Abstract
Background
Twin studies of veterans and adults suggest that approximately 30–46% of the variance in posttraumatic stress disorder (PTSD) is attributable to genetic factors. The remaining variance is attributable to the non-shared environment, which, by definition, includes combat exposure. This study used a gene by measured environment twin design to examine if the effect of genetic and environmental factors that contribute to the etiology PTSD were dependent on level of combat exposure.
Methods
The sample was drawn from the Vietnam Era Twin Registry and included 620 male-male twin pairs who served in the U.S. Military in South East Asia during the Vietnam War era. Analyses were based on data from a clinical diagnostic interview of lifetime PTSD symptoms and a self-report measure of combat exposure.
Results
Biometric modeling revealed that the effect of genetic and non-shared environment factors on PTSD varied as a function of level of combat exposure such that the association between these factors and PTSD was stronger at higher levels of combat exposure.
Conclusions
Combat exposure may act as a catalyst that augments the impact of hereditary and environmental contributions to PTSD. Individuals with the greatest exposure to combat trauma were at increased risk for PTSD as a function of both genetic and other environmental factors. Additional work is needed to determine the biological and environmental mechanisms driving these associations.
The diagnosis of posttraumatic stress disorder (PTSD) requires exposure to a traumatic experience by definition (APA, 1994), with the implication being that trauma exposure exerts a direct causal effect on the development of the disorder. However, there is also evidence that individual differences in heritable factors affect risk for PTSD. Specifically, several twin studies focused on veteran and adult samples have suggested that approximately 30–46% of the variance in PTSD is attributable to genetic factors (True et al. 1993; Xian et al. 2000; Stein et al. 2002; Scherrer et al. 2008; Sartor et al. 2012), while one study of young adult women found that 72% of the variance in PTSD was attributable to genetic factors (Sartor et al. 2011). Twin studies also suggest that aspects of the environment that are not shared across members of a twin pair (e.g., severity and type of trauma exposure) tend to contribute the majority of the variance in risk for PTSD (True et al. 1993; Stein et al. 2002; Sartor et al. 2012).
The effects of combat exposure have been extensively studied among veteran populations. Twin studies suggest that traumatic events that occur in the context of combat exposure partially explain the prevalence and chronicity of PTSD (Goldberg et al. 1990; Roy-Byrne et al. 2004; Gilbertson et al. 2010) and also contribute to the development of other conditions such as depression and substance-dependence (Koenen et al. 2003). As not all individuals exposed to the same traumatic combat events will develop PTSD, it is likely that combat experiences interact with other individual-difference characteristics to affect risk for PTSD and other psychological disorders. One possibility is that combat exposure interacts with an individual’s genetic vulnerability for PTSD to increase risk for the disorder.
Gene by environment interaction (G X E), sometimes referred to as G X E interplay, exists when the effects of a person’s genotype on risk for psychopathology is at least partially dependent on the environment. G X E implies that individuals with genetic risk variants are more sensitive to the negative or positive effects of specific environments than are individuals without the genetic risk (Moffitt et al. 2005). The diathesis-stress model of disease reflects a form of G X E in which a harmful environment interacts with a genetic predisposition towards pathology and increases the risk for the pathology. Thus, the basic concept underlying G X E is that risk for a given trait or disorder is not static, but rather is dependent on the synergy between basic biological and environmental risk factors. The aim of this study was to evaluate the extent to which combat exposure interacts with genetic factors and affects risk for PTSD. We examined this in a sample of U.S. military monozygotic (MZ) and dizygotic (DZ) twins who served in South East Asia during the Vietnam War era.
There are two competing hypotheses to consider regarding the potential effects of combat exposure on the heritability of PTSD. First, exposure to combat may increase the heritability of PTSD if it causes underlying differences in genetic risk variants to exert their pathological effects. This is the classic example of diathesis-stress wherein the genetic vulnerability is manifested only under certain environmental conditions. For example, G X E research suggests that the effect of single nucleotide polymorphisms (SNPs) in the gene FK506 binding protein 5 (FKBP5) on PTSD is dependent on severity of childhood abuse (Binder et al. 2008; Xie et al. 2010), such that the effect is only evident under conditions of abuse. Similar genotype by trauma effects have been obtained for a number of other genes in predicting PTSD, including the serotonin transporter (SLC6A4; Xie et al. 2009), catechol-O-methyltransferase (COMT; Kolassa, et al. 2010) and gammaaminobutyric acid receptor, alpha-2 (GABRA2; Nelson et al. 2009). In these examples, the association between the genotype and PTSD is dependent on exposure to trauma such that the effect is stronger among those exposed to higher levels of trauma.
Alternatively, exposure to combat may decrease the heritability of PTSD. This might occur if the combat experience is so severely traumatic that it overrides the effects of genes (and perhaps other individual difference factors, see below) such that virtually everyone so exposed would develop the disorder (Jang et al. 2007). As proof-of-principle, consider the heritability of height: although adult height is highly heritable (e.g., Dubois et al. 2012), its heritability is decreased significantly under conditions of malnourishment such that lack of adequate nutrition (an environmental variable) becomes a primary contributor to height (Silventoinen, 2003). Similarly, the heritability of intelligence has been shown to be moderated by socioeconomic status among children such that genes contribute negligibly to intelligence under conditions of poverty but contribute the majority of the variance under conditions of affluence (Turkheimer et al. 2003). In theory, a similar relationship could exist between combat exposure, genes, and risk for PTSD.
