Abstract
A popular, if not centric, approach to the study of an event is to first consider that of the simplest cause. When dissecting the underlying mechanisms governing idiopathic diseases, this generally takes the form of an ab initio genetic approach. To date, this genetic ‘smoking gun’ has remained elusive in diabetes mellitus and for many affected by neurodegenerative diseases. With no single gene, or even subset of genes, conclusively causative in all cases, other approaches to the etiology and treatment of these diseases seem reasonable, including the correlation of a systems’ predisposed sensitivity to particular influence. In the cases of diabetes mellitus and neurodegenerative diseases, overlapping themes of mitochondrial influence or dysfunction and iron dyshomeostasis are apparent and relatively consistent. This mini-review discusses the influence of mitochondrial function and iron homeostasis on diabetes mellitus and neurodegenerative disease, namely Alzheimer’s disease. Also discussed is the incidence of diabetes accompanied by neuropathy and neurodegeneration along with neurodegenerative disorders prone to development of diabetes. Mouse models containing multiple facets of this overlap are also described alongside current molecular trends attributed to both diseases. As a way of approaching the idiopathic and complex nature of these diseases we are proposing the consideration of a MIND (mitochondria, iron, neurodegeneration, and diabetes) paradigm in which systemic metabolic influence, iron homeostasis, and respective genetic backgrounds play a central role in the development of disease.
BORROWED EVOLUTION: BETA-CELLS AND NEURONS
Elements influencing the development of diabetes mellitus and a number of neurodegenerative disorders have been well established (see reviews [1–16]), however, given their complexity, a succinct analysis of major underlying themes spanning them has yet to be presented. Approximately 60–70% of the 25.8 million Americans with diabetes develop neurological symptoms and damage [17]. Moreover, more than 20 neurodegenerative syndromes among the 100 characterized so far are associated with diabetes mellitus [18]. In Alzheimer‘s disease (AD) there is an approximately 35% diabetes incidence [19]. Likewise, the presence of diabetes mellitus implies a 65% increased risk of AD [20]. In order to explore these trends and the polygenic influences governing these diseases we are considering two common areas linking them: mitochondria and iron. We will start by making note of the inherent similarities between the neurological and endocrine systems.
In 1869 Paul Langerhans identified and described the endocrine, or hormone secreting, cells of the pancreas [21]. These clusters of cells have since been named the Islets of Langerhans and been described in detail. Islets are composed of 5 known cell types, each playing an independent role in endocrine regulation. In diabetes mellitus the insulin producing and secreting beta-cells are of particular interest since these cells are generally dysfunctional or altogether destroyed in events preceding the onset of diabetes. Interestingly, beta-cell dysfunction in diabetes might provide a unique opportunity when considering different etiological approaches to Alzheimer’s disease. This is due, in part, to an evolving realization that beta-cells and neurons possess striking similarities, both functionally and in genetic profile. In 2011 Arntfield and van der Kooy summarized the similarities between neurons and beta-cells and suggested that in an instance of convergent evolution, beta-cells are “borrowed from the brain” (Table 1, [22–44]). Basically, neurons and beta-cells are derived from different tissue layers, ectoderm and endoderm respectively, but are very similar in the way that they store, respond to, and transmit signaling molecules. Knowledge of the similarities between beta-cells and neurons has existed for some time [35]. As early as the 1970’s it was discovered that the pancreas actually synthesizes, stores, and secretes gamma-aminobutyric acid (GABA), a major inhibitory neurotransmitter [45]. Further investigation revealed that beta-cells store and release GABA through synaptic-like microvesicles [26]. In fact, glucose mediated secretion of GABA by beta-cells inhibits glucagon release from alpha-cells [46]. Conversely, acetylcholine, another major neurotransmitter, is released from alpha-cells resulting in beta-cells that are primed for adequate insulin response [47]. Similar to neurotransmitter release in neurons, insulin secretion in beta-cells is accomplished via membrane depolarization upon external cues. In diabetes, primary metabolic processes governing glucose mediated insulin secretion are impaired. Comparatively, metabolic processes governing synaptic plasticity and transmission are dysfunctional or impaired in AD. It seems evident that diabetes mellitus and Alzheimer’s disease likely possess similarly perturbed mechanisms that govern their pathology. Thus closer analysis of shared changes and defects between these two diseases will prove beneficial.
Table 1.
Similarities between neurons and beta-cells as described by Arntfield et. al [22]. Adapted with permission from John Wiley and Sons, Inc.
