Abstract
The neurogenic cranial placodes are a unique transient epithelial niche of neural progenitor cells that give rise to multiple derivatives of the peripheral nervous system, particularly, the sensory neurons. Placode neurogenesis occurs throughout an extended period of time with epithelial cells continually recruited as neural progenitor cells. Sensory neuron development in the trigeminal, epibranchial, otic, and olfactory placodes coincides with detachment of these neuroblasts from the encompassing epithelial sheet, leading to delamination and ingression into the mesenchyme where they continue to differentiate as neurons. Multiple signaling pathways are known to direct placodal development. This review defines the signaling pathways working at the finite spatiotemporal period when neuronal selection within the placodes occurs, and neuroblasts concomitantly delaminate from the epithelium. Examining neurogenesis and delamination after initial placodal patterning and specification has revealed a common trend throughout the neurogenic placodes, which suggests that both activated FGF and attenuated Notch signaling activities are required for neurogenesis and changes in epithelial cell adhesion leading to delamination. We also address the varying roles of other pathways such as the Wnt and BMP signaling families during sensory neurogenesis and neuroblast delamination in the differing placodes.
Keywords: Cranial placodes, Neurogenesis, Delamination, Notch, FGF, Wnt, BMP
Introduction
Cranial placodes are a unique model of neural development. In vertebrate embryos neurons are generated from three sources, the neuroepithelium of the neural tube, the neural crest, and the ectodermal cranial placodes. Placodes share the epithelial characteristic of the CNS neuroepithelium and the transient migratory nature of the neural crest. Cranial placodes arise from a preplacodal domain of ectodermal progenitor cells. After initial induction of this panplacodal primordium into individual placodes, each placode is specified for a unique sensory fate. While some placodes contribute non-neuronal cell types to cranial sensory organs, the neurogenic placodes that contribute sensory neurons to the PNS include the trigeminal, epibranchial, otic, and olfactory placodes. Placode-derived neurons enter the mesenchyme to co-mingle with neural crest cells to establish cranial ganglia, the sensory nervous system component of cranial nerves. A recent study highlighted the important interactions of neural crest and placode cells in this process (Freter et al., 2013). Two key cellular processes early in placodal sensory neuron development are: 1) neuronal determination, where primed progenitor epithelial cells are selected for a neuronal fate, undergoing neurogenesis and neuronal differentiation; and 2) delamination from the epithelium, whereby cells detach from their epithelial neighbors and escape through breaks in the basement membrane into the mesenchyme as migratory sensory neuroblasts in a process different from the epithelial to mesenchyme transition (EMT) seen in neural crest cells (Graham et al., 2007).
In this focused review we will only briefly introduce the neurogenic placodes, and then comprehensively examine how the Notch, FGF, Wnt, and BMP signaling protein families direct sensory neurogenesis and delamination from the placodal epithelium, where the pathways are conserved, where they diverge, and what we still have to learn about the differentiation process.
Origins and derivatives of neurogenic placodes
Progenitors within the neurogenic placodes give rise to different types of sensory neurons/cells, which contribute to the cranial ganglia, the inner ear, and the olfactory epithelium. Sensory neurons originating from the placodes delaminate from the epithelium, migrate and condense to form the cranial ganglia. The sole derivatives of both the trigeminal and epibranchial placodes are sensory neurons of the cranial ganglia (D'Amico-Martel and Noden, 1983; Harlow and Barlow, 2007). The neural contribution of the otic placode includes both secondary sensory hair cells of the inner ear and sensory neurons of the cochleovestibular ganglion (CVG), which delaminate from the epithelium of the invaginated otic vesicle. The neurogenic portion of the olfactory placode gives rise to delaminating neurons in the migratory mass and chemosensory receptor neurons, which remain in the olfactory epithelium (Beites et al., 2005; Kawauchi et al., 2004).
Trigeminal placode
While some of the cranial placodes produce cell types other than neurons, sensory neurons are the sole derivative of the trigeminal placodes. The trigeminal placode consists of two molecularly distinct sub-placodes, the ophthalmic (opV) and the maxillomandibular (mmV). The opV and mmV placodes each contribute neurons to the distal region of their respective ganglionic lobes, while the neural crest contributes proximal neurons, as well as glial cells (Baker and Bronner-Fraser, 2000; Baker and Bronner-Fraser, 2001; D'Amico-Martel and Noden, 1983; Schlosser, 2006). The trigeminal ganglion, the sensory ganglion of cranial nerve V, is the largest of the cranial ganglia and provides sensation to much of the face and jaw. Trigeminal ganglion neurons are primary sensory neurons, responsible for touch, pain, and temperature sensation from the head.
Fate mapping studies in the chick have shown that the opV placode develops in the ectoderm adjacent to the midbrain and the midbrain-hindbrain boundary (MHB), while the mmV placode is found directly caudal at the rhombomere 2 & 3 level (Xu et al., 2008). In both chick and mouse, trigeminal neurons first develop in the opV placode followed by the mmV placode, while neural crest-derived neurons differentiate at considerably later stages (Covell and Noden, 1989; d'Amico-Martel and Noden, 1980; Moody et al., 1989a; Moody et al., 1989b; Nichols, 1986; Stainier and Gilbert, 1991; Verwoerd et al., 1981).
Epibranchial placodes
Similar to the trigeminal placode, epibranchial placodes give rise solely to sensory neurons of the cranial ganglia; they are located at the hindbrain axial level and develop ventral to the otic placode in the dorsal and caudal margins of the pharyngeal clefts (Begbie et al., 2002; Begbie et al., 1999; Graham et al., 2007; Ladher et al., 2010). The epibranchial placodes consist of the geniculate, petrosal, and nodose placodes which produce neuroblasts in the surface ectoderm that delaminate and migrate, contributing viscerosensory neurons to cranial nerves VII (facial), IX (glossopharyngeal) and X (vagus), respectively, innervating several visceral organs and the taste buds (Northcutt, 2004).
Otic placode
The otic placode gives rise to the entire inner ear, including the sensory hair cells and the innervating sensory neurons of the CVG (Torres and Giraldez, 1998). Each otic placode is located adjacent to rhombomeres 5 and 6 of the posterior hindbrain, and this oval sheet of thickened placodal epithelium invaginates, forming the otic cup, which subsequently closes and detaches from the surface ectoderm as it becomes the otic vesicle. The otic vesicle undergoes continued morphogenesis during early development, ultimately producing all of the structures of the inner ear. The CVG develops from neuroblasts in the otic epithelium that delaminate and migrate from the neurosensory domain of the otic vesicle, and also from a contribution of neural crest cells which differentiate to glial cells (Barald and Kelley, 2004; Carney and Silver, 1983; D'Amico-Martel and Noden, 1983; Rubel and Fritzsch, 2002; Schneider-Maunoury and Pujades, 2007). Progenitors in the neurosensory domain of the otic vesicle appear to be able to differentiate as sensory neurons, hair cells, and supporting cells, making it a more complex model for sensory neurogenesis. Neural crest cells have recently been described as contributing more broadly, first integrating themselves into the otic epithelium, and then differentiating alongside placode-derived cells (Freyer et al., 2011).
Olfactory placode
The olfactory placode, like the otic, invaginates to form the olfactory pit and generates migrating cells including the neuropeptidergic neurons, such as GnRH-secreting neurons that eventually enter into the forebrain and contribute to the neuroendocrine compartments (Tarozzo et al., 1995). Different from other neurogenic placodes, the olfactory placode also gives rise to a dominant group of sensory neurons, the olfactory sensory cells, which do not delaminate from the placode and reside within the olfactory sensory neuroepithelium to transduce odor and pheromone signals to the CNS through their projection axons (the olfactory nerve) (Croucher and Tickle, 1989). Additional cell types derived from the olfactory placode include the basal progenitors and the non-neuronal sustentacular cells residing in the olfactory epithelium, and in a classic view, also include the olfactory ensheathing cells (OECs) which delaminate from the olfactory epithelium to the lamina propria and ensheath the olfactory axons. However, the origins of OECs have recently been challenged by several genetic fate mapping studies as they are likely derived from the neural crest cells (reviewed by Forni and Wray, 2012). Nevertheless, neuronal cells delaminating from the olfactory placodal epithelium are consistent with the properties of delaminating sensory neuroblasts that contribute to cranial ganglia from the other neurogenic placodes.
