bias, and the influence of current affective states on symptom perception and reporting (21). Methods of physiologically detecting hot flashes have rarely been used in the literature examining physical activity and hot flashes, though it is known that correlates of these and self-reported hot flashes differ (20). Associations between acute physical activity and self-reported hot flashes may be more indicative of a role of physical activity in symptom perception and interpretation. Alternatively, associations between acute physical activity and physiologically-detected hot flashes may point to a physiological mechanism linking physical activity and hot flashes, such as increased core body temperature coupled with the hypothalamic thermoregulatory dysregulation.
In the current study, we sought to examine acute changes in daily physical activity as a trigger of self-reported and physiologically-detected hot flashes. We expected to find that increases in physical activity would be more likely prior to hot flashes compared to control times. Differences in this relationship by type of hot flash (e.g., self-reported, physiologic) were also explored. Given the important relationships between negative affect and both hot flash reporting (21) and physical activity (22), we also tested whether associations between acute physical activity and hot flashes would vary by baseline levels of negative affect and habitual physical activity.
Materials and Methods
Participants
The study sample was a subcohort of participants (N = 52) of the Pittsburgh site of the Study of Women's Health across the Nation (SWAN). SWAN is a cohort study designed to characterize the menopausal transition, conducted at seven sites across the United States. Details of SWAN procedures have been reported previously (23). At enrollment (1996-1997), SWAN participants (N = 3,302) were aged 42 to 52 years, had an intact uterus and at least one ovary, were not pregnant or breast feeding, had menstruated within 3 months, and were not using oral contraceptives or hormone therapy.
A subcohort of participants at the Pittsburgh SWAN site participated in SWAN FLASHES, an ancillary study using physiologic measures of hot flashes. SWAN FLASHES assessments occurred from 2008 to 2009, most closely corresponding to SWAN's 10th annual visit. By design, the Pittsburgh site recruited only Caucasian and African-American women. SWAN FLASHES inclusion criteria included reporting any hot flashes or night sweats in the past 2 weeks, having a uterus and both ovaries, not being pregnant, not using hormone therapy or selective serotonin reuptake inhibitors/serotonin norepinephrine uptake inhibitors for 3 months, and not currently undergoing chemotherapy. SWAN FLASHES enrolled 52 women. One woman was excluded from analysis because of missing sternal skin conductance data, for a final sample of 51 women (25 African American, 26 Caucasian women). Procedures were approved by the University of Pittsburgh Institutional Review Board. Participants provided written informed consent.
Design and procedures
Participants in SWAN FLASHES wore physiological hot flash monitors and carried an electronic hot flash diary. The monitoring period totaled approximately 96 hours, conducted in the context of two separate 48-hour ambulatory monitoring sessions within approximately 4 weeks. Participants were instructed to continue their regular routines, but to refrain from vigorous exercise while wearing the monitoring equipment. They also completed questionnaires and provided demographic and anthropometric data during a laboratory visit.
Hot Flashes
Both physiologically-detected and self-reported hot flashes were assessed. Physiologic hot flashes were detected with Biolog sternal skin conductance monitors (model 3991/2-SCL; UFI, Morro Bay, CA). The Biolog measures sternal skin conductance sampled at 1 Hz from the sternum via a 0.5-volt constant voltage circuit passed between two Ag/AgCl electrodes (UFI) filled with 0.05 M KCL Velvachol/glycol paste. Physiologic hot flashes were classified via standard methods, with skin conductance rise of 2 μmho in 30 seconds flagged automatically by UFI software (DPSv3.6) and edited for artifact. Given that some women show submaximal hot flashes failing to reach the 2 μmho criterion, all potential hot flash events were also visually inspected. Events showing the characteristic hot flash pattern but less than 2μmho/30 sec rise were coded as hot flashes. This coding has been shown to be reliable (24,25). A 20-minute hot flash lockout period was implemented after the start of the flash. Physiologically-detected hot flashes were considered reported if met by a self-report of a hot flash within 5 minutes. Participants reported hot flashes as they occurred during waking hours by completing a portable electronic diary (Palm Z22; Palm, Inc., Sunnyvale, CA) and pressing event mark buttons on the hot flash monitor. Hot flashes reported by either method were categorized as a self-report. Four categories were considered for these analyses: physiologic hot flashes, self-reported hot flashes, self-reported hot flashes not corroborated by a physiologic hot flash, and physiologically-detected hot flashes not met by a self-report.