| Category | Similarity | Description |
|---|---|---|
| Physiology & function | APUD phenotype & paraneuron concept | All endocrine cells have the ability to take up and decarboxylate amine precursors, as well as produce polypeptide hormones [23], features that they share with neurons [24]. |
| Neurotransmitters | β-Cells synthesise glutamate and use it for intracellular signalling in glucose-responsive insulin secretion [25]. | |
| Neurotransmitter assembly proteins | β-Cells express glutamic acid decarboxylase, an enzyme found in gamma aminobutyric acid (GABA)-secreting neurons but not other cell types [26]. | |
| Neurotransmitter receptors | β-Cells contain glutamate receptors, which are mainly found in the central nervous system [27]. | |
| Secretory granules & microvesicles | β-Cells store insulin in secretory granules that are secreted from synaptic-like microvesicles [26]. | |
| Action potentials | Pancreatic β-cells are capable of generating action potentials similar to those used by neurons to transmit signals along their axons. These action potentials may cause the release of insulin from b-cells in a manner akin to the release of neurotransmitters from neurons [28]. | |
| Glucose response | Neurons in the hypothalamus can sense blood glucose levels and are stimulated by changes in the same way β-cells are [29]. | |
| Schwann cells | Islets are surrounded and highly penetrated by Schwann cells, the major glial cell of the peripheral nervous system [30]. These Schwann cells may be functioning as support cells for both the islets and innervating neurons [30]. | |
| Cell migration | The migration of pancreatic precursors into the surrounding mesenchyme has been shown to be dependent on the axon guidance protein, netrin-1 [31]. | |
| Adhesion molecules | Endocrine cells of adult mammalian islets associate partially by the expression of neural cell adhesion molecule (NCAM) [32]. | |
| Gene expression | Global gene expression | β-Cells are more similar in global mRNA expression and chromatin methylation pattern to neurons than any other cell type, including pancreatic acinar cells [33]. |
| Sodium channels | Islet cells express the alpha-1 subunit sodium channel mRNA which is primarily expressed in the brain [34]. | |
| Neurofilaments | Dissociated b-cells have been found to synthesise neurofilaments in vitro which may be recapitulating their developmental migration [26]. | |
| REST expression | β-Cells lack expression of repressor element 1 silencing transcription factor (REST) which is expressed in non-neuronal cells and suppresses the neuronal phenotype [35]. | |
| Insulin & other pancreatic endocrine hormones | Insulin, glucagon and ghrelin are expressed in the brain during development and in adulthood [36, 37]. | |
| Glucosetransporters | The β-cell specific glucose transporter, Glut-2, is expressed in certain regions of the brain, including the hypothalamus, one of the sites of insulin action [38]. | |
| Isl-1 | The homeodomain protein Isl-1 is expressed in mature pancreatic endocrine cells, calcitonin-producing thyroid cells and neurons of the peripheral and central nervous systems [39]. | |
| Development | Pax-6 | Pax-6 is involved in development of a-cells of the pancreas and proper insulin secretion from b-cells [40], as well as neurogenesis in the developing central nervous system [41]. |
| Nkx6.1 | Nkx6.1 is a transcription factor involved in the formation of b-cells in the pancreas [40] as well as maturation and migration of hindbrain motor neurons [42]. | |
| Notch | Notch is a transmembrane signalling protein that has been implicated in maintaining pancreatic precursors in a proliferative state, as well as influencing cell fate decisions [40]. Notch has been shown to have similar functions in the developing nervous system [41]. | |
| Neurogenin | The transcription factor neurogenin-3 is repressed by Notch and when activated it contributes to specification of endocrine cells in the pancreas [40]. Notch may also repress neurogenin-1 and 2 which are involved in the specification of neurons from neural progenitors [41]. | |
| HB9 | HB9 is expressed in the embryonic gut and initiates formation of the pancreatic bud and is later expressed in mature b-cells [40]. BHB9 is also expressed in embryonic and adult motor neurons [43]. | |
| PDXI | The pancreatic specific transcription factor PDXI is turned on in the brain during development [44]. | |
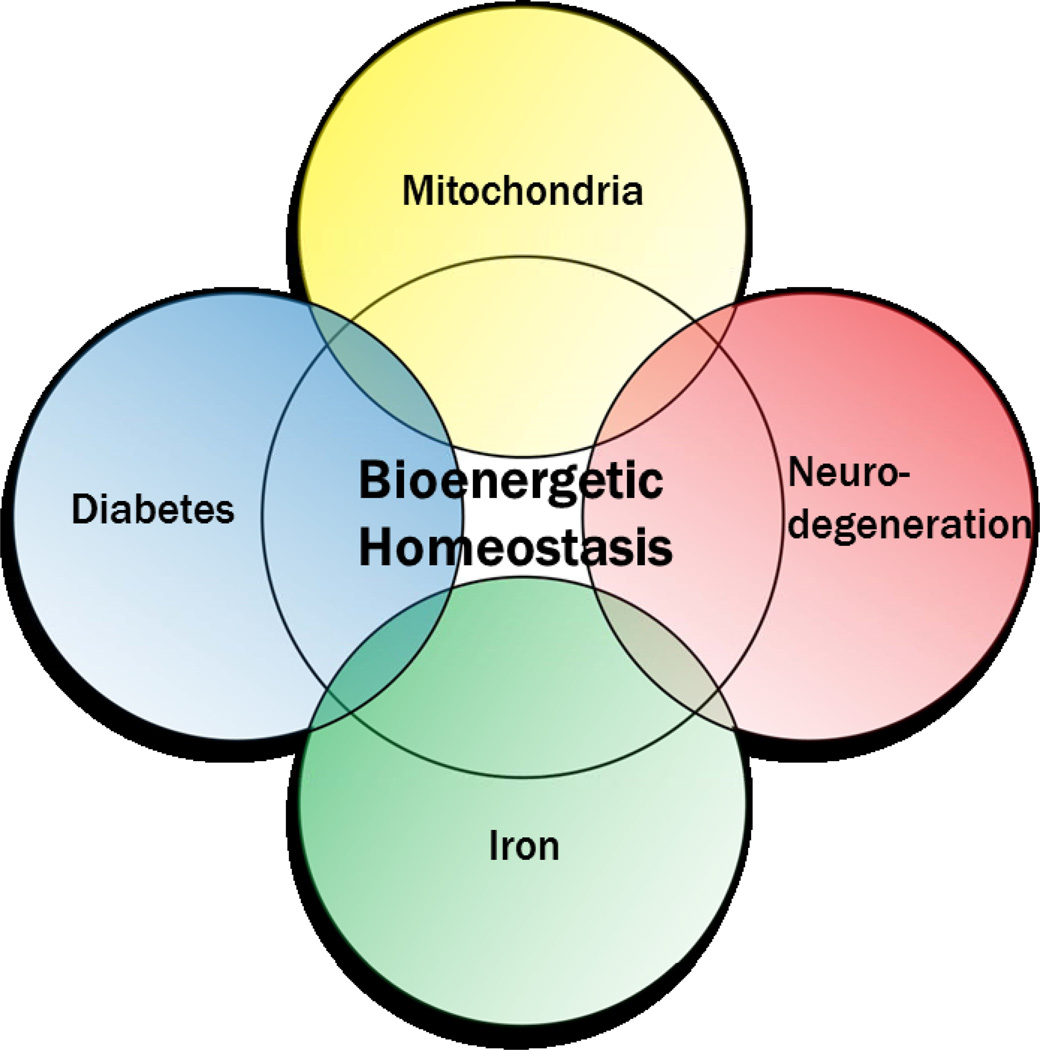
In essence, diabetes and Alzheimer’s disease are simply complex and elicit significant uncertainty in etiological studies. For this reason we are proposing that idiopathic cases arise through each system’s predisposed sensitivity to metabolic influence determined not by a single gene but rather by a genetic background whose composite function determines susceptibility. More accurately, we posit that changes in bioenergetic homeostasis, influenced largely by mitochondrial and iron related pathways, lie at the nexus of neurodegeneration and diabetes (Figure 1).