Signaling pathways critical in placode neurogenesis and delamination
Neurogenic placodes continuously generate neuroblasts within the epithelium over an extended period of time, indicating that the placodes represent specialized epithelial progenitor niches (Graham et al., 2007). Neurogenesis begins within these restricted zones and the primary morphological event of the placode is delamination of neuroblasts from the specified epithelium (Graham et al., 2007; Lassiter et al., 2010; McCabe et al., 2009). Sensory neurons are derived from both the cranial placodes and the neural crest migratory cell populations; however, placodal delamination differs markedly from that of neural crest. The process of sensory neurogenesis in the placodes also differs somewhat from that observed for neural crest. Neurogenesis begins within the epithelium prior to cells delaminating and becoming migratory. This is evidenced by the expression of early neuronal markers (Ngn, Isl1, NeuroD) and by a significant reduction in cycling cells, although some neuronal precursors are not yet post-mitotic. Identifying differentiating neurons morphologically is only possible as they begin to exit the epithelium, at which time delaminating neuroblasts appear to escape the epithelium individually or in small clusters. In the epibranchial placodes, for example, cells emerge from a pseudostratified single-layered epithelium as neuronal cells with distinct neuronal morphology (Graham et al., 2007). At the site of neuroblast exit from the epithelium there is a finite breakdown of the basal lamina. Neurogenesis and neuronal delamination from the trigeminal and epibranchial placodal niche is continuous for an extended period of 2 days, whereas neural crest delamination ceases within 15 hours in chick embryos (Blentic et al., 2011; Graham et al., 2007). Placode-derived cells contribute to the condensing ganglion as post-mitotic neurons that have terminally differentiated. The timing of terminal differentiation occurs within the epithelium prior to delamination, as seen in the ophthalmic trigeminal (McCabe et al., 2009), or shortly after delamination during their migration, observed in mmV, epibranchial, and otic neurons (Begbie et al., 2002; Blentic et al., 2011). Interestingly, epibranchial placode cells appear to be mitotically quiescent during the delamination process (Graham et al., 2007), while neuronal differentiation of placode-derived cells initiates prior to migration (Blentic et al., 2011; Lassiter et al., 2010). Placode neurogenesis begins within the epithelial niche and these cells are committed as neurons upon delamination from the ectoderm, indicating that neuronal cell selection and changes in cell adhesion leading to delamination may be coupled. It is likely that though individual specification of the neurogenic placodes may be differentially regulated, the mechanisms and signaling pathways directing the event of neurogenesis and delamination may be conserved in all neurogenic placodes.
The Notch, FGF, Wnt, and BMP signaling pathways, along with others, play various and multiple roles throughout development of the placodes and because of the dynamic and ongoing nature of this process it is challenging to examine exclusively neuronal selection and cellular delamination distinct from induction, specification, and differentiation. However, it is clear from the literature that both the Notch and FGF pathways specifically direct neurogenesis within the epithelium of all neurogenic placodes and also alter the cellular adhesion properties, resulting in delamination (Fig. 1, Table 1 & references therein). The Wnt and BMP pathways also play important roles in these events, but evidence suggests they may function differently in different placodes (Table 1). We will detail the effects of each of these signal transduction pathways on placode neurogenesis and epithelial delamination.
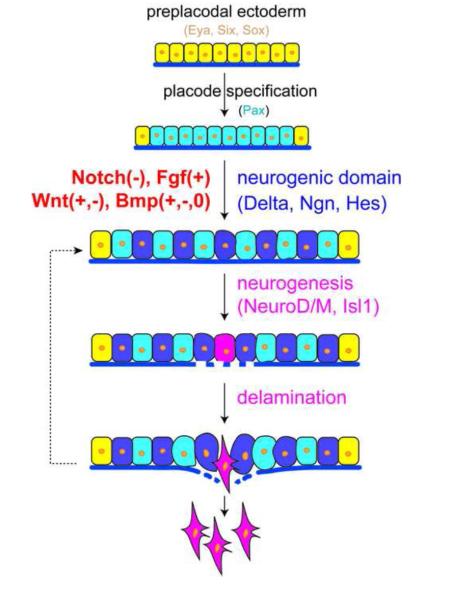
Fig. 1.
A general model of the signaling programs in the neurogenic placodal niche during neurogenesis and delamination. After cranial placodes are specified (by expressing Pax transcription factors) from the preplacodal ectoderm (defined by expressing Eya, Six, or Sox transcription factors), the neurogenic domain is formed by expressing proneurogenic factors, such as Delta/Delta-like, Ngn, and Hes. These neural precursors differentiate to NeuroD/M or Isl1 positive neuroblasts, which subsequently delaminate from the neurogenic placodes. A conserved signaling program with attenuated Notch and activated FGF signaling is critical in reiterative neurogenesis and delamination processes in all neurogenic placodes. Wnt and BMP pathways may play positive, negative, or no roles in different placodes during neurogenesis and delamination. The early role of the signaling pathways in placodal formation and specification are not addressed here. +, activation and upregulation; -, inhibition or downregulation; 0, no role.
Table 1.
Experimental findings of the Notch, FGF, Wnt, and BMP signaling pathways in the neurogenic placodes, in different species, and the effects on neurogenesis and delamination of sensory neuroblasts. Each placode has been assigned a color: trigeminal – yellow, epibranchial – green, otic – blue, and olfactory – pink. GOF, gain-of-function; LOF, loss-of-function; na, not assayed or addressed in the study; NE, no effect; +, increase and/or upregulation; -, inhibition and/or downregulation.
| Notch | LOF | DAPT chemical | trigeminal | chick | Islet1, NF | + | + | Lassiter et al., 2010 |
| LOF | Notch1−/−, RBPjk−/− | trigeminal | mouse | NeuroD, Mash1 |
+ | na | de la Pompa et al., 1997 | |
| LOF | Notch2−/− | trigeminal | mouse | Ngn1 | NE | na | Hamada et al., 1999 | |
| LOF | Notch1−/−, RBPjk−/− | geniculate | mouse | NeuroD | + | na | de la Pompa et al., 1997 | |
| LOF | DAPT chemical | otic | chick Delta 1, | NeuroD | + | + | Abelló et al., 2007 | |
| LOF | mindbomb Ub ligase mutant | otic | zebrafish | Delta1, Islet1 | + | na | Haddon et al., 1998 | |
| LOF | DAPT chemical | olfactory | chick | Ngn, HuC/D |
+ | na | Maier et al., 2011 | |
| LOF | Notch2−/− | olfactory | mouse | Hes1 | NE | na | Hamada et al., 1999 | |
| GOF | NICD electroporation | trigeminal | chick | Islet1 | − | − | Lassiter et al., 2010 | |
| GOF | CAGCreER+;Rosa26-NICDloxp/+ | otic | mouse | Tuj1 | − | na | Liu et al., 2012 | |
| GOF | Foxg1Cre;Rosa-NICD | otic | mouse | na | na | − | Hartman et al., 2010 | |
| GOF | Foxg1Cre;Rosa-NICD | otic | mouse | Islet1, NeuroD, Tuj1 | − | − | Pan et al., 2010 | |
| GOF | NICD electroporation | olfactory | chick | HuC/D, Tuj1 | − | − | Maier et al., 2011 | |
| FGF | LOF | sec-FGFR4 electroporation | trigeminal | chick | Islet1, NeuN, NF | − | − | Lassiter et al., 2009 |
| LOF | SU5402 chemical | trigeminal | chick | Islet1 | − | − | Lassiter et al., 2009 | |
| LOF | SU5402 chemical | trigeminal | chick | Islet1 | − | na | Canning et al., 2008 | |
| LOF | hsp70:dn-FGFR1 | epibranchial | zebrafish | Phox2b | − | − | Nechiporuk et al., 2007 | |
| LOF | SU5402 chemical | epibranchial | zebrafish | Phox2b | − | na | Nechiporuk et al., 2007 | |
| LOF | FGF3 morpholino | epibranchial | zebrafish | Ngn, Phox2a&2 b, Hu |
− | na | Nechiporuk et al., 2005 | |
| LOF | SU5402 chemical | epibranchial | zebrafish | Ngn, Phox2a&2 b |
− | na | Nechiporuk et al., 2005 | |
| LOF | FGFR1n7/n7 | epibranchial | mouse | Ngn, NF | − | − | Trokovic et al., 2005 | |
| LOF | EMD341608 chemical | otic | mouse | Ngn1 | − | na | Brown et al., 2011 | |
| LOF | SU5402 chemical | otic | zebrafish | NeuroD | − | − | Hammond et al., 2011 | |
| LOF | SU5402 chemical | otic | chick | Ngn1 | − | − | Abelló et al., 2010 | |
| LOF | SU5402 chemical | otic | chick | Ngn1, Delta1, NeuroD |
− | na | Alsina et al., 2004 | |
| GOF | FGF8 electroporation (ect) | trigeminal | chick | Islet1 | NE | NE | Lassiter et al., 2009 | |
| GOF | FGF8 electroporation (NT) | trigeminal | chick | Islet1 | + | + | Canning et al., 2008 | |
| GOF | hs-FGF3 or hs-FGF8 | epibranchial | zebrafish | Phox2b | + | na | Nechiporuk et al., 2007 | |
| GOF | FGF8 bead | epibranchial | zebrafish | Phox2b | + | na | Nechiporuk et al., 2007 | |
| GOF | hs-FGF3 | epibranchial | zebrafish | Phox2a | + | na | Nechiporuk et al., 2005 | |
| GOF | hsp70:FGF3 | otic | zebrafish | NeuroD | + | + | Hammond et al., 2011 | |
| GOF | FGF8 electroporation | otic | chick | NeuroD | + | + | Abelló et al., 2010 | |
| GOF | FGF10 beads | otic | chick | NeuroD&M | + | + | Alsina et al., 2004 | |
| GOF | FGF10 electroporation | otic | chick | NeuroD | + | + | Alsina et al., 2004 | |
| Wnt | LOF | DN-TCF4 electroporation | trigeminal | chick | Islet1, NeuN, NF |
− | − | Lassiter et al., 2007 |
| LOF | Pax2cre;βcateninfloxed/del | epibranchial | mouse | Ngn1 | − | na | Ohyama et al., 2006 | |
| LOF | Pax2cre;βcateninfloxed/del | otic | mouse | NeuroD, Tuj1 |
NE | NE | Ohyama et al., 2006 | |
| GOF | CA-βcat electroporation (ect) | trigeminal | chick | na | na | NE | Lassiter et al., 2007 | |