Physical activity
Daily physical activity was assessed continuously over the monitoring period with accelerometer-derived activity counts from the Biolog monitor. The mean activity count in the 10 minutes prior to a hot flash were calculated for each type of hot flash, and categorized as pre-flash activity. Activity during each type of flash and for the 10 minutes following a hot flash was not included in the analysis, as it was expected that women may be likely modify their physical activity pattern during and immediately following a hot flash. The mean activity count during all other waking hours not preceding hot flashes was calculated and categorized as control activity. For the purposes of these analyses, only activity counts during waking hours were used.
Covariates
All covariates were selected on the basis of previously documented associations with vasomotor symptoms and physical activity, and included education, habitual physical activity, race/ethnicity, body mass index, negative affect, age, menopausal status, and time of day of hot flash occurrence. Race/ethnicity and educational level were self-reported in the SWAN screening interview. Habitual physical activity was assessed with the Kaiser Physical Activity Survey, an adaptation of the Baecke physical activity questionnaire (9). A sum score for total activity was derived from responses to questions about physical activity during sports/exercise, household/caregiving tasks, and daily routine in the previous year. BMI was calculated using weight (kg) and height (m)2, and treated as a continuous variable for this analysis. Depressive symptoms were self-reported with the Center for Epidemiological Studies Depression Scale (CESD), a 20-item depression symptom scale (26). Anxiety symptoms were self-reported with the Spielberger State-Trait Anxiety scale (27), a 40-item scale that assesses current anxiety (state anxiety) and typical anxiety levels (trait anxiety). Age was calculated from the participant's date of birth and date of study visit. Menopausal status was defined as perimenopausal (bleeding in the last 12 months, some change in cycle regularity) and postmenopausal (no bleeding in the last 12 months). Time of day was categorized as morning (4 am to noon), afternoon (noon to 4 pm) and evening (4 pm until 4 am). As only those symptoms that occurred during waking hours were including in analysis, morning encompassed symptoms during waking until noon, while evening encompassed symptoms from 4 pm until participants reported going to sleep.
Statistical analyses
Separate hierarchical generalized linear models were used to examine relationships between physical activity and each category of hot flashes (physiologic hot flashes, self-reported hot flashes, self-reported hot flashes without physiologic hot flash, and physiologically-detected hot flashes without self-report). Hierarchical generalized linear modeling utilizes logistic regression methods that adjust for and model the non-independence of repeated measures within individuals, making it appropriate for this assessment of continuous and repeated factors over several days within a sample of women. Associations between mean accelerometer-assessed activity counts in the 10 minute period prior to hot flashes during waking hours was compared to the mean activity counts at all waking non-flash times. Separate non-flash control times were calculated for all hot flash types. Odds ratios of hot flash reporting by each standard deviation increase in activity count were then determined. Final models were adjusted by education, habitual physical activity, race/ethnicity, body mass index, depressive symptoms, age, menopausal status, and time of day hot flashes were reported/measured. Covariates were initially selected a priori due to previously-documented associations between with hot flashes, and subsequently retained in the model with significant univariate associations with hot flash type (p<.05). Depressive symptoms and anxiety were examined separately as covariates given their high correlation (depressive symptoms and trait anxiety r: .84, p<.001) and associated concerns about multicollinearity. The cross-product of negative affect variables and activity count, as well as habitual physical activity and activity count, were also examined in separate final, fully adjusted models investigating the role of negative affect and habitual physical activity on the relationships found between acute increases in physical activity and each hot flash type.