Figure 1.
Exploring the MIND paradigm, bioenergetic homeostasis lies at the nexus between common defects found in neurodegenerative disease and diabetes mellitus. Mitochondria and iron play a critical role in bioenergetic homeostasis, suggesting that disturbances in proper mitochondrial function and iron metabolism result in dysfunction common to both diabetes and neurodegeneration.
FORMING THE MIND PARADIGM: FRATAXIN, NCB5OR, AND THE MONOGENIC CONTRIBUTORS
Friedreich’s ataxia (FA), a largely monogenic disease, provides a critical demonstration of the link between mitochondria and iron in the processes and pathways governing neurodegeneration and diabetes. FA patients experience peripheral nerve and dorsal root ganglia atrophy, ataxia in all four limbs, dysarthria, cardiac abnormalities including arrhythmia, and diabetes mellitus. The primary cause of FA is a significant reduction in the availability of the mitochondria-localized protein frataxin as a result of a large GAA trinucleotide repeat expansion in the first intron of FXN [48–50]. As is the case in other trinucleotide repeat disorders, it is postulated that this expansion results in either delayed transcription or altered transcript processing [51], or altered protein folding and translational efficiency like that observed in Huntington’s disease [52–54]. Various studies suggest that frataxin is a key component in iron-sulfur processing in the mitochondrial matrix [55, 56]. A number of models for Friedreich’s ataxia have been developed, ranging from transgenic mice to immortalized and induced pluripotent stem cell (iPSC) cell lines [57–59].
Other monogenic instances in diabetes and Alzheimer’s disease have been documented in the forms of subtypes and models, representing a small percentage of the population for which a single contributing factor can be identified [60–65]. Upon further examination, it becomes evident that many of these monogenic causes lie in major pathways contributing to metabolic flux, mitochondrial function, and regulation of iron metabolism and homeostasis [64,66–70].
Monogenic Events in Diabetes
Maturity-onset diabetes of the young (MODY) and Maternally Inherited Diabetes and Deafness (MIDD) can be considered two major, albeit rare, monogenic forms of diabetes mellitus [60]. In MIDD, a single A→G mutation in the mitochondrial encoded gene for tRNA leucine results in diabetes and deafness [71]. In parallel, more than half of the 6 genes attributed to the development of MODY [60] impact features and pathways critical to proper metabolic function [72, 73]. Other monogenic diabetes models include animals lacking TFAM and Ncb5or [74]. In the latter case, no significant contribution has been assigned to mutations or variations within the diabetic population [65], yet Ncb5or null mice develop lean diabetes at age 7 weeks as a result of beta-cell demise and display gross mitochondrial morphological changes accompanied by altered lipid metabolism [64, 70, 75]. Interestingly, naturally occurring missense mutations in human Ncb5or lead to improper folding and significantly reduced levels of intracellular Ncb5or [76]. Our recent data also suggest that ablation of Ncb5or leads to iron dyshomeostasis [77]. Preliminary findings indicate neuromuscular junction defects and behavioral changes associated with altered neurological function in Ncb5or null mice (Stroh et. al, unpublished data). Since Ncb5or is ubiquitously expressed, such cell specific phenotypes from an otherwise non cell specific gene provide a useful framework to study correlation and causality in disease.
Monogenic Events in Alzheimer’s Disease
Alzheimer’s disease is the most prevalent neurodegenerative disorder, affecting 13% of the population over the age of 65 [78]. Mutations in the amyloid precursor protein (APP), the precursor to amyloid plaques at times considered to be a possible cause of Alzheimer’s disease, have been associated with early onset, familial Alzheimer’s disease (FAD). The Swedish mutation in APP leads to premature and accelerated cleavage of APP by β-secretase resulting in accumulation of amyloid oligomers [62]. The toxicity and function of β-amyloids has long been debated but recent evidence suggests that APP possesses ferroxidase and iron export activity [67], which may indicate APP normally participates in iron homeostasis. Regulatory elements in the APP mRNA directly link its function to the cellular iron status [66].
One of the best known genetic contributors to late onset Alzheimer’s disease (LOAD) is that of the ApoEε4 allele. Patients who possess ApoEε4 have a significantly elevated risk, and account for approximately 40% of the LOAD population [79]. ApoEε4 carriers also demonstrate a reduced ability to clear Aβ42 amyloid deposits compared to those possessing the ε2 and ε3 alleles [80]. Interestingly, evidence outlining metabolic brain abnormalities in young and old ApoEε4 carriers alike suggests that metabolic decline, mitochondrial dysfunction, and eventual hypometabolism are critical risk factors in the development of ApoEε4 associated LOAD [81–88]. Less robust cognitive test performance in individuals harboring the allele can be observed in middle adulthood [89]. These observations lend to the notion that changes in the metabolic environment occur much earlier than the development of the histologic hallmarks of Alzheimer’s disease, pointing to possible upstream pathologies.
MITOCHONDRIA AND IRON IN SPORADIC ALZHEIMER’S DISEASE
Neurodegeneration is a generalized term used to describe loss of neuronal mass, locally or globally, as well as systemic malfunction and eventual loss of efficient neural transduction and communication. In either case these events can result from changes in extra-neuronal networks by which the ideal neural environment is maintained, as is the case in multiple sclerosis [90], to changes in individual neuronal environments directly impacting their function. While it is obvious that genetic background plays a significant role in both mechanisms, contemplating the impact of compounded genetic variability on disease susceptibility is not so trivial. Changes in metabolic function and iron regulation are observed in a number of neurological diseases yet the diseases remain pathologically distinct [91]. For this reason, variables governing mitochondrial function and iron regulation warrant further investigation. While many neurodegenerative diseases display abnormalities in mitochondria and iron homeostasis, our focus will largely remain on changes in Alzheimer’s disease.