Just as individual-level genetic vulnerabilities may interact with the effects of combat exposure to increase or decrease the risk of PTSD, it is also possible that the effect of the environment on risk for PTSD may vary as a function of combat exposure (i.e., E X E). G X E twin studies provide the opportunity to evaluate if the effects of unmeasured environmental factors (reflected as the common environment and non-shared environment) are dependent on an observed environmental variable. The effects of combat exposure could increase the importance of other environmental variables that have previously been shown to affect risk for PTSD. For example, exposure to combat may amplify the risk for PTSD that is associated with exposure to early childhood trauma (i.e., a childhood trauma by combat exposure interaction). This could manifest in greater severity of childhood trauma-related PTSD symptoms in those exposed to subsequent combat trauma. Alternatively, it is conceivable that combat exposure could decrease the importance of other environmental factors because combat exposure trumps other environmental variables in importance for determining risk for PTSD (e.g., in the same way that extreme exposure might override any genetic effects). In sum, there are reasons to hypothesize that combat exposure may either increase or decrease the strength of the genetic and/or environmental pathways that predict PTSD. This study allowed us to test these competing hypotheses about the effects of measured combat exposure on the genetic and environmental etiology of PTSD.
Method
Participants
This sample was drawn from the larger, nationally-representative Vietnam Era Twin Registry (VETR), which began with 14,738 male-male twin pairs born between 1939 and 1957 who served in the U.S. military during the Vietnam War era (see Tsai et al. 2012). The data analyzed in this study were collected as part of the 1992 Harvard Twin Study of Drug Abuse and Dependence, which included 3,372 complete twin pairs (Tsuang et al. 1996). As we were interested in the moderating role of combat exposure on liability for PTSD, we limited our analyses to twin pairs in which both members served in South East Asia during the Vietnam War (and thus had the potential to be exposed to combat; n = 1240 veterans, comprised of 394 MZ and 226 DZ twin pairs). The mean age of this subsample was 43 (range: 35–53); self-reported racial identity in the sample was 90.16% White, 4.03% Black, 1.77% Hispanic, 0.81% Native American, 0.32% Asian American or Pacific Islander, and 2.90% other. Fourteen percent met DSM-III-R (APA, 1987) criteria for lifetime PTSD (12.9% of MZ and 14.8% of DZ twins).
Procedure and Measures
Diagnostic interviews were completed over the telephone using the Mental Health Diagnostic Interview Schedule (Version III—Revised; DIS–III–R; Robins et al. 1998), as described in greater detail by Koenen et al. (2002) and Lyons et al. (1998). We evaluated DSM-III-R lifetime symptom counts for PTSD wherein each of the 17 DSM criteria was scored as present or not. Inter-rater reliability for the PTSD diagnosis, as determined by re-interview of 146 participants (with a mean interval of 466 days between assessments), was κ = .54. Given the length of time between assessments, this coefficient reflects both rater agreement and the reliability of the diagnosis.
Combat was assessed using the 18-item Combat Exposure Index which assessed personal history of specific combat experiences (e.g., retrieving dead bodies, receiving incoming fire, being wounded; Janes, et al. 1991). Participants completed this survey by mail. It has previously demonstrated good internal consistency and predictive validity (e.g., association with receipt of military combat medal; Janes et al. 1991). For these analyses, we divided the 18-item measure into three cut-points to reflect no combat exposure (i.e., a score of zero on the measure; n = 325), at or below this sample’s rounded non-zero mean of 4 on the combat exposure index (n = 536), and above the nonzero sample mean on combat exposure (n = 379). This was done for four reasons: (1) because there was low frequency of endorsement of scores in the upper range of the scale; (2) because the use of the three-point scale simplified the computation of the latent moderation variables; (3) because this approach offered a simple way to interpret the interaction results; and (4) because there was no differential strength of association between PTSD and the full combat scale versus the three-point one, suggesting that the use of the shorter-scale did not result in significant loss of information.
Trauma exposure was assessed during the study interview: participants reported on their exposure (yes/no) to a maximum of three self-identified “worst” traumatic events from a pre-defined list of eleven types of traumatic experiences (military combat, rape, physical assault, seeing someone hurt or killed, natural disaster, threat, narrow escape, sudden injury, news of a sudden death, other personal shock, or shock to someone else). Approximately 26% of the sample reported no exposure to combat on the self-report Combat Exposure Index; of these individuals 10% endorsed combat exposure during the telephone interview but not on the self-report measure, 7% endorsed sudden injury, 6% endorsed seeing someone hurt, and 3% endorsed physical assault as the “worst” traumatic event that PTSD symptoms were linked to (other events were endorsed at a prevalence < 3%). Individuals who did not endorse exposure to any traumatic event on the interview were coded as zero on PTSD. Zygosity was determined through a questionnaire and blood-group matching approach that achieved 95% accuracy (Eisen et al. 1989).
Statistical Analyses
As a general overview, twin studies examine the extent to which genetic (A), common environmental (C), and non-shared environmental (E) factors contribute to a phenotype. These associations can be evaluated because of known relationships among MZ and DZ twins; namely, that MZ twins are genetically identical while, on average, 50% of genetic variation is shared across DZ twin pairs. In addition, the common environment is, by definition, 100% shared across all twin pairs reared together while the non-shared environment is fully unshared across members of a twin pair. G X E models are used to evaluate if the strength of the genetic and environmental factors predicting the phenotype is dependent on a measured environmental variable.