Results
Characteristics of sample
Participants were relatively highly educated, postmenopausal, and overweight (Table 1). By design, the sample was half Caucasian and half African American. All participants had at least one self-reported or physiologically-detected hot flash during the observed period. Participants reported fewer hot flashes than were detected by the physiologic hot flash monitors (mean of 3.41 self-reported hot flashes per participant per day; mean of 5.09 physiological hot flashes per participant per day). During waking hours a total of 1,067 hot flashes were reported, and 1,587 physiologic hot flashes were detected.
Table 1.
Characteristics of the sample (n=51).
| N (%) | |
|---|---|
| Education | |
| High school or less | 10 (19.6) |
| Some college or more | 41 (80.4) |
| Race/ethnicity | |
| Caucasian | 26 (51) |
| African American | 25 (49) |
| Menopausal status | |
| Perimenopausal | 5 (9.8) |
| Postmenopausal | 46 (90.2) |
| Mean (SD) | |
| Hot flashes± | |
| Self-reported hot flashes | 20.92 (13.60) |
| Physiologic hot flashes | 31.12 (18.83) |
| Self-reported hot flashes without physiologic evidence | 4.71 (7.38) |
| Physiologic hot flashes without self-report | 14.90 (12.34) |
| Activity count* | 24.6 (96.7) |
| Habitual physical activity (KPAS total score) | 6.8 (1.3) |
| Body mass index | 29.8 (5.0) |
| Depressive symptoms (CESD) | 7.5 (6.5) |
| Trait anxiety (STAI trait) | 35.1 (8.1) |
| State anxiety (STAI state) | 31.1 (8.6) |
Mean number of daytime hot flashes over monitoring period
Activity count derived from physiologic monitor (range 1 - 3,623).
KPAS: Kaiser Physical Activity Survey
CESD: Center for Epidemiological Studies Depression Survey
STAI: State-Trait Anxiety Inventory
Increased activity as a predictor of hot flashes
We tested the likelihood of each of the four types of hot flashes following increases in physical activity. We observed no relationships between physiologic hot flashes (OR: 1.00, 95% CI: 1.00-1.00, p=.06), self-reported hot flashes (OR: 1.00, 95% CI: 1.00-1.00, p=.50), or physiologically-monitored hot flashes not met by self-report (OR: 0.99, 95% CI: 0.99-1.00, p=.66) and physical activity in multivariable models. However, self-reported hot flashes not corroborated by a physiologic hot flash were more likely following increases in physical activity (OR: 1.04, 95% CI: 1.00-1.10, p=.02) in multivariable models. Higher habitual physical activity (OR: 1.27, 95% CI: 1.13-1.41, p<.001), higher BMI (OR: 1.1, 95% CI: 1.06-1.17, p<.001) more depressive symptoms (OR: 1.05, 95% CI: 1.01-1.06, p=.02), and STAI state anxiety (OR: 1.03, 95% CI: 1.01-1.06, p<.01) were also associated with self-reported hot flashes not corroborated by a physiologic hot flash (table 2).
Table 2.
Self-reported hot flashes without physiologic evidence (n=240) predicted by 1 standard deviation increase in activity count.
| Odds Ratios (95% Confidence Intervals) | p-values | |
|---|---|---|
| Education | ||
| Some college or more (compared to high school or less) | 1.41 (.91-1.99) | 0.11 |
| Habitual physical activity (KPAS total score) | 1.27 (1.13-1.41) | <.001 |
| Race/ethnicity | ||
| African-American (compared to Caucasian) | 0.83 (.49-1.25) | 0.32 |
| Body mass index | 1.10 (1.06-1.17) | <.001 |
| Depressive symptoms (CESD) | 1.05 (1.01-1.06) | 0.02 |
| Age | 0.99 (.90-1.06) | 0.80 |
| Menopausal status | 0.64 (.32-1.67) | 0.19 |
| Postmenopausal (compared to perimenopausal) | ||
| Time hot flash reported | ||
| Morning (compared to evening)# | 1.05 (.82-1.35) | 0.66 |
| Afternoon (compared to evening)# | 0.73 (.58-.91) | <.01 |
| Activity count (change per standard deviation)* | 1.04 (1.00-1.10) | 0.02 |
All variables entered simultaneously in hierarchical generalized linear models.