In AD, the complexity of the disease is unmistakable with progressive cognitive decline consistently out of sync with historical pathological mechanisms like altered APP processing and amyloid fibril formation. In fact, significant amyloid plaque burden can be detected in elderly but cognitively normal individuals [92]. Even so, the most current and popular approaches to investigation and treatment are to simply analyze, understand, and eventually remove amyloid plaque deposits. So far this strategy has yielded negative or inconclusive results in clinical trials. Aβ oligomer deposition is a unique characteristic ascribed to Alzheimer’s disease, however, inconsistencies in the effects of senile plaques call for reassessing the role of toxic amyloid species in AD etiology [93] Not discounting the downstream role of APP processing in the pathogenesis of Alzheimer’s disease, we would like to focus on pathways that are potential targets for therapeutic intervention prior to amyloid deposition and which aim to clarify susceptibility to the more common form of Alzheimer’s disease, LOAD. With the aforementioned correlation between Alzheimer’s disease and diabetes mellitus we will explore pathways that influence both diseases.
Mitochondrial and Metabolic Influence
Mitochondrial-derived metabolic dysfunction has been documented in multiple neurodegenerative disorders [2, 94, 95]. One of the most recognized of these disorders is Alzheimer’s disease, for which a mitochondrial cascade hypothesis has been proposed [96–99]. Altered function in critical citric acid cycle components and electron transport chain (ETC) complexes as well as altered glucose metabolism and insulin resistance have all been observed in AD [100–103]. It has even been suggested that Alzheimer’s disease is in fact a form of type III diabetes. This assertion is based on evidence for impaired insulin response pathways and insulin resistance in the Alzheimer’s brain [103, 104]. The most reported functional deficiencies in ETC complexes arise in complex IV (cytochrome c oxidase; COX) Vmax activities, with complex I and III activities closely following suit. Reduced complex IV activity was first reported in mitochondria isolated from AD subject platelets [105]. Further investigations probing COX activity in the AD brain also showed decreased activity similar to that observed in platelet-derived mitochondria [106, 107]. This raised questions as to whether mitochondrial dysfunction could influence neurofibrillary tangles and amyloid plaque deposition. Early studies provided evidence that altered metabolism impacted APP processing [108, 109]. Cytoplasmic hybrid (cybrid) studies, in which platelet mitochondria isolated from AD patients are transferred to mitochondria-deficient (ρ0) neuronal (neuroblastoma or teratocarcinoma) cells, have also reported changes in APP processing and amyloid species production [110, 111].
In addition, cybrid studies show a profound shift in overall cellular bioenergetic equilibrium with reductions in membrane potential and altered calcium homeostasis [111]. Changes in calcium homeostasis are consistent with changes in mitochondrial regulatory pathways and support observations that mice harboring presenilin 1 mutations, an established cause of familial AD (FAD), display mitochondrial dysfunction and altered cytosolic calcium homeostasis [112]. Such observations also provide evidence for mitochondria as an upstream affecter in the production of β-amyloid since altered amyloid processing has been correlated with altered calcium homeostasis [113]. Although there are conflicting accounts as to whether β-amyloid itself induces or alters the calcium changes observed in AD [114], cybrid studies provide insight into mechanisms that may nurture β-amyloid production prior to its potential effects on intracellular calcium.
It is widely accepted that ETC activity naturally produces reactive oxygen species (ROS) [115, 116]. ROS production and oxidative stress rapidly increases during times of cellular stress and mitochondrial dysfunction, leading to significant damage to cellular organelles and DNA. The frontal cortex of AD patients display oxidative stress [117] along with increased levels of 8-hydroxydeoxyguanosine and 8-hydroxyguanosine in DNA and RNA, respectively [118], which are also indicative of oxidative stress. Recently, investigators demonstrated that, while multiple components of the ETC are sites of ROS production, the contribution to total ROS production from each site depends largely on the substrates being oxidized and not solely upon the protein function per se [118]. This provides further insight into possible dietary and environmental factors that were not considered in previous studies.
Modes of inheritance for Alzheimer’s disease and Parkinson’s disease are sparsely Mendelian, lending to investigations of inheritance patterns in non-monogenic populations. These investigations have yielded ample data suggesting a maternal pattern of inheritance in Alzheimer’s disease [119–121], consistent with maternal inheritance of mitochondrial DNA (mt-DNA) [122]. The synthesis of this data in whole supports a mitochondrial cascade hypothesis in which mitochondria and cell bioenergetics lie at the forefront of AD pathology.
Iron Homeostasis in Neurodegeneration
Iron metabolism and dyshomeostasis is implicated in a number of neurodegenerative disorders including Parkinson’s disease, Alzheimer’s disease, neuroferritinopathy, aceruloplasminaemia, and neurodegeneration with brain iron accumulation (NBIA) [91]. Among them, AD and PD are the most prevalent forms without a single known cause. The role for iron in the pathogenesis of PD is being investigated but remains elusive with changes in iron and iron regulatory pathways consistently observed but spatially and temporally diffuse [123]. However, ample evidence supports consistent changes in redox-active metals in the brains of AD patients [124, 125].
Early reports of iron accumulation in the AD brain [126] have led to interest in the interaction and association of iron with the histologic hallmarks of the disease: neurofibrillary tangles (NFT’s) containing hyperphosphorylated tau and Aβ containing senile plaques. Iron accumulation in NFT’s and amyloid plaques, as well as in the broader cortex, have been observed in AD patients and AD transgenic mouse models [127, 128]. Indeed, hyperphosphorylated tau-associated NFT formation has been shown to be induced and reversed by Fe3+ and Fe2+, respectively [129]. In 2010, Duce et al. addressed questions surrounding the function of APP by demonstrating ferroxidase activity in “a conserved H-ferritin-like active site” within APP [67]. It was concluded that APP loads oxidized iron into transferrin and demonstrates a significant interaction with ferroportin. These authors also reported the inhibition of APP mediated iron export by Zn2+. Most relevant to observations in AD patients, APP knockout mice demonstrated insufficient iron export capacity and significant retention of iron in cortical neurons [130–132]. The intracellular retention of iron likely potentiates a toxic cascade as Aβ mediated reduction of iron has been shown to generate ROS, which in turn leads to increases in both APP and Aβ production [130–132]. Recently it was discovered that increased iron content from brain microbleeds enhanced the neurotoxic effects of Aβ [133]. Also, changes in serum iron and copper homeostasis were found to correlate and accurately predict cognitive decline [134]. It seems evident that iron’s role in the pathogenesis of AD lies either upstream or in tandem with Aβ. In comparison, the latter seems less likely as over expression of APP in trisomy 21 does not result in early life fibrillar amyloid accumulation [135]. Instead, it is more likely that the effect of iron accumulation on Aβ induced toxicity is the result of progressive insult to cellular iron processing.