| GOF | Wnt1 electroporation (NT) | trigeminal | chick | Islet1, NF | + | na | Canning et al., 2008 | |
| GOF | Wnt3a medium | trigeminal | chick | Islet1 | + | na | Canning et al., 2008 | |
| GOF | LiCl chemical | otic | mouse | Ngn1 | − | na | Brown et al., 2011 | |
| GOF | Foxg1cre;Catnblox(ex3) | otic | mouse | Ngn1, NeuroD, NF |
− | na | Freyer et al., 2010 | |
| GOF | Pax2cre;Catnblox(ex3) | otic | mouse | NeuroD | − | na | Ohyama et al., 2006 | |
| BMP | LOF | PRDC electroporation | epibranchial | chick | Delta1, Phox2a, HuC |
− | na | Kriebitz et al., 2009 |
| LOF | Noggin | epibranchial | zebrafish | Phox2b | − | na | Holzschuh et al., 2005 | |
| LOF | Follistatin beads | epibranchial | chick | NF-M | − | na | Begbie et al., 1999 | |
| LOF | Noggin electroporation | olfactory | chick | Tuj1, HuC/D |
− | NE | Maier et al., 2011 | |
| GOF | CA-BMPR1b electroporation | epibranchial | chick | NeuroD | + | + | Tripathi et al., 2009 | |
| GOF | BMP4 electroporation | epibranchial | chick | Phox2a | + | na | Kriebitz et al., 2009 | |
| GOF | BMP4 beads | epibranchial | zebrafish | Phox2b | + | na | Holzschuh et al., 2005 | |
| GOF | BMP7 medium | epibranchial | chick | Phox2a, NF-M |
+ | na | Begbie et al., 1999 | |
| GOF | CA-ALK3 electroporation | otic | chick | Islet1 | NE | NE | Abelló et al., 2010 | |
| GOF | CA-BMPR1b electroporation | olfactory | chick | Tuj1, HuC/D | − | − | Maier et al., 2011 | |
Notch signaling
All placodes express members of the Notch/Delta signaling pathway that are confined to the epithelium and are downregulated as the neuroblasts enter the mesenchyme, indicating that they are likely essential for neuronal selection and possibly delamination in all placodes (Begbie et al., 2002; Haddon et al., 1998; Schwarting et al., 2007) (Fig. 2). In both the PNS and CNS, neurogenesis becomes a delicate balance between cells in a proliferative progenitor state and cells selected to initiate neuronal differentiation. Numerous genes are involved in priming the field of precursor cells towards a neuronal fate allowing for the transition from stem cell to differentiated neuron, including the Sox, Fox, Ngn, and Hes gene families (Schlosser, 2010). Significantly, many of these genes are regulators and effectors of the Notch juxtacrine signaling pathway. Notch signaling is a key regulator of neurogenesis. Briefly, through lateral inhibition, cells expressing the Delta ligand promote cleavage of the Notch intracellular domain in adjacent cells, activating Hes genes that repress neuronal differentiation through blocking Ngn, a proneural factor involved in the upregulation of the Delta ligand.
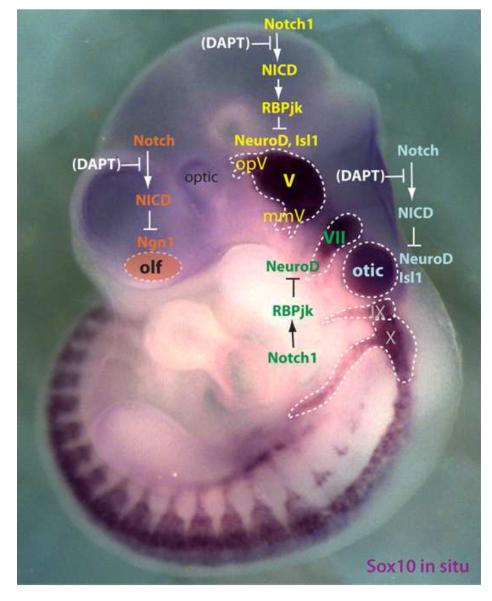
Fig. 2.
Experimentally verified Notch signaling in neurogenic placodes. Notch1-RBPjk signaling negatively regulates neurogenesis and delamination in the trigeminal (V) and geniculate (VII) placodes. Activation or inhibition of NICD down- or up-regulates neurogenic factors in the olfactory (olf), trigeminal, and otic placodes, respectively. DAPT shown in brackets is a pharmacological antagonist for Notch signaling via inhibition of gamma-secretase. mmV & opV, maxillomandibular & ophthalmic trigeminal placodes. All neurogenic placodal regions (except the olfactory) are visualized by Sox10 in situ signals on an E9.5 mouse embryo.
In the trigeminal, epibranchial, otic, and olfactory placodes, Notch signaling modulates which cells will be chosen from the neural progenitor field to undergo neurogenesis and exit the epithelium as differentiating neurons (Table 1). Experimental constitutive activation of the Notch pathway or gain-of-function (GOF), after initial induction and specification of the individual placodes prevents both neurogenesis and delamination. The progenitor cells do not express early or late neuronal markers such as Dll1 (Delta-like 1), Ngn, Isl1, HuC/D, Tuj1, or Neurofilament (NF). In contrast, inhibition of Notch signaling or loss-of-function (LOF) allows for precocious and premature neurogenesis and delamination.
In the trigeminal placode, Notch LOF carried out in chick head explant cultures resulted in a dramatic increase in neurogenesis within placodal epithelium and in the mesenchyme, in both the opV and mmV trigeminal placodes (Lassiter et al., 2010). Inhibition of Notch led to a substantial increase of ectodermal cells expressing Isl1 and NF, as well as a vast sheet of neuroblasts delaminating from the epithelium with an abundant amount of differentiating neurons also observed in the mesenchyme. Interestingly, this significant enhancement in neurogenesis and delamination did not extend beyond the normal specified placode domain suggesting that Notch signaling is regulating a neuronal fate choice at this developmental time point and is not involved in specifying the competent neurogenic domain. In the trigeminal placodes, blocking Notch activity with the gamma-secretase inhibitor DAPT resulted in precocious neurogenesis, including ectopic neuronal differentiation within the epithelium (Lassiter et al., 2010). The spatiotemporal expression patterns of Delta/Notch pathway components were also described in this study, and most expression was observed only within the placodal ectoderm and not in migratory cells. This is similar to their expression patterns in neural crest cells (Begbie et al., 2002; Lassiter et al., 2010; Schlosser and Northcutt, 2000) While experimental inhibition of Notch signaling causes differentiation in the absence of cellular delamination, most would argue that during normal embryogenesis, delamination may be the primary endogenous mechanism used to remove cells from the oscillating Notch signaling environment, thereby allowing for terminal differentiation (Kageyama et al., 2008; Kageyama et al., 2009; Shimojo et al., 2008).
Mouse genetic approaches have revealed that Notch1/RBPjk signaling is crucial in negative regulation of neurogenesis in both CNS and cranial placodes (Fig. 2 & Table 1). Increased expression of NeuroD in the trigeminal and geniculate placodes, and ectopic expression of NSCL1 in the geniculate were detected in the Notch1-LOF or RBPjk-LOF mutant mouse embryos (de la Pompa et al., 1997). Downregulated Hes5 and upregulated Dll1 expression occurred systematically in these mutants. The RBPjk-LOF mutants exhibited stronger effects on the alteration of neurogenesis than that in the Notch1-LOF mutants, suggesting a functional redundancy of Notch genes in neurogenesis. Interestingly, the Notch2-LOF shows no effects on neurogenesis in the olfactory and trigeminal placodes of mice (Hamada et al., 1999), indicating that other Notch genes, such as Notch3 or Notch4, may play redundant roles with Notch1 in placode neurogenesis.
Cell-autonomous Notch GOF via electroporation of the Notch intracellular domain (NICD) into the specified chick trigeminal placode completely short-circuits both neurogenesis and cell delamination (Lassiter et al., 2010). All transfected cells fail to express neuronal markers and remain stalled in the epithelium. Ectopic Notch activation therefore prevents neuronal cell fate selection while simultaneously blocking changes in cell adhesion, demonstrating a requirement for attenuation of Notch signaling in placode neurogenesis and delamination.
Analogous outcomes from Notch GOF and LOF experiments have been demonstrated in the otic placode of mouse, chick, and zebrafish (Table 1). Notch signaling functions in various ways in the otic placode, likely due to the multiple derivatives generated from this placode. Notch signaling is implicated in otic development in establishing the neurosensory domain, hair cell determination, and neurogenesis of delaminating CVG neurons. Importantly, Delta(+) cells are confined to the otic epithelium and not present in the CVG (Adam et al., 1998; Alsina et al., 2004). Early evidence from the mindbomb zebrafish mutant, in which Notch signaling is inhibited, show a twofold excess in the number of Isl1(+) cells in the CVG, leading the authors to strongly suggest that normal otic neurogenesis is regulated at an early step by lateral inhibition (Haddon et al., 1998). In agreement with this, LOF experiments in the chick otic placode showed that Notch is required in the proneural domain for inhibiting neuronal fate through the mechanism of lateral inhibition (Abello and Alsina, 2007). Notch inhibition via DAPT resulted initially in an increased number of neuronal precursors without affecting the specification of the proneural domain. In addition, a substantial increase in NeuroD(+) neuroblasts was observed in the otic epithelium. These neuroblasts also were observed exiting the epithelium early and in amplified numbers, suggesting a regulation of cell adhesion mechanisms by Notch signaling, similar to the findings in the trigeminal placode. Also, despite the increase in the number of neuroblasts after Notch inhibition, neuroblasts are always restricted to the proneural domain of the otic placode, again similar to observations in the trigeminal.