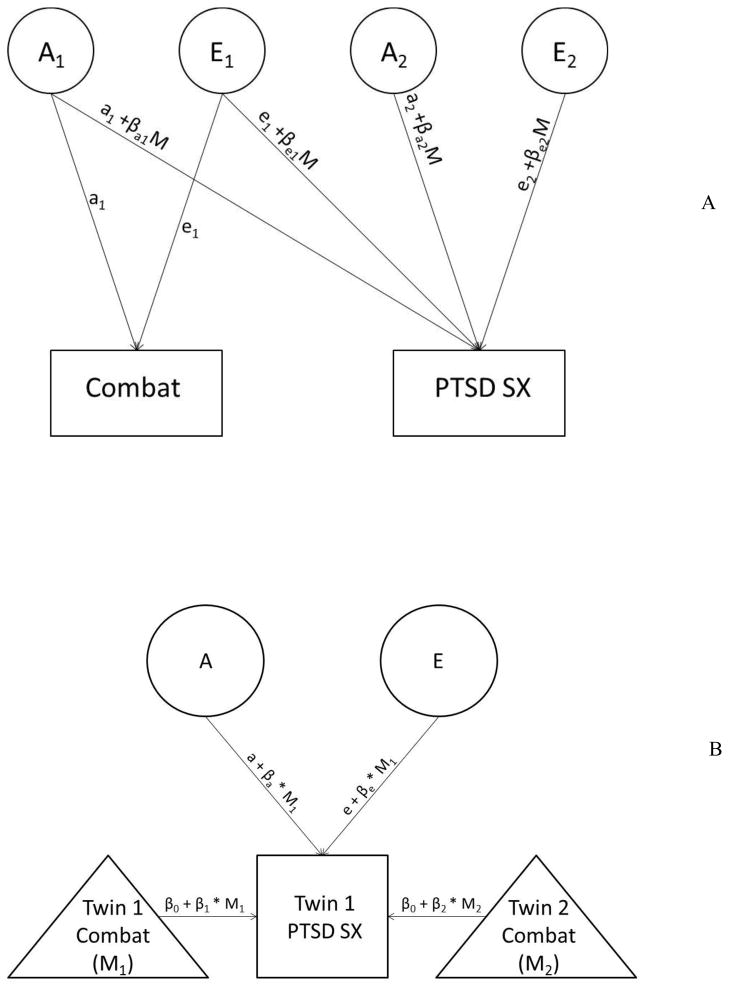
There are several analytic approaches for examining G X E in twin designs (Purcell, 2002; Rathouz et al. 2008; van der Sluis, et al. 2012). We evaluated the evidence for G X E in these data using two of these analytic methods. The first was a bivariate model described in detail by Purcell (2002). This model, depicted in Figure 1, Panel A, accounts for the extent to which the same versus different genetic and environmental factors contribute to the moderator (combat) and the phenotype (PTSD) by modeling this overlap directly. When the same genetic factors account for variance in the moderator and the phenotype, this is referred to as gene-environment correlation (rGE; see Plomin et al. 1977). RGE as it applies to this study would occur if the same genetic factors that influence risk for combat also influence likelihood of PTSD. This could occur, for example, if genetically-influenced temperament led an individual to volunteer for more dangerous combat missions and also put the individual at risk for PTSD following combat exposure. It is important to account for potential rGE because it may erroneously be reflected as G X E (Purcell, 2002). It is also relevant because prior studies using the VETR showed evidence for rGE between combat and PTSD (McLeod et al. 2001; Scherrer et al. 2008) and studies using other datasets have similarly found overlapping genetic variance between assaultive trauma and PTSD (Jang et al. 2003). This bivariate analytic approach is commonly employed in G X E of psychopathology research (see Hicks et al. 2009; Distel et al. 2011; South & Krueger, 2011) and Rathouz et al. (2008) and Van Hulle et al. (2013) indicated that it is appropriate when there is a temporal ordering of the moderator and the phenotype (e.g., combat before PTSD). In our bivariate model, both the genetic and environmental paths that are shared across combat and PTSD and those that are unique to PTSD were examined to determine if they were dependent on combat exposure (see Figure 1, Panel A).
Figure 1.
Panel A shows the bivariate model that was evaluated. The baseline bivariate model included genetic (A) and non-shared environment (E) latent variables that were common to combat and PTSD (A1 and E1) and A and E factors that were specific to PTSD (A2 and E2). All paths implicated in the etiology of PTSD were also moderated by combat exposure. Panel B shows the univariate model that was evaluated. This model included regressive paths from combat exposure in both twins to PTSD in twin 1 (the complimentary model for twin 2 is not show in the figure). The model also includes A and E effects for PTSD and moderation of these effects by combat exposure. In both types of models the cross-twin correlation between the A factors was set to 1.0 and .50 for MZ and DZ twins, respectively, and the cross-twin correlation between the E factors was set to 0. M = moderator; sx = symptoms.