Iron-sulfur clusters (ISCs) and heme are iron-containing prosthetic groups at the catalytic centers of many critical metabolic enzymes, including all four of the mitochondrial ETC complexes, and are essential to enzymes responsible for H2O2 removal and maintaining general cellular homeostasis. Mitochondria serve as the center stage for synthesis of ISCs and heme [136]. As discussed earlier, mitochondrial and metabolic dysfunction are observed early in AD [137]. Normally, aging is accompanied by increased mt-DNA copy number which is likely a compensatory mechanism for an aged and dysfunctional mitochondrial population [138, 139]. However, in the late stages of AD amplifiable levels of intact mt-DNA are reduced, possibly through down regulation of PGC1α and mitophagy-induced degradation of mitochondria [140]. Given the relationship between iron, APP, and senile plaques, it seems likely that the intracellular recycling of large amounts of iron containing mitochondrial protein coupled with reduced APP and thus reduced or impaired iron export capability may compound bioenergetic decline in Alzheimer’s disease.
MITOCHONDRIA AND IRON IN IDIOPATHIC DIABETES
Diabetes mellitus can be characterized as a metabolic disorder for which three major types are known: Type 1 (T1D), Type 2 (T2D), and gestational diabetes mellitus. T1D (or insulin dependent diabetes mellitus) manifests as the result of autoimmune targeted destruction of insulin-producing beta-cells in the pancreatic islets, leading to progressive loss of insulin secretion resulting in absolute insulin deficiency. T2D, most often associated with obesity, is characterized as hyperglycemia in the context of insulin resistance and relative insulin deficiency. Gestational diabetes results in hyperglycemia and insulin resistance during the term of pregnancy in formerly non-diabetic women. T2D accounts for 90% of diabetes mellitus, whereas T1D and gestational diabetes, along with known and unknown monogenic diabetes, comprise the remaining 10%.
Insulin is a 5.8 kDa hormone produced by pancreatic beta-cells and in the brain in response to elevated blood glucose levels. Insulin stimulation results in multiple complex, dynamic changes and cascades within a cell ranging from changes in metabolic pathways and bioenergetic flux to targeted transcriptional changes in the nucleus (for review see ref. [141]). One of the primary changes that occur in diabetes mellitus is that of glucose intolerance due to changes in insulin response. Many different, and often opposing, theories have arisen in an attempt to target the mechanisms responsible, however due to the complexity and variation within the disease little progress has been made. Diabetes mellitus results in dramatic changes to global metabolic regulation and substrate utilization for reasons that are undetermined, however significant evidence suggests that disturbed mitochondrial function and iron homeostasis play a significant role.
Mitochondrial and Metabolic Influence
T2D is characterized by relative insulin resistance and impaired insulin secretion in the presence of glucose. Changes in insulin secretion and response suggest attempts to compensate for or regulate potentially harmful changes in metabolism. Mitochondria play a significant role in regulating insulin secretion in beta-cells through regulating ATP levels responsible for facilitating membrane depolarization and insulin granule, as well as anaplerosis coupled, exocytosis (for reviews see ref. [142, 143]). Rapid, dramatic changes in mitochondrial morphology upon glucose stimulated insulin secretion have been observed as well as GTP modulated insulin secretion via changes in mitochondrial metabolism and calcium homeostasis [144, 145]. Changes in mitochondrial and metabolic status in events preceding the onset of diabetes have been recognized for some time and have led to multiple theories proposing different roles for the observed mitochondrial dysfunction, as a consequence or otherwise. Interestingly, changes in lipid oxidation and fatty acid storage occur just prior to the disease with increased lipolysis accompanied by changes in circulating free fatty acids [146]. It is debated whether this change constitutes a state of compensation or mitochondrial dysfunction. This mainly stems from inconsistent observations of the effects of fatty acid metabolism and altered mitochondrial function in different diabetic populations (i.e. obese vs. lean) [147]. Normally, glucose stimulated insulin secretion from beta-cells increases in the presence of fatty acids; however this has been shown to decrease during prolonged fatty acid incubation [148]. In contrast, some studies have found that increased fatty acid availability stimulates mitochondria-mediated fatty acid oxidation in muscle [146]. This suggests that insulin resistance in the periphery is not necessarily a product of mitochondrial dysfunction but rather a non-canonical shift in primary bioenergetic pathways based on substrate availability. Still, mitochondria play a critical role in regulating pathways mediating insulin secretion and response, making them central to investigations of perturbed bioenergetic flux in diabetes.