Activity count derived from physiologic monitor (range: 1 - 3,623; SD: 96.7).
KPAS: Kaiser Physical Activity Survey
CESD: Center for Epidemiological Studies Depression Survey
STAI: State-Trait Anxiety Inventory
Morning: Waking-12:00 pm
Afternoon: 12:01-4:00 pm
Evening: 4:01 pm-bed
The relationship between physical activity and self-reported hot flashes lacking physiologic evidence varied by levels of negative affect, such that the relationship between increased activity and self-reported hot flashes lacking physiologic evidence was stronger among women with higher depressive symptom scores (OR: 1.005, 95% CI: 1.001-1.009, p: .02) or higher trait anxiety (OR: 1.003, 95% CI: 1.000-1.007, p: .05). Stratified analyses confirmed that the increased likelihood of self-reported hot flashes lacking physiologic evidence following physical activity was most apparent among women with higher CESD scores or trait anxiety. Habitual physical activity did not modify relationships between acute increases in activity and any type of hot flash (data not shown).
Additional findings deserve mention. In multivariable models, African-American women (vs. Caucasian, OR: 1.56, 95% CI: 1.18-2.07, p<.001), and postmenopausal women (vs. perimenopausal OR: 1.66, 95% CI: 1.22-2.44, p<.01) were most likely to show physiologically monitored hot flashes. Higher depressive symptoms were associated with increased likelihood of self-reported hot flashes (CESD OR: 1.03, 95% CI: 1.01-1.05, p<.01). Younger (OR: 0.92, 95% CI: 0.85-0.99, p: .03) and postmenopausal women (OR: 1.49, 95% CI: 1.20-1.85, p<.01) had a higher likelihood of physiologic hot flashes without self-report.
Discussion
In this investigation of daily physical activity and hot flashes in midlife women, modest increases in physical activity were associated with subsequent self-reported hot flashes lacking physiologic evidence. This relationship was modified by negative affect, such that women with higher levels of depressive symptoms and anxiety showed the highest propensity to report hot flashes lacking physiologic evidence following increases in physical activity. In contrast, acute increases in daily physical activity were not associated with physiologically-monitored hot flashes. Therefore, rather than acting as a trigger of physiologically-detected hot flashes, acute increases in physical activity were linked to a subsequent reporting of hot flashes lacking physiologic evidence.
These findings add to the limited existing literature on physical activity and hot flashes. Physical activity is frequently discussed as both a protective factor and an acute trigger of hot flashes, but there has been relatively little rigorous empirical investigation of this association. Few published studies have utilized both self-report and physiologic measures of both hot flashes and physical activity to test the commonly posited association between physical activity and hot flashes. This addition is important, as self-reported and physiologically-detected assessments of both hot flashes (20) and physical activity (28) are not highly correlated. Further, the risk and protective factors associated with these self-reported and physiologically-detected variables often differ. Some existing studies using physiologic measures of hot flashes and/or physical activity have shown a similar relationship between aerobic exercise (3) and diary-reported physical exertion (20) with increases in hot flashes without physiologic evidence. Limitations of these studies include self-reported physical activity measures (20) and an ethnically homogenous, relatively normal-weight sample with limited generalizeability (3). Thus, this study adds importantly to the literature, indicating that the association between acute increases in physical activity may be most linked to the reporting of hot flashes, specifically those lacking physiologic evidence. Taken as a whole, these findings indicate that symptom perception and interpretation likely plays a key role in the relationship between physical activity and hot flash reporting.