In the mouse, numerous Notch GOF studies indicate that activation of the Notch pathway prevents both neurogenesis and delamination (Hartman et al., 2010; Liu et al., 2012; Pan et al., 2010). In the Rosa26-NICD;Foxg1-Cre, the neural markers TuJ1, NeuroD, and Isl1 are severely reduced or absent, whereas the otic specification marker Pax2 is unaffected (Pan et al., 2010). Delamination of cells was also completely prevented and the CVG was not present. In a similar study utilizing the Notch1-GOF;Foxg1-Cre mouse, delaminating neuroblasts were significantly reduced (Hartman et al., 2010). However, activation of Notch signaling at early developmental stages is required for the prosensory specification of the otic vesicle. Jag1-LOF;Foxg1-Cre mouse embryos exhibit severe defects in the otic sensory progenitor domains (Kiernan, et al., 2006). Conversely, Notch1-GOF in early mouse embryos leads to fully expansion of the sensory domains to the entire otic vesicle, or causes ectopic formation of sensory cells in non-sensory regions via lateral induction (Hartman et al., 2010). Evidence from another Notch-GOF transgenic mouse also demonstrates that constitutive Notch signaling prevented neurogenesis and instead generated ectopic hair cells in the CVG area (Liu et al., 2012). These results highlight opposing effects of Notch signaling on prosensory specification and subsequent neurogenesis.
As in the otic placode, the consensus is that olfactory placode produces multiple derivatives. The majority of evidence shows that coincident with invagination of the olfactory placode there are early forming sensory neurons that migrate away from the olfactory epithelium referred to as the migratory mass (Croucher and Tickle, 1989; Fornaro et al., 2003; Maier and Gunhaga, 2009; Mendoza et al., 1982). Notch experiments in the chick olfactory placode are consistent with the trigeminal and otic placodes. In Notch LOF embryo explants, the neuronal marker HuC/D is dramatically upregulated. Electroporation of NICD into the olfactory epithelium resulted in a significant decrease in HuC/D migratory neurons and targeted cells did not delaminate or enter the mesenchyme but remained stalled in the epithelium (Maier et al., 2011).
Members of the Notch signaling family and downstream targets are found in the epibranchial placodes coincident with expression of the early neuronal marker Phox2a. In the chick, epibranchial specification and neurogenesis begins at about 18 to 20 somite-stage (ss), coincident with onset of Delta-1 expression. As in the trigeminal placodes, Delta-1 is restricted to individual cells within epibranchial placode epithelium and is not expressed by cells that have delaminated and migrated away from the placodes (Begbie et al., 2002). Notch regulation in the epibranchial placodes remains little understood. A significant work early in epibranchial research was performed in mouse embryos null for the Notch effector Ngn2 gene (Fode et al., 1998). Without Ngn, specified cells within the epibranchial placode remain in the ectoderm and do not delaminate or differentiate further as neurons. Delta expression, directly regulated by Ngn, is also lost in Ngn mutant mice. Also in zebrafish, when Ngn is blocked by morpholino injection, neuronal expression is lost (Nechiporuk et al., 2005). It is likely that Notch regulation of neurogenesis and cell adhesion is conserved in epibranchial placodes consistent with trigeminal, otic, and olfactory placodes. However, future experiments are needed to confirm this hypothesis. In summary, Notch regulation of sensory neurogenesis is a key checkpoint mechanism in all placodes, where downregulation of Notch signaling in individual cells causes them to quickly differentiate as sensory neurons.
FGF signaling
The FGF signaling pathway is important throughout the development of the cranial placodes. FGF signaling, including the FGF8 ligand in particular, is thought to be crucial for the induction or specification of all neurogenic placodes: trigeminal, epibranchial, otic, and olfactory (Bailey et al., 2006; Canning et al., 2008; Kawauchi et al., 2005; Maier et al., 2010; Nechiporuk et al., 2007; Nikaido et al., 2007; Sun et al., 2007). A defining study in the chick (Bailey et al., 2006) proposed that lens placode specification is the ground default state for all sensory placodes. Their findings indicate that FGF signaling (specifically FGF8 in the olfactory placode) represses the lens fate in precursors of all other placodes while simultaneously activating properties responsible for their induction. Though FGFs are vital for initial induction of the neurogenic placodes, they also play a clear and distinct role after specification of the individual placodes, directing neurogenesis and neuroblast delamination (Fig. 3 & Table 1).
Fig. 3.
Experimentally verified FGF signaling in neurogenic placodes. FGF8-Fgfr4 signaling in the trigeminal (V), FGF3/FGF8/FGF10 signaling in the otic, and FGF3/FGF15-Fgfr1 signaling in the epibranchial (VII, IX, & X) placodes positively regulate neurogenesis and delamination. FGF8 and Fgfr1/Fgfr2 signaling in the olfactory placode is known to regulate placode formation and specification. An autoregulatory mechanism may exist within the neurogenic placodes (i.g. epibranchial placode) via FGF receptor (Fgfr1) signaling that is initiated by endodermal FGF lignads (FGF3) and then induces the placode ectodermal FGF ligands (FGF3/FGF15) for subsequent neurogenesis and delamination. The pharmacological antagonists shown in brackets are the inhibitors of the FGF receptor tyrosine kinase domain.
In the trigeminal placode, Fgfr4 is transiently expressed in opV placode cells. After initial induction and specification, demarcated by Pax3, Fgfr4 is upregulated in the trigeminal epithelium and is subsequently downregulated upon neuroblast delamination (Stark et al., 1997). Fgfr4 expression is coincident with Ngn2 expression. Inhibition of FGF signaling in the trigeminal placode of chick embryos demonstrated that FGF signaling is not necessary to maintain trigeminal identity and does not affect specification of the placode, however, it is necessary for subsequent neurogenesis and delamination of trigeminal sensory neurons (Lassiter et al., 2009). Cells targeted with an inhibitory Fgfr4 construct did not express neurogenic markers and remained stalled in the epithelium again indicating that neurogenesis and changes in cell adhesion are fundamentally linked. In chick explant experiments of FGF-LOF, inhibition of neurogenesis is also observed (Canning et al., 2008). In the same study, activation of FGF signaling through electroporation of the FGF ligand into the adjacent neural tube results in increased and premature neurogenesis and delamination in both the opV and mmV trigeminal placodes. Interestingly, electroporation of the FGF ligand directly into the placodal ectoderm produced no effect (Lassiter et al., 2009). These contrary results suggest a potentially non-cell-autonomous role of FGF-GOF on the trigeminal placode.
Similar findings are seen in the epibranchial placodes (Fig. 3 & Table 1). In zebrafish, FGF3, emanating from the pharyngeal endoderm is implicated as a determining factor required for neurogenesis and delamination in the epibranchial placodes (Nechiporuk et al., 2007; Nechiporuk et al., 2005). Although other FGF signals earlier in development are necessary, neither pharyngeal endoderm nor FGF3 are required for initial induction and specification of the epibranchial placodes, suggesting a later function. FGF3 morpholino inhibition results in loss of Ngn1, Phox2a, Phox2b, and Hu, demonstrating that blocking FGF signaling short circuits neuronal differentiation in the epibranchial placodes. In the mouse, an inhibitory form of Fgfr1 in the epibranchial epithelium results in a significant reduction of Ngn and NF (Trokovic et al., 2005). FGF3 is sufficient to induce Phox2a(+) ectopic neurons in wild-type embryos and to rescue Phox2a(+) neurons in mutants lacking endodermal tissue (Nechiporuk et al., 2005). FGF3 is expressed in the pharyngeal endoderm of zebrafish, chick, and mouse immediately adjacent to the presumptive epibranchial placodes.
Consistent with the trigeminal and epibranchial placodes, FGF signaling is essential for instructing neurogenesis and regulating delamination in the otic placode of mouse, chick, and zebrafish (Fig. 3 & Table 1). Distinguishing the roles of signaling families in otic placode development can be complicated due to its multiple derivatives. We have attempted to focus on the region and developmental stages of the otic placode that give rise to the delaminating sensory neurons of the CVG. In zebrafish, LOF experiments utilizing the FGF inhibitor SU5402 during a finite stage in development resulted in a reduction of NeuroD(+) delaminating neuroblasts while otic vesicle specification markers Pax2 and Eya1 were expressed normally (Hammond and Whitfield, 2011). These data support the findings that FGF signaling is required for neurogenesis and delamination during a short critical time window, between 10 to 20 ss, that is distinct from its earlier role in otic induction. Evidence in the FGF-LOF mouse shows consistent downregulation of neurogenic markers in the otocyst (Brown and Epstein, 2011). In the chick, FGF-LOF via chemical inhibition leads to a specific reduction in both neurogenesis and delamination of neuroblasts without affecting otic specification (Abello et al., 2010; Alsina et al., 2004). Of note, despite drastic inhibition of NeuroD in the epithelium of the otic vesicle treated with SU5402, neuroblasts within the CVG continue to express NeuroD. This implies that once neuronal selection occurs from the neural progenitor domain and neuroblasts exit the epithelium, they are no longer dependent upon FGF signaling. NeuroD(+) neuroblasts are fully committed to the neuronal fate, migration to, and proliferation within the CVG (Alsina et al., 2004).