In 1901 Eugene Opie discovered protein deposition in the pancreas of patients with hyperglycemia, describing “hyaline degeneration of the islands of Langerhans” [149]. It wasn’t until 1986 that islet amyloid polypeptide (IAPP) was identified and found to be a primary component of these deposits [150]. It has since been discovered that more than 90% of patients with T2D display IAPP associated protein deposition in the pancreas [151, 152]. The human form of IAPP (hIAPP) is secreted with insulin, although its exact function remains unknown. Interestingly, IAPP and brain-associated APP share 90% structural similarity [18, 152], with hIAPP also possessing structural characteristics that allow it to form amyloid fibrils. hIAPP has been shown to induce loss of beta-cell mass through mitochondrial dysfunction-induced activation of apoptotic pathways [153]. Results regarding the potential of an FDA approved treatment for T2D in a transgenic FAD mouse model were released in September of 2013. Liraglutide, a glucagon mimetic and GLP-1 agonist used to stimulate insulin secretion in the presence of glucose, was shown to result in decreased tau phosphorylation, decreased neurofilament formation, and increased memory performance and ability in mice [154]. This is consistent with previous findings that demonstrated reduced plaque formation and oxidative stress in transgenic mice treated with GLP-1 [155]. It is not surprising that exendin-4, a GLP-1 agonist, protects beta-cells from hIAPP-associated cell death through improved mitochondrial function and biogenesis [156]. Exendin-4 also increases adult neurite outgrowth of sensory neurons [157], a result important in addressing diabetic neuropathy (vide infra). To our knowledge, the effects of GLP-1 agonists on mitochondrial function and oxidative stress in the AD brain have yet to be studied, although these results provide significant reason to suspect altered and improved metabolic status as a result of GLP-1 receptor modulation.
Iron Homeostasis in Diabetes
The effects of iron overload and dyshomeostasis in the pathophysiology of diabetes is relatively understudied. However, an increased interest has been generated with multiple groups finding an increased risk of T2D and insulin resistance associated with alterations in tissue iron content, iron metabolism, and iron overload [158–160]. Increased serum ferritin concentration, an indicator of tissue iron status, was positively correlated with the risk of developing T2D [161]. More studies have confirmed the increased risk of diabetes with high normal or above normal serum ferritin levels and suggested that iron deficiency may not provide protection against this risk [162]. A recent study evaluated ferritin and transferrin saturation in pre-diabetes concluded that high ferritin combined with low transferrin saturation was associated with a higher risk of developing pre-diabetes [163].
Hereditary hemochromatosis, or iron overload, is commonly caused by mutations in the HFE gene and provides the strongest evidence of systemic iron changes leading to T2D. An allelic variant of HFE linked to hemochromatosis, termed the ‘D allele’, was shown to increase the risk for diabetes [164]. HFE associated diabetes suggests that changes in systemic iron homeostasis, particularly iron overload is associated with diabetes incidence. This has been strengthened with observations that dietary intake of red meat, a significant source of heme iron, is positively correlated with an increased risk of T2D [165]. Also, frequent blood donations and iron chelation therapy are shown to improve diabetes in T2D patients [166].
With little indication as to the exact role of iron overload in the risk for diabetes it is difficult to say whether iron dyshomeostasis is a catalyst or artifact of the disease. Studies on frataxin and Ncb5or pathways will help to provide novel insights into roles of iron metabolism in diabetes.
ALZHEIMER’S DISEASE, PARKINSON’S DISEASE, AND DIABETIC NEUROPATHY
Diabetic neuropathy is a generalized term describing changes and defects in the nervous system of patients diagnosed with diabetes mellitus. Diabetic neuropathy does not discriminate between T1D and T2D. Several forms exist and can be divided into two distinct groups: generalized symmetric polyneuropathies (acute sensory, chronic sensorimotor, autonomic), and focal and multifocal neuropathies (cranial, truncal, focal limb, proximal motor, chronic inflammatory demyelinating polyneuropathy) [167]. Numerous studies have observed changes in bioenergetic pathways and mitochondrial function in diabetic neuropathy-associated neurodegeneration for both T1D and T2D (for reviews see [168, 169]). In fact, improving mitochondrial bioenergetics through NF-κB activation prevents sensory neuropathy in mouse models of T1D [170]. The glycolytic byproduct methylglyoxal, a reactive form of pyruvate that plays a role in the development of advanced glycation endproducts (AGE’s), is of particular interest to diabetes and diabetic neuropathy [171]. AGE’s have been positively correlated with diabetic nephropathy along with a number of different diabetes associated complications [171] [172]. A recent investigation found that methylglyoxal mediated modification of the Nav1.8 sodium channel is associated with hyperalgesia in diabetic neuropathy [173], implicating a direct role for methylglyoxal in diabetes-induced nociceptive pain. In addition, a novel neurotoxin known as ADTIQ (1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline) discovered in the brains of PD patients has been found to be the result of methylglyoxal reacting with dopamine [174]. It has been suggested that this neurotoxin, the result of altered bioenergetic homeostasis and a specific neurotransmitter, might be a common pathogen between Parkinson’s disease and diabetes.
Both T1D and T2D result in impaired memory and learning, however the distinctions between T1D- and T2D have led to much debate as to the underlying cause. Traditionally, impaired insulin signaling was primarily attributed to T2D and thought to be a larger risk factor for developing AD compared to T1D. However, recent studies have demonstrated worsened AD associated phenotypes in the context of T1D [175, 176], blurring the lines between T1D and T2D in AD. Still, little is known of the effects of AD on peripheral neuropathy. Interest has been generated due to reports of the underrepresented population of dementia patients who experience pain [177], but it is not immediately clear whether pain associated with dementia is the result of true neuropathy or that of altered pain perception. In 2012, Jolivalt et al. provided physiological evidence that APP transgenic mice demonstrated “similar patterns of peripheral neuropathy” when compared to insulin-deficient mouse models [178]. This was attributed to impaired or altered insulin signaling through lowered insulin receptor phosphorylation as well as altered GSK3β-mediated metabolic regulation. Changes in insulin signaling and regulation are found in both diabetes and AD (for review see [179]). Deficiencies in metabolic processes and pathways are clearly identified in both diseases, thus the presence of peripheral neuropathy like phenotypes in AD mouse models suggests shared pathogenic mechanisms.
GENETIC VARIATION, DISEASE SUSCEPTIBILITY, AND POTENTIAL THERAPEUTICS
A variety of neurodegenerative diseases display characteristics of MIND paradigm-associated defects. Still, with all of the data presented and available, the polygenic nature of these diseases makes identifying viable therapeutic targets the rate limiting step in treatment. Traditionally, successful treatment development relies on two inherent criteria: disease incidence/impact and identifiable therapeutic targets. In complex, sporadic diseases like Alzheimer’s disease or Parkinson’s disease, the incidence of the disease is profound and indisputable. However, progress falls short when treatment is focused on therapeutic targets which are not major contributors to the majority of the disease.