This study underscores the potential importance of depressive and anxiety symptoms in the association between acute physical activity and hot flash reporting. Other work has shown that self-reported and physiologically monitored hot flashes may differ, and that women with high levels of negative affect (e.g., depressive symptoms, anxiety) are more likely to report physiologically-undetected hot flashes (20,24). Another study showed that self-reported hot flashes decreased following increases in habitual physical activity over time among women with a history of depression (12). However, previous work has not examined negative affect as a modifier of relationships between physical activity and hot flashes, and our study is the first to examine the role of negative affect on the relationship between acute physical activity and the occurrence of hot flashes. The current study contributes to this small literature with additional evidence that negative affective states are influential in the occurrence of hot flash reporting following acute increases in physical activity.
This study also examined factors commonly associated with hot flashes as selected covariates, with the expectation that the mechanisms contributing to physiologically-detected and self-reported hot flashes would differ. Supporting this concept, race/ethnicity and menopausal status were associated with physiologically-detected hot flashes, but not with self-reported hot flashes. In contrast, negative affect, known to influence symptom perception, was associated with self-reported hot flashes, but not with physiologically-detected hot flashes. While habitual physical activity was related to self-reported hot flashes not corroborated by physiologic hot flashes, physiologically-detected and self-reported hot flashes were not associated with habitual physical activity in this study. The latter relationships are consistent with most studies of habitual physical activity. While other studies have supported a role of cardiorespiratory fitness in modifying the relationship between hot flashes and physical activity (3), levels of habitual physical activity did not influence relationships between acute activity and any type of hot flash. However, our measure of habitual physical activity cannot be considered a proxy for fitness. In a closer comparison of differences in associations by method of hot flash assessment, covariates associated with physiologic hot flashes without corroborating self-report mirror those of physiologic hot flashes in general, while self-reported hot flashes that were not corroborated by a physiologic hot flash were also associated with higher habitual physical activity, higher BMI, and higher levels of negative affect. Overall, this pattern of findings supports the concept that different methods of measuring hot flashes capture varied information that can contribute to our understanding of these common symptoms.
This study had several strengths. The thorough measurement of hot flashes is particularly notable, and differs from the larger literature that primarily utilizes retrospective self-report of these common symptoms. Diary and event marker self-reporting of hot flashes throughout the observed period reduces the retrospective reporting biases common to typical measurement. The inclusion of physiologic hot flash monitoring allows for the assessment and comparison of self-reported and physiologically-detected hot flashes. This comparison enables an examination of the mechanisms contributing both to physiological changes and the interpretation and subjective experience of symptoms. The reliability and external validity of these findings was enhanced by ambulatory monitoring during participants' normal daily activities. Finally, this study was conducted with a well-characterized sample of African-American and Caucasian women who have been followed throughout the menopausal transition.
Several limitations of this study should also be noted. Intensity of physical activity and changes in energy expenditure cannot be interpreted from accelerometer-derived activity counts, the objective measure of physical activity utilized in this study. Future study would benefit from detailed assessments of activity that could identify these aspects of activity change, in order to clarify the level and intensity of acute activity necessary to exhibit effects on symptom reporting. Overall, women in this study exhibited low levels of physical activity during the observed period, consistent with protocol recommendations to reduce artifact in hot flash measures. These levels may not reflect participants' typical activity levels, which may have masked relations that would be observed with more intense bouts of physical activity. Further, these low levels of physical activity, along with the frequent measurement of small units of time assessed, may have contributed to the small point estimates seen in this analysis. While established parameters were followed in coding hot flashes, these methods are subject to error. Finally, this sample is relatively small, and includes only women who typically report hot flashes and are willing and able to comply with the protocol. This may limit the generalizability of these findings to other samples.