Data from FGF-GOF experiments in the zebrafish using a heat-shock inducible FGF3 (hsp70:FGF3) transgenic line clearly demonstrate that FGF signaling is sufficient for neurogenesis and delamination in the otic vesicle (Hammond and Whitfield, 2011). Delaminating NeuroD(+) neuroblasts are increased in the anteroventral domain and also ectopically expressed from the nonneural posteroventral otic domain. The otic induction/specification genes Pax2a and Eya1 are expressed normally; further indicating that FGF signaling via FGF3 is instructive in neuronal selection and regulation of cell adhesion leading to delamination of neuroblasts from the ectoderm. GOF experiments in the chick by in ovo overexpression of FGF10 with microbeads, or the addition of FGF10 to otic explants increases the number of delaminating cells expressing NeuroD and NeuroM within the proneurosensory domain with no ectopic or aberrant sites of delamination in otic vesicles (Alsina et al., 2004). Interestingly, electroporation of FGF8 into otic placode ectoderm did expand NeuroD(+) expression in the otic cup beyond its normal anteroventral domain. This supports multiple roles for FGFs throughout placode development with different FGF ligands specifying the neurogenic domain while others are distinctly required for the transition of neuronal determination (Abello et al., 2010).
Intriguingly, in hypomorphic Fgfr1 mutants, FGF ligands (FGF3 and FGF15) found in the epibranchial epithelium are also downregulated (Trokovic et al., 2005). This may hint to a possible autoregulatory mechanism within the placodal epithelial niche where initial endodermal FGF3 induces epibranchial ectoderm via Fgfr1, which in turn activates expression of FGF ligands (FGF3 and FGF15) within the placode (Fig. 3). This same mechanism may be occurring with BMP signaling (discussed below) in the placode. Initial BMP signaling originates from surrounding tissues with subsequent upregulation of BMP ligands within the placodal epithelium. It is possible that after initial induction/specification, later events like sensory neurogenesis and delamination may be regulated through autocrine signaling and confined to the placodal niche. This is also supported by studies in the otic placode where the otic vesicle, when isolated, can autonomously produce all inner ear and CVG cell types.
Wnt signaling
Wnt signaling plays varied roles in neurogenic placodes (Table 1 & Fig. 4). Cell-autonomous electroporation of dominant-negative (DN) Tcf/Lef transcription factors of Wnt/ß-catenin pathway in chick embryos prevented opV cells from delaminating from the ectoderm and also from becoming neurons (Lassiter et al., 2007). Cells did not contribute to the trigeminal ganglion but instead remained stalled in the epithelium and did not express neuronal markers. However, different from the FGF and Notch pathways, Wnt signaling is required for maintained expression of the trigeminal specification marker Pax3. We have recently demonstrated that Wnt/ß-catenin signaling directly modulates Pax3 promoter activities (Zhao et al., 2013). After initial induction of the trigeminal placode, though Wnt signaling continues to be essential for maintenance of Pax3 expression, neurogenesis, and delamination at opV, the neural tube source of Wnts is no longer required (Baker et al., 1999; Canning et al., 2008). This confirms early explant experiments, which found Pax3(+) opV placode cells to be committed after the 10 ss (Baker et al., 2002; Baker et al., 1999). It is possible that after initial induction from the neural tube, continued autocrine regulation by Wnt signaling occurs within the epithelium. Wnt signaling GOF within the epithelium alone, however, is likely insufficient for opV induction or neurogenesis. In chick, cell-autonomous constitutive intracellular activation of canonical Wnt signaling in placodal ectoderm showed no difference from the wild-type; the placode domain was not expanded, the number of opV-derived trigeminal neurons did not increase, and ectopic neurogenesis was not observed (Lassiter et al., 2007). However, misexpression of Wnt signaling into the isthmus of the neural tube lead to increased and premature delamination and neuronal differentiation (Canning et al., 2008). In placode explant cultures, activation of Wnt signaling also resulted in neurogenesis but when FGF signaling was simultaneously chemically inhibited, Wnt activation failed to upregulate the neuronal gene Isl1 (Canning et al., 2008). Interestingly, premature differentiation was seen in both the opV and mmV branches, providing the first insight into the signaling regulation of the mmV placode. Taken together, these experiments indicate that the gain-of-function of Wnt signaling may indirectly act through the isthmic FGF signaling to promote trigeminal placode neurogenesis and delamination.
Spatiotemporal gene expression of chick Frizzled receptors has been observed in the epibranchial ectoderm after specification and segregation of the placodes at a time coincident with neurogenesis, delamination, and differentiation (Stark et al., 2000). However, there are few studies that address the role of Wnt signaling in these epibranchial placodes. In chick and mouse, modulation of Wnt signaling in the posterior placodal region prior to neurogenesis distinguishes otic and epibranchial fates, with Wnt activation resulting in otic competence and Wnt inhibition in epibranchial precursors (Freter et al., 2008; Ohyama et al., 2006). Conditional ß-catenin-LOF;Pax2-Cre mouse embryos showed a significant reduction of NeuroD in the epibranchial placodes, whereas in these same mice NeuroD expression in delaminated neuroblasts originating from the otic vesicle was unaltered (Ohyama et al., 2006). Genetic activation of Wnt signaling in mouse otic vesicle blocked neurogenesis. In ß-catenin-GOF;Foxg1-Cre mutants, expression of Ngn1 and NeuroD was reduced at E9.5 and lost by E10.5 in the otic vesicle (Freyer and Morrow, 2010). In ß-catenin-GOF;Pax2-Cre mutants NeuroD expression was also significantly reduced (Ohyama et al., 2006). Mouse embryos cultured in the Wnt signaling agonist LiCl also showed a consistent and profound downregulation of Ngn1 in the anterior otocyst (Brown and Epstein, 2011).
Although little is known for the role of Wnt signaling in olfactory placode neurogenesis and delamination, conditional ablation of ß-catenin with Foxg1-Cre caused dramatic loss of the upper jaw and nasal primordia as a possible consequence of FGF8 inactivation in the anterior neural ridge and facial ectoderm during early embryogenesis (Wang et al., 2011a). Molecular biological approaches demonstrated that FGF8 is a transcriptional target of Wnt/ß-catenin signaling, indicating that Wnt signaling may act through FGF signaling to regulate olfactory placodal formation and specification (Fig. 4). This is in line with the possible regulatory loop of Wnt/FGF signaling for the trigeminal placodal specification and neurogenesis (Canning et al., 2008). At a later developmental stage, a mouse transgenic approach revealed that the Wnt/ß-catenin signaling reporter TOPeGFP was predominantly activated in the olfactory epithelial stem cells and/or sensory neural progenitors, and in vitro approaches demonstrated a critical role of Wnt signaling in olfactory sensory neurogenesis (Wang et al., 2011c).
Fig. 4.
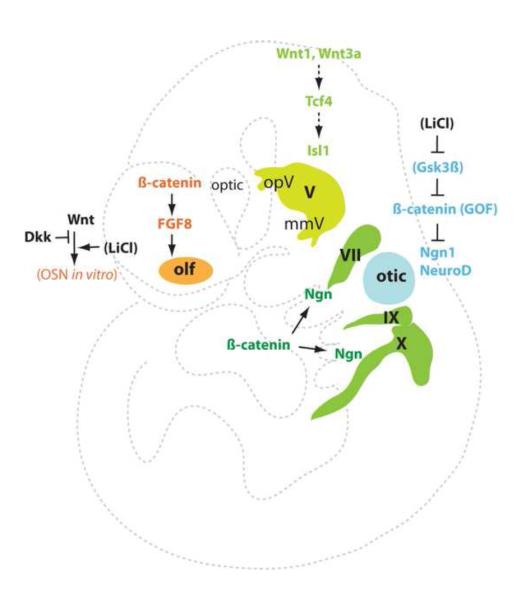
Experimentally verified Wnt signaling in placode neurogenesis. Wnt1/3a-Tcf4 signaling promotes trigeminal (V) placodal neurogesis in chicks, but ß-catenin signaling represses otic placode neurogenesis in mice. Wnt/ß-catenin signaling may enhance neurogenesis in the epibranchial placodes and regulate FGF signaling in the olfactory placode in vivo, and also promote olfactory epithelial neurogenesis in vitro. The role of Wnt signaling in delamination remains unclear. The pharmacological agonist lithium ion shown in brackets is a Gsk3ß inhibitor preventing ß-catenin degradation.
In sum, evidence from the various placodes from different species implies that Wnt signaling may not have a conserved role during placode neurogenesis and delamination or that Wnt signaling may regulate FGF signaling and play context-dependent roles in neurogenic placodes.