While the bivariate approach is commonly employed and is a sophisticated method for distinguishing rGE from G X E, the model also has several limitations, including risk of false positive moderation results (Rathouz et al. 2008; van der Sluis et al. 2012). It is also computationally more demanding, less parsimonious, and achieves less statistical power than a univariate model described originally by Purcell (2002) and recently extended by van der Sluis and colleagues (2012).1 The univariate approach employed in this study (see van der Sluis et al. 2012) models the genetic and environmental contributions to the phenotype and controls for rGE by regressing the phenotype on the moderator for both twins, and allowing these regression coefficients to vary across MZ and DZ twins. This model is shown in Figure 1, Panel B. Van der Sluis et al. (2012) demonstrated that this approach reduces the risk of false positive G X E effects when the moderator and phenotype are correlated and the moderator is also correlated across twins, which is the case in these data. Given the different strengths and limitations of each type of model, we examined the data both ways to evaluate the replicability of the findings across these different analytic approaches.
The common environment (C) was not modeled in either set of analyses because preliminary analyses found no such effect (details available from first author), consistent with other studies of this type (True et al. 1993; Stein et al. 2002; Sartor et al. 2012). For both types of models, the interaction between combat and the latent genetic and environmental factors was modeled as a latent interaction term (scripts available from first author).
In both models we tested the necessity of the moderated paths by setting them to zero in nested models and determining if doing so significantly damaged model fit using the χ2 difference test (i.e., −2* loglikehood value) and by comparing the values of the Akaike (Akaike, 1987) and Bayesian (Schwartz, 1978) information criteria (AIC and BIC, respectively), with lower relative values indicating the preferred solution on these latter two indices. We also compared the fit of the moderated models with a null model which included no moderation.
Finally, we conducted secondary analyses to determine whether including non-combat trauma as a covariate impacted our main results. To do so, we reevaluated the best fitting univariate model and regressed PTSD not just on combat exposure but also on a dichotomous variable which reflected exposure to non-combat trauma.
All analyses were conducted using Mplus 7 (Muthén & Muthén, 2012) with maximum likelihood (ML) estimation. All reported parameter estimates are unstandardized as recommended by both Purcell (2002) and Rathouz et al. (2008) as standardized results in G X E analyses can be misleading since all variance components must sum to 1.0 (therefore, by definition, as standardized variance attributable to one factor increases the standardized variance attributable to the other must decrease). We also report 95% confidence intervals (CIs) for the parameter estimates for the final models to aid interpretation of the results.
Results
Table 1 shows the cross-twin correlations between PTSD symptom count and the three-point combat exposure index for MZ and DZ twins separately. The within-twin phenotypic correlation between combat exposure and PTSD was r = .32 for MZ and r = .34 for DZ twins. A simple AE model of PTSD revealed that the heritability of PTSD was 23% (CI: 17% – 28%), and the variance attributed to the non-shared environment was 77% (CI: 75% – 79%). The fit of the model is shown in the first row of Table 2.
Table 1.
Cross-Twin Correlations for PTSD Symptom Count and Combat Exposure
| Variable | PTSD
|
Combat
|
||
|---|---|---|---|---|
| MZ | DZ | MZ | DZ | |
| PTSD | .29 | .14 | ||
| Combat | .08 | .03 | .43 | .20 |
Note. PTSD = posttraumatic stress disorder; MZ = monozygotic; DZ = dizygotic.
Table 2.
Fit of Biometric Models
| Model | Loglikelihood value (# free parameters) | AIC | BIC | Model comparison | Δχ2 (Δ df) | p |
|---|---|---|---|---|---|---|
| AE for PTSD | −3603.305 (3) | 7213 | 7226 | |||
| Bivariate Models | ||||||
| 1a. Full Moderation | −4860.90 (12) | 9746 | 9799 | |||
| 2a. No Moderation | −4900.443 (8) | 9817 | 9852 | 1a. vs. 2a. | 79.09 (4) | < .001 |
| 3a. Common AE moderated paths to 0 | −4863.059 (10) | 9746 | 9790 | 1a. vs. 3a. | 4.32 (2) | .12 |
| 4a. PTSD-specific moderated A path to 0 | −4866.223 (9) | 9750 | 9790 | 3a vs. 4a. | 6.33 (1) | .01 |
| 5a. PTSD-specific moderated E path to 0 | −4876.387 (9) | 9771 | 9811 | 3a. vs. 5a. | 26.66 (1) | < .001 |
| Univariate Models | ||||||
| 1b. Full Moderation | −4862.619 (13) | 9751 | 9809 | |||
| 2b. No Moderation | −4899.861 (11) | 9822 | 9870 | 1b. vs. 2b. | 74.48 (2) | < .001 |
| 3b. Moderated A path to 0 | −4865.805 (12) | 9756 | 9809 | 1b. vs. 3b. | 6.36 (1) | .01 |
| 4b. Moderated E path to 0 | −4875.682 (12) | 9775 | 9829 | 1b vs. 4b. | 26.13 (1) | < .001 |
Note. The p-value column reflects the statistical significance of the difference in χ2 values across competing models. AIC = Akaike information criterion; BIC = Bayesian information criterion; A = genetic; E = non-shared environment; vs. = versus; PTSD = posttraumatic stress disorder.