Conclusions
The relationship between daily, low-intensity physical activity and hot flashes seems to be limited to self-reported hot flashes that are not corroborated by a physiologic hot flash. This is particularly true among women with higher levels of negative affect, who are also known to be more likely to report more frequent, severe, and bothersome hot flashes in midlife. The commonly posited relationship between acute physical activity and hot flashes may therefore be more tied to symptom perception and reporting than to any physiological mechanism. Women in midlife reporting hot flashes often seek treatment options and recommendations to manage their symptoms, but may be wary of behavioral change thought to worsen their symptom experience. Clinicians should consider the potentially modifiable role of affect and the perception of hot flashes in the relationship between physical activity and hot flashes among women when making treatment recommendations for their symptomatic patients.
Figure 1.
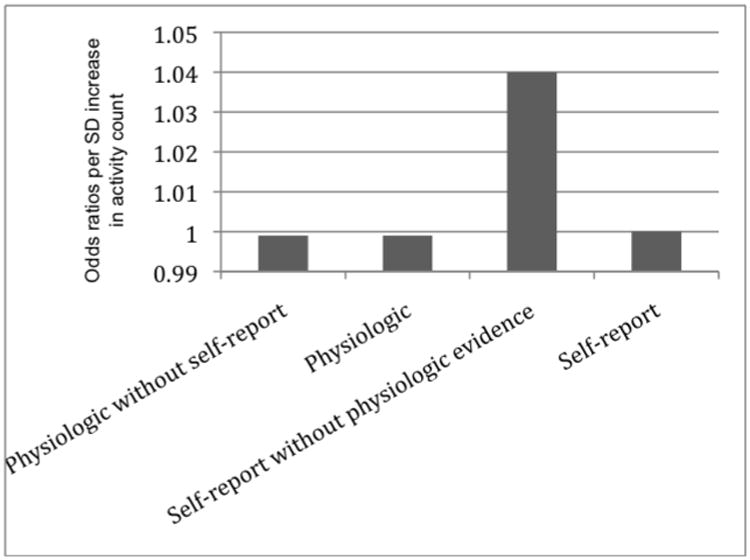
Odds of reporting different types of hot flashes with increases in physical activity.
Acknowledgments
The authors would like to thank Ben Paul, MS, for statistical assistance.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN FLASHES has additional funding through K23AG029216 (PI: Rebecca Thurston, PhD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006 Jul;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greendale GA, Gold EB. Lifestyle factors: are they related to vasomotor symptoms and do they modify the effectiveness or side effects of hormone therapy? Am J Med. 2005 Dec 19;118(12B):148–54. doi: 10.1016/j.amjmed.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 3.Elavsky S, Gonzales JU, Proctor DN, Williams N, Henderson VW. Effects of physical activity on vasomotor symptoms: examination using objective and subjective measures. Menopause. 2012 Oct;19(10):1095–103. doi: 10.1097/gme.0b013e31824f8fb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivarsson T, Spetz AC, Hammar M. Physical exercise and vasomotor symptoms in postmenopausal women. Maturitas. 1998 Jun 3;29(2):139–46. doi: 10.1016/s0378-5122(98)00004-8. [DOI] [PubMed] [Google Scholar]
- 5.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas. 2005 Dec;52(3-4):374–85. doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Luoto R, Moilanen J, Heinonen R, Mikkola T, Raitanen J, Tomas E, et al. Effect of aerobic training on hot flushes and quality of life--a randomized controlled trial. Ann Med. 2012 Sep;44(6):616–26. doi: 10.3109/07853890.2011.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindh-Astrand L, Nedstrand E, Wyon Y, Hammar M. Vasomotor symptoms and quality of life in previously sedentary postmenopausal women randomised to physical activity or estrogen therapy. Maturitas. 2004 Jun 15;48(2):97–105. doi: 10.1016/S0378-5122(03)00187-7. [DOI] [PubMed] [Google Scholar]
- 8.Slaven L, Lee C. Mood and symptom reporting among middle-aged women: the relationship between menopausal status, hormone replacement therapy, and exercise participation. Health Psychol. 1997 May;16(3):203–8. doi: 10.1037//0278-6133.16.3.203. [DOI] [PubMed] [Google Scholar]
- 9.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999 Mar;28(3):313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Holm K. Physical activity alone and in combination with hormone replacement therapy on vasomotor symptoms in postmenopausal women. West J Nurs Res. 2003 Apr;25(3):274–288. doi: 10.1177/0193945902250413. discussion 289–293. [DOI] [PubMed] [Google Scholar]