BMP signaling
BMP signaling also plays varied roles in different neurogenic placodes (Table 1 & Fig. 5). Evidence in chick and zebrafish indicate a key role for the BMP signaling in epibranchial neurogenesis. Early LOF in vitro experiments in chick epibranchial ectoderm explants show that BMP inhibition via follistatin beads prevented NF expression (Begbie et al., 1999). Genetic LOF in vivo studies in the zebrafish snailhouse BMP7 mutant also report a severe reduction in NeuroD(+) epibranchial neurons (Holzschuh et al., 2005). Another study in chick specifically addressed the role of the BMP pathway after initial induction and specification of the epibranchial placodes (Kriebitz et al., 2009). Inhibition of BMP4 through the inhibitor PRDC led to a loss of neurogenic markers Dll1, Phox2a, NeuroM, and HuC but did not affect the expression of the Pax2 epibranchial induction marker.
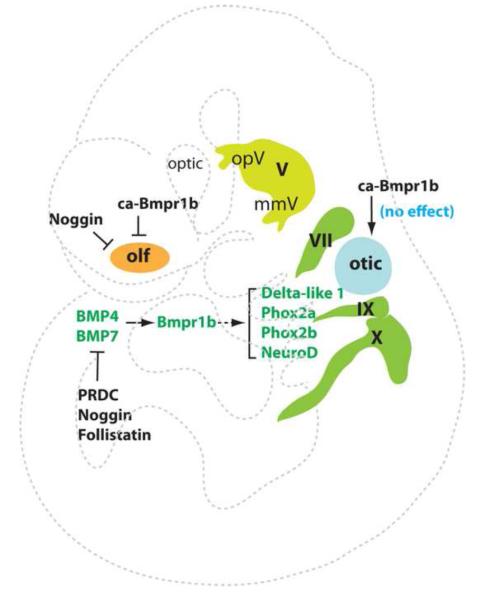
Fig. 5.
Experimentally verified BMP signaling in neurogenic placodes. BMP4/7-Bmpr1b signaling exerts positive roles in the epibranchial neurogenesis, while constitutively active (ca) Bmpr1b (ALK3) shows no effects on the otic placode, and a negative role in the olfactory placode neurogenesis or delamination. The inhibitory proteins PRDC, Follistatin, and Noggin repress the epibranchial neurogenesis, and Noggin also represses the olfactory neurogenesis.
In chick GOF in vitro epibranchial explant experiments, BMP7 is sufficient to upregulate Phox2a and NF (Begbie et al., 1999). This suggests an important role for BMP signaling in epibranchial neurogenesis and also hints at the neurogenic potential contained within the placodal epithelium. At this stage, in the chick, the ectoderm activated by BMP signaling contains all necessary components to produce differentiated neurons. Consistent with this, misexpression of constitutively active BMP-receptor1 elicits increased NeuroD expression and also significant delamination of these cells from the epithelium, indicating BMP signaling is key in directing both neurogenesis and delamination (Tripathi et al., 2009). Interestingly, responsiveness is restricted to the epibranchial specified ventrocaudal ectoderm suggesting a distinct post-specification role for BMP. This correlates with findings in zebrafish where BMP beads (GOF) induce ectopic neurogenesis but only within the branchial ectoderm (Holzschuh et al., 2005). Also in chick, misexpression of BMP4-GOF results in increased neurogenesis; together with LOF studies these data suggest that BMP7, emanating from the endoderm, initiates the neurogenic field in the epibranchial placodes and that BMP4 (constrained by PRDC) is then upregulated and in turn directs neurogenesis and delamination of placodal neurons through autocrine signaling within the ectoderm (Kriebitz et al., 2009).
BMP signaling does not seem to have a critical impact on otic placode sensory neurogenesis. GOF experiments in the chick, where high levels of BMP were expressed in the otic ectoderm had no affect on neural fate acquisition (Abello et al., 2010). The authors indicate that BMP activity may be positively regulating neurogenesis as seen in the epibranchial placodes instead of inhibiting neuronal fate in the otic as hypothesized.
In the olfactory placode BMP activity has previously been shown to play a key role in early specification of the placode and neuronal differentiation in the sensory epithelium (Maier et al., 2010; Shou et al., 2000; Shou et al., 1999; Sjodal et al., 2007). Recent evidence in chick, examining the distinct effects of BMP signaling on migratory neurons from the olfactory epithelium revealed that both LOF and GOF of BMP signaling in ovo electroporation experiments reduce the number of migratory neurons (Maier et al., 2011). Interestingly, inhibition of BMP signaling reduced the determination of neurons, but did not affect the ability of the neuroblasts to delaminate from the olfactory epithelium, whereas, elevated BMP activity in the epithelium suppressed neuroblast delamination, thereby reducing the number of migratory neurons.
Conclusion & Prospective
We have discussed the important roles of the Notch, FGF, Wnt, and BMP pathways in placodal sensory neurogenesis and the morphological changes allowing for delamination of neuroblasts from the placodal epithelium. A core signaling program, the attenuated Notch and activated FGF, is likely conserved for neurogenesis and delamination in all neurogenic placodes. Wnt and BMP pathways play varied roles in placode neurogenesis and delamination. Interestingly, a different combination of signaling activities, the attenuated BMP and perhaps Wnt, together with the activated FGF, is required for pre-placodal region formation (Ahrens and Schlosser, 2005; Kwon et al., 2010; Litsiou et al., 2005). A recent study with human embryonic stem cells (ESCs) supported the importance of attenuated BMP signaling, where only certain concentrations of BMP promoted transient expression of pre-placodal ectoderm (PPE) marker expression, while subsequent modulation of this and other signals specified region-specific placode marker expression (Leung et al., 2013). Once the PPE is established, Wnt signaling may regulate FGF signaling in regional placode specification, ultimately leading to modulation of Notch signaling during the final step toward neuronal differentiation and delamination. Additionally, other signaling pathways not discussed here may play pivotal roles in differentiation. For example, an important role for retinoic acid signaling was recently described in the early steps of olfactory neurogenesis (Paschaki et al., 2013), and hedgehog signaling was shown to help regulate regional identity in human ESCs that had been previously programmed toward a PPE identity (Leung et al., 2013). How these pathways interact during the several stages of placode development remains poorly understood. Significantly, most of these signaling pathways are critical regulators of tissue/organ-specific stem cells including neural stem cells (Li and Clevers, 2010; Wang et al., 2011b), and Wnt/ß-catenin, Shh, Notch, and FGF signaling pathways are likely integrated by Gsk3 proteins in CNS neural stem cell/progenitor homeostasis (Kim et al., 2009). Future studies may uncover the interactive and integrative mechanisms among these signaling pathways during placode neurogenesis and delamination.
Though a vast number of studies have investigated the effect of these signaling pathways on neuronal genes, very few have addressed the genes regulating the cell adhesion changes simultaneously occurring. Placodes have a great potential as a key model for investigating the conserved regulatory pathways involved in changes in cell adhesion during neurogenesis. The coupling of neurogenesis and delamination is observed not only in placodes but also during development of the neuroepithelium of the CNS, neural stem cells, and in metastatic cancer. During spinal cord motor neuron development, Foxp proteins concomitantly regulate both neurogenesis and detachment of the neural progenitor cells from the neuroepithelium (Rousso et al., 2012). In neural stem cell cultures isolated from the adult olfactory bulb an increase in neurogenic gene expression and migration is observed when exposed to FGF2 (Vergano-Vera et al., 2009). In colorectal cancer, a higher degree of neurogenesis occurs with highly metastatic cells and is an indicator of cancer progression and outcome (Albo et al., 2011).
There are a few likely cell adhesion candidate genes that may work via these same signaling pathways to play a role during neuroblast delamination in the placodes. Intriguingly, ß-catenin exerts dual roles in Wnt signaling and in cell adhesion. Conditional ablation of ß-catenin with hGFAP-Cre mice disrupted both Pax6 signaling and ventricular organization of the cortical radial glia/neural stem cells (Gan et al., 2013). Nevertheless, it remains unclear whether ß-catenin plays a direct role in placode cell delamination. Several important molecules, such as ephrins, integrins, tetraspanins, and cadherins, have all been implicated in signaling and cell adhesion, and in the delamination process (Babb-Clendenon et al., 2006; Davies, 2007; Hong et al., 2012; McCabe and Bronner, 2011; Saeger et al., 2011). Their role in neural crest cell EMT and migration has been studied significantly (Kerosuo and Bronner-Fraser, 2012) more than in the placodes, where the most comprehensive works on placode cell delamination are primarily descriptive (Graham et al., 2007; Shiau et al., 2011). These molecules are potential downstream effectors or interactive partners of the signaling pathways during neurogenic placodal development. Indeed, several signaling pathways discussed above have been shown to interact with integrins, tetraspanins, ephrins, and cadherins to regulate cell adhesion (Bhat and Riley, 2011; Chong et al., 2000; Glazier et al., 2008; Karsan, 2008; Kerosuo and Bronner-Fraser, 2012; Saravanamuthu et al., 2009; Toledo et al., 2005). The placode model is well suited to investigate general questions of delamination, including cell adhesion changes, basal lamina breakdown, and cell motility. It is also an ideal system to investigate the molecular links between delamination and differentiation. Such a link was proposed for the role of FGF signaling in trigeminal placode cells (Lassiter et al., 2009), and was observed in the same placode after Notch inhibition, where enhanced neurogenesis occurred concomitantly with epithelial fragmentation (Lassiter et al., 2010). Future investigations can address the untapped potential of cranial placodes in revealing the mechanisms regulating both neurogenesis and cell adhesion and possibly a conserved role in CNS and PNS neuronal development, including neural stem cells and neural crest cells, and in metastatic cancers.