Bivariate Models
The fit of the full bivariate model with moderation of both the genetic and non-shared environment paths common to combat and PTSD and specific to PTSD (i.e., Figure 1, Panel A) is shown in Table 2 (Model 1a). Results yielded significant shared genetic effects on combat (β = .50, p < .001) and PTSD (β = .99, p < .001), and common non-shared environmental effects on combat (β = .57, p < .001) and PTSD (β = .60, p = .014). This suggests that that some of the genetic and environmental risk for combat exposure and PTSD is shared. In addition, the model yielded genetic (β = 1.28, p < .001) and non-shared environmental effects (β = 2.92, p < .001) specific to PTSD. Finally, the model revealed no significant moderation by combat of the genetic (β = .36, p = .18) or non-shared environmental (β = .18, p = .45) paths that were common to combat and PTSD but did suggest that combat moderated the genetic (β = .71, p = .012) and non-shared environmental (β =.75, p < .001) paths that were specific to PTSD. The results of this model suggested that the genetic and environmental contributions shared across combat and PTSD were not dependent on level of combat exposure while the strength of the PTSD-specific genetic and environmental risk factors varied as a function of level of combat exposure. The fit of this full moderation model provided substantially better fit compared to a model which included no moderated effects as shown by the significant χ2 difference test in Table 2, Model 2a.
Given that there was no evidence for significant moderation of the genetic and non-shared environment paths common to combat and PTSD, we set the moderated portion of these paths to zero in a nested model to test if doing so significantly degraded model fit. The fit of this model is shown in Table 2 (Model 3a); the χ2 test indicated that eliminating these paths did not degrade model fit (see Table 2). Next, we evaluated the necessity of moderation of the genetic path specific to PTSD. As shown in Table 2 (Model 4a), eliminating the effect of the moderator on this path resulted in significant degradation of model fit and no improvement in AIC or BIC values. This suggests that the PTSD-specific genetic by combat exposure interaction was significantly different from 0 and important for overall model fit. Finally, we tested the necessity of the moderated portion of the non-shared environmental path that was specific to PTSD. Eliminating the moderated portion of this path also significantly degraded model fit and was associated with higher (worse) AIC and BIC (Model 5a, Table 2).
Thus, the final model included significant genetic and non-shared environmental effects common to combat and PTSD as well as significant genetic and non-shared environmental effects that were specific to PTSD. The effect of these PTSD-specific factors was dependent on level of combat exposure.2 The parameter estimates for this model are shown in Table 3.
Table 3.
Parameter Estimates for the Best-Fitting Bivariate and Univariate G X E Models
| Parameter | Bivariate Model
|
Univariate Model
|
||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Common A path to Combat | .50 (.44 – .55) | < .001 | N/A | |
| Common A path to PTSD | 1.26 (.84 – 1.67) | < .001 | N/A | |
| Common E path to Combat | .57 (.53 – .61) | < .001 | N/A | |
| Common E path to PTSD | .75 (.39 – 1.11) | < .001 | N/A | |
| Unique A path to PTSD | 1.26 (.60 – 1.93) | < .001 | 1.31 (.68 – 1.93) | < .001 |
| Unique E path for PTSD | 2.92 (2.61 – 3.23) | < .001 | 2.93 (2.62 – 3.24) | < .001 |
| Unique A * Combat | .75 (.22 – 1.28) | .006 | .74 (.19 – 1.28) | .008 |
| Unique E * Combat | .75 (.46 – 1.03) | < .001 | .74 (.43 – 1.05) | < .001 |
| MZ: CombatT1 to PTSDT1 | N/A | 1.59 (1.19 – 1.98) | < .001 | |
| MZ: CombatT2 to PTSDT1 | N/A | .37 (.00 – .74) | .05 | |
| DZ: CombatT1 to PTSDT1 | N/A | 1.99 (1.37 – 2.60) | < .001 | |
| DZ: CombatT2 to PTSDT1 | N/A | .08 (−.40 – .56) | .74 | |
Note. All β are unstandardized coefficients. A = genetic; E = non-shared environment; PTSD = posttraumatic stress disorder; CI = confidence interval; MZ = monozygotic; DZ = dizygotic; T1 = twin 1; T2 = twin 2; N/A = not applicable.
Univariate Models
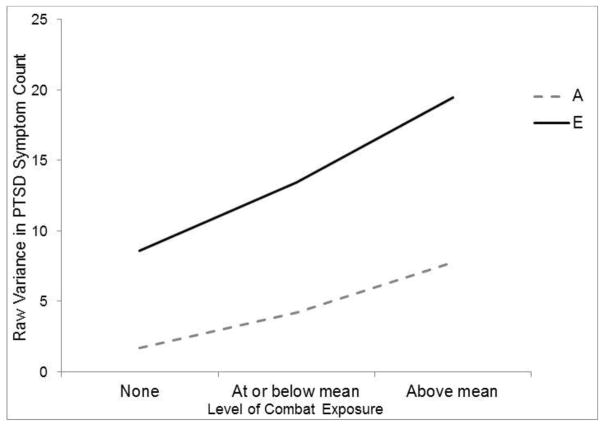
We next conducted a series of parallel analyses using the van der Sluis et al. (2012) extended univariate approach. The first model included combat exposure in both twins as a predictor of PTSD, A and E factors for PTSD, and moderation of both factors by combat exposure. The parameter estimates for this model are shown on the right side of Table 3. This model yielded significant genetic and non-shared environmental effects on PTSD as well as significant moderation of both these paths; the fit of this model is shown in Table 2 (Model 1b). In addition, the within-twin regression of PTSD on combat exposure was significant for MZ and DZ twins while the cross-twin regression of PTSD on combat exposure was not. We then tested a model with both moderated effects set to zero and determined that this model yielded significantly degraded fit compared to the model with both moderated effects (see Table 2, Model 2b). Next, we tested a model which set only the moderated genetic path to zero (Table 2, Model 3b) and found that this model yielded worse fit compared to the model with both moderated paths. Finally, we tested a model which set only the moderated non-shared environmental path to zero (Table 2, Model 4b) and found that this, too, yielded worse fit compared to the model with both moderated paths. Given this, the model with moderated A and E paths was retained. The results were very similar whether evaluated using the bivariate or univariate analytic approach. The moderated effects from the final univariate model are depicted graphically in Figure 2, which shows that the heritability and non-shared environmental contributors to PTSD increased with higher levels of combat exposure. The figure depicting the pattern of results when using the bivariate analytic approach (not shown) was near identical to Figure 2.