The popular belief that Aβ initiates AD pathogenesis set off a plethora of anti-amyloid based treatments [180–182]. So far targeting senile plaques in clinical trials has failed to stop or even conclusively slow disease progression [183–185]. This implies amyloid deposition may represent a consequence, as opposed to cause, of the disease. A recent study of microbleed events associated with β-amyloid immunization has concluded that immunization actually facilitates cerebral microbleeds and worsens iron deposits in the choroid plexus [186].
In light of these failed treatments, new approaches are needed. This can likely be achieved through targeted manipulation of multiple pathways based on currently unidentified endophenotypes. In AD, mitochondria-associated endophenotypes have been considered due to the maternal inheritance associated with sporadic AD and correlative maternal mt-DNA inheritance [187]. With enough evidence supporting a mitochondrial and metabolic role in disease, a novel approach to treatment strategy has been proposed. Termed “bioenergetic medicine”, the rationale behind the approach involves the manipulation or support of those pathways that influence or are directly involved in bioenergetic flux [188]. This includes previously described mitochondrial medicine approaches in which mitochondria and mitochondrial regulated cellular processes are targeted. These approaches include manipulating ETC components based on the functional state of mitochondria or cell energy status, influencing mitophagy events, changing mitochondrial mass, and even directing mitochondrial mediated apoptotic events [187]. Targeting bioenergetic flux for treatment might be better understood in the context of AD pathology.
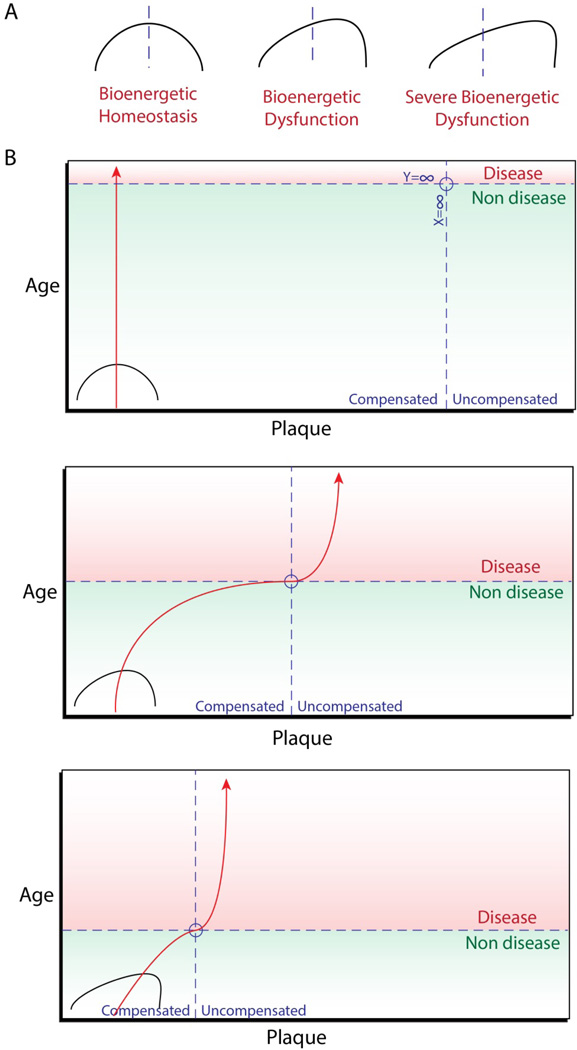
During the early stages of Alzheimer’s, prior to mild cognitive impairment syndrome, β-amyloid deposition is abundant. It isn’t until after senile plaque formation drastically slows that the disease manifests. It has been suggested that the early deposition of senile plaques is coupled with a state of compensated metabolic function [99]. That is, compensation for dysfunctional metabolic processes is responsible for β-amyloid production in the events preceding cognitive impairment, representing a form of compensated brain aging. At the point at which the framework supporting these compensatory actions is stressed to fracture, metabolic processes are down regulated and hypometabolism sets in. At this stage, plaque deposition halts and uncompensated brain aging occurs, resulting in the onset of the clinical symptoms of Alzheimer’s disease (Figure 2). The notion of compensated vs. uncompensated states of dysfunction can also be applied to patterns in diabetes, with changes in metabolic flux, beta-cell mass, and global metabolic regulation differing from prediabetes to clinical onset [189–193]. In addition, pre-diabetic Ncb5or knockout mice show a marked increase in metabolic rate prior to the onset of diabetes [194]. It is possible, and even probable, that mechanisms similar to those responsible for compensated brain aging play a role in the events preceding the development of diabetes mellitus. Therefore, investigating pathways contributing to metabolic flux and dysfunction in both AD and diabetes will provide novel therapeutic insights.
Figure 2.
A.) Visual representation of bioenergetic homeostasis, with the midline indicating an ideal flux/balance in bioenergetic pathways. Distorted mitochondrial function and iron metabolism result in bioenergetic dysfunction, shifting away from ideal bioenergetic homeostasis. B.) Graphical representation of compensated vs. uncompensated states of bioenergetics in AD. Ideally, bioenergetic homeostasis results in compensated aging and no plaque deposition (top). However, in instances of bioenergetic dysfunction plaque deposition occurs during compensated brain aging. At the point at which compensatory mechanisms fail, plaque deposition slows or stops and disease manifests (middle). Severe bioenergetic dysfunction results in the same trend, however this occurs at a much earlier age (bottom).
The mitochondrial cascade hypothesis has helped to provide a broad overarching perspective to evidence of altered brain bioenergetics and mitochondrial function in AD. However, identifying targets for therapeutic intervention is as complex as the disease itself. This is due, in part, to the infancy of risk estimation incorporating both familial history and genetic background in complex diseases [195]. The identification of endophenotypes and eventually multiple and specific targets in complex diseases will require personalized approaches to treatment, complicating the issue further. In short, complex diseases will require complex treatment. So with the development of complex, personalized treatment on the seemingly distant horizon, progress toward effective therapy can likely be made through evaluating the MIND paradigm.