Highlights.
▶ A core signaling program exists throughout the neurogenic placodes.
▶ Attenuated Notch signaling initiates placode neurogenesis and delamination.
▶ Active FGF signaling also triggers placode neurogenesis and delamination.
▶ Wnt and BMP pathways exert context-dependent roles in neurogenic placodes.
Acknowledgements
This work was supported by the NIH/NIDCR (R01DE021696 to CZ and R01HD046475 to MS) and the Shriners Hospitals for Children (86100 & 87500 to CZ). We thank Yongping Wang and Jacob Voelkel for their contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abello G, Alsina B. Establishment of a proneural field in the inner ear. Int J Dev Biol. 2007;51:483–93. doi: 10.1387/ijdb.072343ga. [DOI] [PubMed] [Google Scholar]
- Abello G, Khatri S, Radosevic M, Scotting PJ, Giraldez F, Alsina B. Independent regulation of Sox3 and Lmx1b by FGF and BMP signaling influences the neurogenic and non-neurogenic domains in the chick otic placode. Dev Biol. 2010;339:166–78. doi: 10.1016/j.ydbio.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sense-organ development. Development. 1998;125:4645–54. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Ahrens K, Schlosser G. Tissue and signals invovled in the induction of placodal Six1 expression in Xenopus laevis. Dev Biol. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Albo D, Akay CL, Marshall CL, Wilks JA, Verstovsek G, Liu H, Agarwal N, Berger DH, Ayala GE. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117:4834–45. doi: 10.1002/cncr.26117. [DOI] [PubMed] [Google Scholar]
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–34. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Babb-Clendenon S, Shen YC, Liu Q, Turner KE, Mills MS, Cook GW, Miller CA, Gattone VH, 2nd, Barald KF, Marrs JA. Cadherin-2 participates in the morphogenesis of the zebrafish inner ear. J Cell Sci. 2006;119:5169–77. doi: 10.1242/jcs.03299. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–17. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–56. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Bronner-Fraser M. Pax3-expressing trigeminal placode cells can localize to trunk neural crest sites but are committed to a cutaneous sensory neuron fate. Dev Biol. 2002;249:219–36. doi: 10.1006/dbio.2002.0767. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–56. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131:4119–30. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–11. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Begbie J, Brunet JF, Rubenstein JL, Graham A. Induction of the epibranchial placodes. Development. 1999;126:895–902. doi: 10.1242/dev.126.5.895. [DOI] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306:309–16. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Bhat N, Riley BB. Integrin-alpha5 coordinates assembly of posterior cranial placodes in zebrafish and enhances FGF-dependent regulation of otic/epibranchial cells. PLoS One. 2011;6:e27778. doi: 10.1371/journal.pone.0027778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blentic A, Chambers D, Skinner A, Begbie J, Graham A. The formation of the cranial ganglia by placodally-derived sensory neuronal precursors. Mol Cell Neurosci. 2011;46:452–9. doi: 10.1016/j.mcn.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Brown AS, Epstein DJ. Otic ablation of smoothened reveals direct and indirect requirements for Hedgehog signaling in inner ear development. Development. 2011;138:3967–76. doi: 10.1242/dev.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning CA, Lee L, Luo SX, Graham A, Jones CM. Neural tube derived Wnt signals cooperate with FGF signaling in the formation and differentiation of the trigeminal placodes. Neural Dev. 2008;3:35. doi: 10.1186/1749-8104-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PR, Silver J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J Comp Neurol. 1983;215:359–69. doi: 10.1002/cne.902150402. [DOI] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, Daar IO. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol Cell Biol. 2000;20:724–34. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covell DA, Jr., Noden DM. Embryonic development of the chick primary trigeminal sensory-motor complex. J Comp Neurol. 1989;286:488–503. doi: 10.1002/cne.902860407. [DOI] [PubMed] [Google Scholar]
- Croucher SJ, Tickle C. Characterization of epithelial domains in the nasal passages of chick embryos: spatial and temporal mapping of a range of extracellular matrix and cell surface molecules during development of the nasal placode. Development. 1989;106:493–509. doi: 10.1242/dev.106.3.493. [DOI] [PubMed] [Google Scholar]
- d'Amico-Martel A, Noden DM. An autoradiographic analysis of the development of the chick trigeminal ganglion. J Embryol Exp Morphol. 1980;55:167–82. [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. Am J Anat. 1983;166:445–68. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–82. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–48. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–94. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Geuna S, Fasolo A, Giacobini-Robecchi MG. HuC/D confocal imaging points to olfactory migratory cells as the first cell population that expresses a post-mitotic neuronal phenotype in the chick embryo. Neuroscience. 2003;122:123–8. doi: 10.1016/j.neuroscience.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Forni PE, Wray S. Neural crest and olfactory system: new prospective. Mol Neurobiol. 2012;46:349–60. doi: 10.1007/s12035-012-8286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter S, Fleenor SJ, Freter R, Liu KJ, Begbie J. Cranial neural crest cells form corridors prefiguring sensory neuroblast migration. Development. 2013;140:3595–600. doi: 10.1242/dev.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter S, Muta Y, Mak SS, Rinkwitz S, Ladher RK. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development. 2008;135:3415–24. doi: 10.1242/dev.026674. [DOI] [PubMed] [Google Scholar]
- Freyer L, Morrow BE. Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev Dyn. 2010;239:1708–22. doi: 10.1002/dvdy.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer L, Aggarwal V, Morrow BE. Dual embryonic origin of the mammalian otic vesicle forming the inner ear. Developent. 2011;138:5403–5414. doi: 10.1242/dev.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q, Lee A, Suzuki R, Yamagami T, Stokes A, Nguyen B, Pleasure D, Wang J, Chen HW, Zhou CJ. Pax6 mediates ß-catenin signaling for self-renewal and neurogenesis by neocortical radial glial stem cells. Stem Cells. 2013 doi: 10.1002/stem.1561. doi:10.1002/stem.1561 [pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier JA, Zhang Y, Swat M, Zaitlen B, Schnell S. Coordinated action of N-CAM, N-cadherin, EphA4, and ephrinB2 translates genetic prepatterns into structure during somitogenesis in chick. Curr Top Dev Biol. 2008;81:205–47. doi: 10.1016/S0070-2153(07)81007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Blentic A, Duque S, Begbie J. Delamination of cells from neurogenic placodes does not involve an epithelial-to-mesenchymal transition. Development. 2007;134:4141–5. doi: 10.1242/dev.02886. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–44. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–24. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- Hammond KL, Whitfield TT. FGF and Hh signalling act on a symmetrical pre-pattern to specify anterior and posterior identity in the zebrafish otic placode and vesicle. Development. 2011;138:3977–87. doi: 10.1242/dev.066639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Barlow LA. Embryonic origin of gustatory cranial sensory neurons. Dev Biol. 2007;310:317–28. doi: 10.1016/j.ydbio.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15792–7. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, Schilling TF. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–42. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Hong IK, Jeoung DI, Ha KS, Kim YM, Lee H. Tetraspanin CD151 stimulates adhesion-dependent activation of Ras, Rac, and Cdc42 by facilitating molecular association between beta1 integrins and small GTPases. J Biol Chem. 2012;287:32027–39. doi: 10.1074/jbc.M111.314443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–51. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic regulation of Notch signaling in neural progenitor cells. Curr Opin Cell Biol. 2009;21:733–40. doi: 10.1016/j.ceb.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Karsan A. Notch and integrin affinity: a sticky situation. Sci Signal. 2008;1:pe2. doi: 10.1126/stke.12pe2. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Beites CL, Crocker CE, Wu HH, Bonnin A, Murray R, Calof AL. Molecular signals regulating proliferation of stem and progenitor cells in mouse olfactory epithelium. Dev Neurosci. 2004;26:166–80. doi: 10.1159/000082135. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. FGF8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Kerosuo L, Bronner-Fraser M. What is bad in cancer is good in the embryo: importance of EMT in neural crest development. Semin Cell Dev Biol. 2012;23:320–32. doi: 10.1016/j.semcdb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–7. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebitz NN, Kiecker C, McCormick L, Lumsden A, Graham A, Bell E. PRDC regulates placode neurogenesis in chick by modulating BMP signalling. Dev Biol. 2009;336:280–92. doi: 10.1016/j.ydbio.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6:e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher RK, O'Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–85. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Lassiter RN, Ball MK, Adams JS, Wright BT, Stark MR. Sensory neuron differentiation is regulated by notch signaling in the trigeminal placode. Dev Biol. 2010;344:836–48. doi: 10.1016/j.ydbio.2010.05.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter RN, Dude CM, Reynolds SB, Winters NI, Baker CV, Stark MR. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter RN, Reynolds SB, Marin KD, Mayo TF, Stark MR. FGF signaling is essential for ophthalmic trigeminal placode cell delamination and differentiation. Dev Dyn. 2009;238:1073–82. doi: 10.1002/dvdy.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Kent Morest D, Li JY. Differential BMP signaling controls formation and differentiation of multipotent preplacodal ectoderm progenitors from human embryonic stem cells. Dev Biol. 2013;379:208–20. doi: 10.1016/j.ydbio.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–62. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Liu Z, Owen T, Fang J, Zuo J. Overactivation of Notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PLoS One. 2012;7:e34123. doi: 10.1371/journal.pone.0034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier E, Gunhaga L. Dynamic expression of neurogenic markers in the developing chick olfactory epithelium. Dev Dyn. 2009;238:1617–25. doi: 10.1002/dvdy.21966. [DOI] [PubMed] [Google Scholar]