Figure 2.
The figure shows that results from the best-fitting univariate model. The figure shows how the variance in PTSD attributable to genetic (A) and non-shared environment (E) factors is dependent on level of combat exposure such that the importance of genetic and non-shared environment factors are increased at higher levels of combat exposure. PTSD = posttraumatic stress disorder.
Effects of Other Trauma Exposure
inally, we conducted secondary analyses to examine the effects of additional forms of trauma exposure on our main results. Exposure to any non-combat trauma was reported by 23% of the sample; 24% of those who reported combat exposure reported additional exposure to non-combat trauma. Results of the univariate model controlling for exposure to other forms of trauma revealed that this variable was a significant predictor of PTSD (β = 3.53, p < .001); however, inclusion of this covariate did not effect the pattern of results from the univariate model reported in the preceding paragraph (details available from first author).
Discussion
The results of this study suggest that the genetic and environmental influences on the development of PTSD in Vietnam veterans were moderated by the level of exposure to traumatic combat experiences. The genetic and non-shared environment factors showed stronger associations with PTSD symptom counts at higher levels of combat exposure. Rather than exerting a fixed influence on risk for PTSD, these results, consistent across two different analytic procedures, suggest that the influences of genes and environment on PTSD vary as a function of level of exposure to a measured environmental pathogen (i.e., combat). Combat exposure may act as a catalyst to amplify the pathological effects of both biological and environmental risk factors for the development of PTSD.
Our study cannot directly speak to the mechanisms via which combat exposure alters the strength of genetic and environmental risk factors for PTSD and thereby affects risk and resilience to the disorder. With respect to G X E, one possibility is that combat trauma has an effect on the function of one or more neurobiological systems underlying PTSD (i.e., a main effect) that is moderated by genotype (G X E). For example, the effects of combat on neurobiology may be greater among individuals with genetic risk variants that are associated with either exaggerated or inadequate responses to the challenges of combat stress. Epigenetics, i.e., the process by which environmental factors affect gene expression by turning genes “on” or “off,” is another possible mechanism of G X E (see Bagout & Meaney, 2010) that has been implicated in the etiology of PTSD (see Uddin et al. 2010, 2011; Koenen et al. 2011; Smith et al. 2011; Chang et al. 2012). It is conceivable that combat stress operates through epigenetic processes, causing some genes to be expressed and/or others inhibited in a fashion that confers increased risk for the development of PTSD.
A similar model may be useful in conceptualizing how the non-shared environment exerts greater influence on the development of PTSD at higher versus lower levels of combat exposure (i.e., E X E). For example, exposure to childhood trauma may sensitize an individual to the effects of subsequent combat trauma yielding a synergetic effect on neurobiological functioning and psychological symptoms. This hypothesis (Hammen et al. 2000) is supported by research showing that the effect of adverse life events on risk for PTSD is heightened among individuals with greater childhood trauma exposure (Breslau et al. 1999; McLaughlin et al. 2010).
G X E and E X E processes may occur independently of each other and/or be dependent on one another (i.e., G X E X E). For example, early childhood trauma may negatively affect the functioning of the neurobiological systems underlying stress response and subsequent combat trauma may further augment this pathological process in individuals with at risk genotypes. Consistent with this general notion, Kilpatrick et al. (2007) reported a three-way interaction between genotype, trauma exposure, and social support (G X E X E) in predicting PTSD such that the short allele variant in SLC6A4 was associated with PTSD among hurricane-exposed individuals only when hurricane exposure was high and social support was low. Similar G X E X E results were obtained for the regulator of G-protein signaling 2 (RGS2) gene by Amstadter and colleagues (2009).
Results of this study provide support for diathesis-stress models of PTSD, which posit that individuals with a genetic vulnerability are more sensitive to the pathological effects of an adverse environmental condition (e.g., combat). They are also compatible with results from molecular genetic studies suggesting that the effects of genetic variation on PTSD are observed only among those with sufficient trauma exposure (see Binder et al. 2008 and Xie et al. 2010). In our study, the non-shared environment also exerted an effect on risk for PTSD across levels of combat exposure, suggesting multiple pathways to the development of the disorder.
The heritability of PTSD, independent of combat exposure, in this sample was 23%. This estimate was somewhat lower than what has previously been reported in other investigations of PTSD, including others using the VETR (35% per Xian et al. 2000, 33% per Scherrer et al. 2008, and 13–34% per True et al. 1993). Differences in the sampling strategy and methodology between our study and these other investigations likely account for this variation. Specifically, we restricted our analyses to twin pairs concordant for South East Asia service and used an interview-based dimensional symptom count of PTSD, whereas prior estimates were based on larger samples using dichotomous PTSD diagnostic variables or self-report inventories. That said, the magnitude of the difference may be trivial given that the confidence interval for the heritability of PTSD in this study overlaps with those reported in prior investigations (Xian et al. 2000; Scherrer et al. 2008).