- 11.Mirzaiinjmabadi K, Anderson D, Barnes M. The relationship between exercise, Body Mass Index and menopausal symptoms in midlife Australian women. Int J Nurs Pract. 2006 Feb;12(1):28–34. doi: 10.1111/j.1440-172X.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Thurston RC, Joffe H, Soares CN, Harlow BL. Physical activity and risk of vasomotor symptoms in women with and without a history of depression: results from the Harvard Study of Moods and Cycles. Menopause. 2006 Aug;13(4):553–60. doi: 10.1097/01.gme.0000227332.43243.00. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DB, Sammel MD, Freeman EW, Lin H, Gracia CR, Schmitz KH. Effect of physical activity on menopausal symptoms among urban women. Med Sci Sports Exerc. 2008 Jan;40(1):50–8. doi: 10.1249/mss.0b013e318159d1e4. [DOI] [PubMed] [Google Scholar]
- 14.Moilanen J, Aalto AM, Hemminki E, Aro AR, Raitanen J, Luoto R. Prevalence of menopause symptoms and their association with lifestyle among Finnish middle-aged women. Maturitas. 2010 Dec;67(4):368–74. doi: 10.1016/j.maturitas.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Wilbur J, Miller AM, McDevitt J, Wang E, Miller J. Menopausal status, moderate-intensity walking, and symptoms in midlife women. Res Theory Nurs Pract. 2005;19(2):163–80. [PubMed] [Google Scholar]
- 16.McAndrew LM, Napolitano MA, Albrecht A, Farrell NC, Marcus BH, Whiteley JA. When, why and for whom there is a relationship between physical activity and menopause symptoms. Maturitas. 2009 Oct 20;64(2):119–25. doi: 10.1016/j.maturitas.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Elavsky S, McAuley E. Personality, Menopausal Symptoms, and Physical Activity Outcomes in Middle-Aged Women. Pers Individ Dif. 2009 Jan;46(2):123–8. doi: 10.1016/j.paid.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaCroix A, Sternfeld B, Caan B, Newton K, Reed S, Cohen L, et al. Presented at the North American Menopause Society Annual Meeting, Orlando, FL. Orlando, FL: 2012. Results from the MsFLASH Randomized Controlled Trial of Yoga, Aerobic Exercise & Omega-3 Supplementation for Relief of Vasomotor Symptoms. [Google Scholar]
- 19.Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999 Jul;181(1):66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- 20.Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005 Feb;67(1):137–46. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- 21.Gibson CJ, Thurston RC, Bromberger JT, Kamarck T, Matthews KA. Negative affect and vasomotor symptoms in the Study of Women's Health Across the Nation Daily Hormone Study. Menopause. 2011 Dec;18(12):1270–7. doi: 10.1097/gme.0b013e3182230e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, Coons MJ, et al. Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosom Med. 2011 Mar;73(2):127–33. doi: 10.1097/PSY.0b013e31820433a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sowers MR, Crawford S, Sternfeld B. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 24.Thurston RC, Matthews KA, Hernandez J, De La Torre F. Improving the performance of physiologic hot flash measures with support vector machines. Psychophysiology. 2009 Mar;46(2):285–92. doi: 10.1111/j.1469-8986.2008.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurston RC, Hernandez J, Del Rio JM, De La Torre F. Support Vector Machines to improve physiologic hot flash measures: application to the ambulatory setting. Psychophysiology. 2011 Jul;48(7):1015–21. doi: 10.1111/j.1469-8986.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977 Jun 1;1(3):385–401. [Google Scholar]
- 27.Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the state-trait anxiety inventory, Consulting Psychologists Press. Consulting Psychologists Press; 1983. [Google Scholar]
- 28.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]