- Maier E, Nord H, von Hofsten J, Gunhaga L. A balance of BMP and notch activity regulates neurogenesis and olfactory nerve formation. PLoS One. 2011;6:e17379. doi: 10.1371/journal.pone.0017379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier E, von Hofsten J, Nord H, Fernandes M, Paek H, Hebert JM, Gunhaga L. Opposing FGF and BMP activities regulate the specification of olfactory sensory and respiratory epithelial cell fates. Development. 2010;137:1601–11. doi: 10.1242/dev.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Bronner M. Tetraspanin, CD151, is required for maintenance of trigeminal placode identity. J Neurochem. 2011;117:221–30. doi: 10.1111/j.1471-4159.2011.07190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe KL, Sechrist JW, Bronner-Fraser M. Birth of ophthalmic trigeminal neurons initiates early in the placodal ectoderm. J Comp Neurol. 2009;514:161–73. doi: 10.1002/cne.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza AS, Breipohl W, Miragall F. Cell migration from the chick olfactory placode: a light and electron microscopic study. J Embryol Exp Morphol. 1982;69:47–59. [PubMed] [Google Scholar]
- Moody SA, Quigg MS, Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotype-specific anti-beta-tubulin monoclonal antibody. J Comp Neurol. 1989a;279:567–80. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- Moody SA, Quigg MS, Little CD. Extracellular matrix components of the peripheral pathway of chick trigeminal axons. J Comp Neurol. 1989b;283:38–53. doi: 10.1002/cne.902830105. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Poss KD, Raible DW. Specification of epibranchial placodes in zebrafish. Development. 2007;134:611–23. doi: 10.1242/dev.02749. [DOI] [PubMed] [Google Scholar]
- Nechiporuk A, Linbo T, Raible DW. Endoderm-derived FGF3 is necessary and sufficient for inducing neurogenesis in the epibranchial placodes in zebrafish. Development. 2005;132:3717–30. doi: 10.1242/dev.01876. [DOI] [PubMed] [Google Scholar]
- Nichols DH. Mesenchyme formation from the trigeminal placodes of the mouse embryo. Am J Anat. 1986;176:19–31. doi: 10.1002/aja.1001760103. [DOI] [PubMed] [Google Scholar]
- Nikaido M, Doi K, Shimizu T, Hibi M, Kikuchi Y, Yamasu K. Initial specification of the epibranchial placode in zebrafish embryos depends on the fibroblast growth factor signal. Dev Dyn. 2007;236:564–71. doi: 10.1002/dvdy.21050. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Taste buds: development and evolution. Brain Behav Evol. 2004;64:198–206. doi: 10.1159/000079747. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development. 2006;133:865–75. doi: 10.1242/dev.02271. [DOI] [PubMed] [Google Scholar]
- Pan W, Jin Y, Stanger B, Kiernan AE. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A. 2010;107:15798–803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschaki M, Cammas L, Muta Y, Matsuoka Y, Mak SS, Rataj-Baniowska M, Fraulob V, Dolle P, Ladher RK. Retinoic acid regulates olfactory progenitor cell fate and differentiation. Neural Dev 8, 13. 2013 doi: 10.1186/1749-8104-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousso DL, Pearson CA, Gaber ZB, Miquelajauregui A, Li S, Portera-Cailliau C, Morrisey EE, Novitch BG. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron. 2012;74:314–30. doi: 10.1016/j.neuron.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Saeger BM, Suhm M, Neubuser A. Ephrin/ephrin receptor expression during early stages of mouse inner ear development. Dev Dyn. 2011;240:1578–85. doi: 10.1002/dvdy.22632. [DOI] [PubMed] [Google Scholar]
- Saravanamuthu SS, Gao CY, Zelenka PS. Notch signaling is required for lateral induction of Jagged1 during FGF-induced lens fiber differentiation. Dev Biol. 2009;332:166–76. doi: 10.1016/j.ydbio.2009.05.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–51. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Making senses development of vertebrate cranial placodes. Int Rev Cell Mol Biol. 2010;283:129–234. doi: 10.1016/S1937-6448(10)83004-7. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Northcutt RG. Development of neurogenic placodes in Xenopus laevis. J Comp Neurol. 2000;418:121–46. [PubMed] [Google Scholar]
- Schneider-Maunoury S, Pujades C. Hindbrain signals in otic regionalization: walk on the wild side. Int J Dev Biol. 2007;51:495–506. doi: 10.1387/ijdb.072345ss. [DOI] [PubMed] [Google Scholar]
- Schwarting GA, Gridley T, Henion TR. Notch1 expression and ligand interactions in progenitor cells of the mouse olfactory epithelium. J Mol Histol. 2007;38:543–53. doi: 10.1007/s10735-007-9110-9. [DOI] [PubMed] [Google Scholar]
- Shiau CE, Das RM, Storey KG. An effective assay for high cellular resolution time-lapse imaging of sensory placode formation and morphogenesis. BMC Neurosci. 2011;12:37. doi: 10.1186/1471-2202-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Shou J, Murray RC, Rim PC, Calof AL. Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development. 2000;127:5403–13. doi: 10.1242/dev.127.24.5403. [DOI] [PubMed] [Google Scholar]
- Shou J, Rim PC, Calof AL. BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor. Nat Neurosci. 1999;2:339–45. doi: 10.1038/7251. [DOI] [PubMed] [Google Scholar]
- Sjodal M, Edlund T, Gunhaga L. Time of exposure to BMP signals plays a key role in the specification of the olfactory and lens placodes ex vivo. Dev Cell. 2007;13:141–9. doi: 10.1016/j.devcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Stainier DY, Gilbert W. Neuronal differentiation and maturation in the mouse trigeminal sensory system, in vivo and in vitro. J Comp Neurol. 1991;311:300–12. doi: 10.1002/cne.903110210. [DOI] [PubMed] [Google Scholar]
- Stark MR, Biggs JJ, Schoenwolf GC, Rao MS. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93:195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Stark MR, Sechrist J, Bronner-Fraser M, Marcelle C. Neural tube-ectoderm interactions are required for trigeminal placode formation. Development. 1997;124:4287–95. doi: 10.1242/dev.124.21.4287. [DOI] [PubMed] [Google Scholar]
- Sun SK, Dee CT, Tripathi VB, Rengifo A, Hirst CS, Scotting PJ. Epibranchial and otic placodes are induced by a common FGF signal, but their subsequent development is independent. Dev Biol. 2007;303:675–86. doi: 10.1016/j.ydbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Tarozzo G, Peretto P, Fasolo A. Cell migration from the olfactory placode and the ontogeny of the neuroendocrine compartments. Zool Sci. 1995;12:367–383. doi: 10.2108/zsj.12.367. [DOI] [PubMed] [Google Scholar]
- Toledo MS, Suzuki E, Handa K, Hakomori S. Effect of ganglioside and tetraspanins in microdomains on interaction of integrins with fibroblast growth factor receptor. J Biol Chem. 2005;280:16227–34. doi: 10.1074/jbc.M413713200. [DOI] [PubMed] [Google Scholar]
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Tripathi VB, Ishii Y, Abu-Elmagd MM, Scotting PJ. The surface ectoderm of the chick embryo exhibits dynamic variation in its response to neurogenic signals. Int J Dev Biol. 2009;53:1023–33. doi: 10.1387/ijdb.082780vt. [DOI] [PubMed] [Google Scholar]
- Trokovic N, Trokovic R, Partanen J. Fibroblast growth factor signalling and regional specification of the pharyngeal ectoderm. Int J Dev Biol. 2005;49:797–805. doi: 10.1387/ijdb.051976nt. [DOI] [PubMed] [Google Scholar]
- Vergano-Vera E, Mendez-Gomez HR, Hurtado-Chong A, Cigudosa JC, Vicario-Abejon C. Fibroblast growth factor-2 increases the expression of neurogenic genes and promotes the migration and differentiation of neurons derived from transplanted neural stem/progenitor cells. Neuroscience. 2009;162:39–54. doi: 10.1016/j.neuroscience.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Verwoerd CD, van Oostrom CG, Verwoerd-Verhoef HL. Otic placode and cephalic neural crest. Acta Otolaryngol. 1981;91:431–5. doi: 10.3109/00016488109138524. [DOI] [PubMed] [Google Scholar]
- Wang Y, Song L, Zhou CJ. The canonical Wnt/beta-catenin signaling pathway regulates FGF signaling for early facial development. Dev Biol. 2011a;349:250–60. doi: 10.1016/j.ydbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Plane JM, Jiang P, Zhou CJ, Deng W. Concise review: Quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells. 2011b;29:907–12. doi: 10.1002/stem.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao T, Hamad S, Lott P, Schnittke N, Schwob JE, Zhou CJ. Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci. 2011c;124:1553–63. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Dev Biol. 2008;317:174–86. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Zhao T, Gan Q, Stokes A, Lassiter RNT, Wang Y, Chan J, Han JX, Pleasure DE, Epstein JA, Zhou CJ. ß-catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation. Development. 2013 doi: 10.1242/dev.101550. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]