Limitations
The generalizability of this study was limited by our focus on male-male twin pairs who served in South East Asia during the Vietnam War, and it is not clear if these results generalize to other demographic or trauma exposed groups (i.e., women, civilians, individuals who experienced sexual assault). In addition, participants were assessed approximately 30 years after the Vietnam War, raising questions about possible recall bias in reports of combat exposure. Diagnostic determinations were based on DSM-III-R definitions of PTSD, and this is a concern given differences in the criteria between DSM-III-R and DSM-IV. However, this concern is offset by our focus on symptom counts, as the same symptoms appeared in both manuals. Analytically, we could not distinguish between error variance and variance attributed to the non-shared environment, a problem common to all twin models using observed indicators of the phenotype. Aspects of combat exposure not covered in the Combat Exposure Index also would be reflected in the non-shared environment. Related to this, the finding that about 10% of veterans with a score of zero on the self-report Combat Exposure Index endorsed exposure to combat during the PTSD interview likely reflects unreliability of one or both assessment instruments. Finally, we could not fully evaluate the role of other trauma types and whether this might also moderate the risk for PTSD, thus this and other unmeasured variables are collectively reflected in the non-shared environmental pathways. That said, preliminary analyses did indicate that exposure to other forms of trauma did not account for the primary results obtained in the study. These analyses were considered preliminary due to concerns about the limited breath and specificity of the assessment of other forms of trauma, and the need to collapse all other forms of trauma exposure into a simple dichotomous index.
Conclusions and Future Directions
This was the first twin study to evaluate the effects of combat exposure severity on the role of the genetic and environmental pathways implicated in risk for PTSD. Results revealed that the roles of both heritability and the non-shared environment increased at higher levels of combat exposure. One implication of these findings is that studies aimed at examining the specific genetic and environmental factors that contribute to risk and resilience to PTSD among veterans may be more powerful when conducted in high combat- or trauma-exposed samples. Future work should aim to identify the specific molecular, biological, neurological, and socio-cultural mechanisms responsible for the effects observed in this study. Twin and molecular genetic studies that include neuroimaging parameters could be helpful in determining how individual differences in genetic risk interact with trauma exposure to yield symptoms of PTSD. More work is also needed to clarify other aspects of the environment that might similarly moderate the genetic and environmental etiology of PTSD. It would be particularly helpful to have a better understanding of protective factors that might be associated with reduced genetic and environmental risk in order to promote resiliency and wellbeing. Such work in combat-related PTSD might focus on the putative role of modifiable protective factors such as social support, military unit cohesion, and psychological intervention that might reduce the likelihood of PTSD, even among those with increased risk for the disorder.
Acknowledgments
Erika Wolf’s contribution to this project was supported by a VA CSR&D Career Development Award. Karen Mitchell’s contribution was supported by K01MH093750. Karestan C. Koenen’s contribution was supported by MH093612. Mark Miller’s effort was supported by National Institute on Mental Health award MH079806 and the Department of Veterans Affairs Merit Award Program. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry. All statements, opinions, or views are solely of the author(s) and do not necessarily reflect the position or policy of the VA or the United States Government. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
It is difficult to make overarching statements about the statistical power associated with a general type of model because power is always heavily dependent on the specific patterns of relationships among the variables (see MacCallum et al. 1999). We conducted a post-hoc Monte Carlo simulation study to examine power for the moderated effects reported in our univariate results and found that power to detect genetic moderation was just under 80% while power to detect environmental moderation was over 90%.
We also tested the final model in the larger sample of twins (n = 2024 MZ and 1309 DZ) that was not limited based on service in South East Asia using a dichotomous index of combat exposure. Results revealed a very similar pattern to that reported in our main analyses such that there was significant moderation of the genetic and non-shared environment factors that were specific to PTSD.
All authors report no financial conflicts of interest on this project.
References
- Akaike H. Factor analysis and the AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 3. APA; Washington, DC: 1987. rev. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. APA; Washington, DC: 1994. [Google Scholar]
- Bagout RC, Meaney MJ. Epigenetics and the biological basis of gene x environment interactions. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RD, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit area survey of trauma. The American Journal of Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson EL, Schultz LR. A second look at prior trauma and the posttraumatic stress disorder effects of subsequent trauma. Archives of General Psychiatry. 2008;65:431–437. doi: 10.1001/archpsyc.65.4.431. [DOI] [PubMed] [Google Scholar]
- Chang S-C, Koenen KC, Galea S, Aiello AE, Soliven R, Wildman DE, Uddin M. Molecular variation at the SLC6A3 locus predicts lifetime risk of PTSD in the Detroit Neighborhood Health Study. PLoS ONE. 2012;7(6):e39184. doi: 10.1371/journal.pone.0039184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Middeldorp CM, Trull TJ, Derom CA, Willemsen G, Boomsma DI. Life events and borderline personality features: The influence of gene-environment interaction and gene-environment correlation. Psychological Medicine. 2011;41:849–860. doi: 10.1017/S0033291710001297. [DOI] [PubMed] [Google Scholar]
- Dubois L, Kyvik KO, Girard M, Tatone-Tokuda F, Perusse D, Hjelmborg J, Skytthe A, Rasmussen F, Wright MJ, Lichtenstein P, Martin NG. Genetic and environmental contributions to weight, height, and BMI from birth to 19 years of age: An international study of over 12,000 twin pairs. PLoS ONE. 2012;7:e30153. doi: 10.1371/journal.pone.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: An approach using questionnaires. Clinical Genetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, McFarlane AC, Weathers FW, Keane TM, Yehuda R, Shalev AY, Lasko NB, Goetz JM, Pitman RK. Is trauma a causal agent of psychopathologic symptoms in posttraumatic stress disorder? Findings from identical twins discordant for combat exposure. The Journal of Clinical Psychiatry. 2010;71:1324–1330. doi: 10.4088/JCP.10m06121blu. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True WR, Eisen SA, Henderson WG. A twin study of the effects of the Vietnam War on posttraumatic stress disorder. Journal of the American Medical Association. 1990;263:1227–1232. [PubMed] [Google Scholar]
- Hammen C, Henry R, Daley SE. Depression and sensitization to stressors among young women as a function of childhood adversity. Journal of Consulting and Clinical Psychology. 2000;68:782–787. [PubMed] [Google Scholar]
- Hicks BM, South SC, Dirago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. Journal of Clinical Psychology. 1991;47:80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Jang KL, Stein MB, Taylor S, Asmundson GJ, Livesley WJ. Exposure to traumatic events and experiences: aetiological relationships with personality function. Psychiatry Research. 2003;120:61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Jang KL, Taylor S, Stein MB, Yamagata S. Trauma exposure and stress response: Exploration of mechanisms of cause and effect. Twin Research and Human Genetics. 2007;10:564–572. doi: 10.1375/twin.10.4.564. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Harley R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, Eisen S, Tsuang M. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. Journal of Nervous and Mental Disease. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toorney R, Eisen SA, True WR, Cloitre M, Wolfe J, Tsuang MT. A high risk twin study of combat-related PTSD comorbidity. Twin Research. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Uddin M, Chang S-C, Aiello AE, Wildman DE, Goldmann E, Galea S. SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depression and Anxiety. 2011;28:639–647. doi: 10.1002/da.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I-T, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ-F. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-O-methyltransferase Val158Met polymorphism. Biological Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Merla-Ramos M, Tsuang MT. A registry-based twin study of depression in men. Archives of General Psychiatry. 1998;55:468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychological Methods. 1999;4:84–99. [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DS, Koenen KC, Meyer JM, Lyons MJ, Eisen S, True W, Goldberg J. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. Journal of Traumatic Stress. 2001;14:259–275. doi: 10.1023/A:1011157800050. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Muthén & Muthén; Los Angeles, CA: 2012. [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, Todd RD, Martin NG, Heath AC, Goate AM, Montgomery GW, Madden PAF. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Molecular Psychiatry. 2009;14:234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, Lahey BB. Specification, testing, and interpretation of gene-by-mesaured-environment interaction models in the presence of gene-environment correlation. Behavior Genetics. 2008;38:301–315. doi: 10.1007/s10519-008-9193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Cottler L, Golding E. National Institute of Mental Health Diagnostic Interview Schedule Version III—Revised. Department of Psychiatry, Washington University in St. Louis; St. Louis, MO: 1998. [Google Scholar]
- Roy-Byrne P, Arguelles L, Vitek ME, Goldberg J, Keane TM, True WR, Pitman RK. Persistence and change of PTSD symptomatology – a longitudinal co-twin control analysis of the Vietnam Era Twin Registry. Social Psychiatry and Psychiatric Epidemiology. 2004;39:681–685. doi: 10.1007/s00127-004-0810-0. [DOI] [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PAF, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychological Medicine. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, Bucholz KK, Madden PAF, Heath AC, Martin NG, Nelson EC. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Archives of General Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Xian H, Lyons MJ, Goldberg J, Eisen SA, True WR, Tsuang MT, Bucholz KK, Koenen KC. Posttraumatic stress disorder; combat exposure; and nicotine dependence, alcohol dependence, and major depression in male twins. Comprehensive Psychiatry. 2008;49:297–304. doi: 10.1016/j.comppsych.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. Estimating the dimension of a model. The Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Silventoinen K. Determinants of variation in adult body height. Journal of Biosocial Science. 2003;35:263–285. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2011;156:700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SC, Krueger RF. Genetic and environmental influences on internalizing psychopathology vary as a function of economic status. Psychological Medicine. 2011;41:107–117. doi: 10.1017/S0033291710000279. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. American Journal of Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Tsai M, Mori AM, Forsberg CW, Waiss N, Sporleder JL, Smith NL, Goldberg J. The Vietnam Era Twin Registry: A quarter century of progress. Twin Research and Human Genetics. 2012;16:429–436. doi: 10.1017/thg.2012.122. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True WR, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onorio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proceedings of the National Academy of Sciences. 2010;107:9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Galea S, Chang S-C, Aiello AE, Wildman DE, de los Santos R, Koenen KC. Gene expression and methylation signatures of MAN2C1 are associated with PTSD. Disease Markers. 2011;30:111–121. doi: 10.3233/DMA-2011-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in G × E modelling of twin data. Behavior Genetics. 2012;42:170–86. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle CA, Lahey BB, Rathouz PJ. Operating characteristics of alternative statistical methods for detecting gene-by-measured environment interaction in the presence of gene-environment correlation in twin and sibling studies. Behavior Genetics. 2012;43:71–84. doi: 10.1007/s10519-012-9568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and Alcohol Dependence. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]