CONCLUDING REMARKS
Understanding the nature of sporadic, complex diseases has long been pursued through approaches that are designed for simple explanation. Unfortunately, this has left advancements in treatments for diseases like Alzheimer’s disease slow moving and at times even retrograde. This is where analyzing the multifactorial nature spanning multiple diseases, such as the MIND paradigm, can make a significant contribution to changes in epidemiology and eventually therapy. Diverging from traditional modes of thought is inherently difficult, especially in scientific investigation. To better pursue balance it is essential to first define a fulcrum. In complex diseases this comes in the form of defining cellular or systemic homeostasis by genetic composition. Adequate treatment can be designed only when recognizing that balance is not universal in the biological processes of non-uniform populations.
Acknowledgements
Supported in part by grants and a fellowship from the National Institutes of Health: P30 AG035982 and R03 NS077852 (R.H.S.), RO1 DK67355 (H.Z.), and Ruth L. Kirschstein National Research Service Award T32 HD057850 (M.A.S.). Additional support came from The School of Health Professions at University of Kansas Medical Center and The Lied Basic Science Program at University of Kansas Medical Center Research Institute (H.Z.). Authors thank John Wiley and Sons, Inc. for permission (#3240270463068) to reproduce Table 1 and Stanton Fernald for artistic assistance with figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Swerdlow RH. Brain aging, Alzheimer's disease, and mitochondria. Biochimica et biophysica acta. 2011;1812(12):1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009;17(4):737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Sun L, Tan Y, Wang G, Lin X, Cai L. Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications. Curr Med Chem. 2009;16(1):113–129. doi: 10.2174/092986709787002862. [DOI] [PubMed] [Google Scholar]
- 5.Van Campenhout A, Van Campenhout C, Lagrou AR, Moorkens G, De Block C, Manuel-y-Keenoy B. Iron-binding antioxidant capacity is impaired in diabetes mellitus. Free Radic Biol Med. 2006;40(10):1749–1755. doi: 10.1016/j.freeradbiomed.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 7.Batista-Nascimento L, Pimentel C, Menezes RA, Rodrigues-Pousada C. Iron and neurodegeneration: from cellular homeostasis to disease. Oxid Med Cell Longev. 2012;2012:128647. doi: 10.1155/2012/128647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakabayashi K. Cellular pathology of neurodegenerative disorders. Rinsho Shinkeigaku. 2013;53(8):609–617. doi: 10.5692/clinicalneurol.53.609. [DOI] [PubMed] [Google Scholar]
- 9.Sharma J. Alzheimer's disease: an update. Nurs J India. 2012;103(6):245–248. [PubMed] [Google Scholar]
- 10.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12(6):609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 11.Tieh P, Dreimane D. Type 2 Diabetes Mellitus in Children and Adolescents. Indian J Pediatr. 2013 doi: 10.1007/s12098-013-1193-6. [DOI] [PubMed] [Google Scholar]
- 12.Scott LK. Presence of type 2 diabetes risk factors in children. Pediatr Nurs. 2013;39(4):190–196. 180. [PubMed] [Google Scholar]
- 13.Wu J, Nie SD, Wang S. Tau pathology in diabetes mellitus. Pharmazie. 2013;68(8):649–652. [PubMed] [Google Scholar]
- 14.Yamashima T. Reconsider Alzheimer's disease by the 'calpain-cathepsin hypothesis'-A perspective review. Progress in Neurobiology. 2013;105:1–23. doi: 10.1016/j.pneurobio.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Tiiman A, Palumaa P, Tougu V. The missing link in the amyloid cascade of Alzheimer's disease - Metal ions. Neurochemistry International. 2013;62(4):367–378. doi: 10.1016/j.neuint.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Grover HS, Luthra S. Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. J Indian Soc Periodontol. 2013;17(3):292–301. doi: 10.4103/0972-124X.115642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. 2011 [Google Scholar]
- 18.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82(8):510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 19.Janson J, Laedtke T, Parisi JE, O'Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 21.Langerhans P. Inaugural-dissertation. 1869. Beitrage zur mikroscopischen anatomie der bauchspeichel druse. [Google Scholar]
- 22.Arntfield ME, van der Kooy D. Beta-cell evolution: How the pancreas borrowed from the brain. Bioessays. 2011;33(8):582–587. doi: 10.1002/bies.201100015. [DOI] [PubMed] [Google Scholar]
- 23.Pearse AG, Polak JM. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut. 1971;12(10):783–788. doi: 10.1136/gut.12.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita T, Kobayashi S, Yui R. Paraneuron concept and its current implications. Adv Biochem Psychopharmacol. 1980;25:321–325. [PubMed] [Google Scholar]
- 25.Maechler P, Wollheim CB. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402(6762):685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 26.Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. GABA and pancreatic beta-cells: colocalization of glutamic acid decarboxylase (GAD) and GABA with synaptic-like microvesicles suggests their role in GABA storage and secretion. EMBO J. 1991;10(5):1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonoi T, Mizuno N, Inagaki N, Kuromi H, Seino Y, Miyazaki J, et al. Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem. 1994;269(25):16989–16992. [PubMed] [Google Scholar]
- 28.Henquin JC, Meissner HP. Significance of Ionic Fluxes and Changes in Membrane-Potential for Stimulus-Secretion Coupling in Pancreatic B-Cells. Experientia. 1984;40(10):1043–1052. doi: 10.1007/BF01971450. [DOI] [PubMed] [Google Scholar]
- 29.Yang XJ, Kow LM, Funabashi T, Mobbs CV. Hypothalamic glucose sensor - Similarities to and differences from pancreatic beta-cell mechanisms. Diabetes. 1999;48(9):1763–1772. doi: 10.2337/diabetes.48.9.1763. [DOI] [PubMed] [Google Scholar]
- 30.Sunami E, Kanazawa H, Hashizume H, Takeda M, Hatakeyama K, Ushiki T. Morphological characteristics of Schwann cells in the islets of Langerhans of the murine pancreas. Archives of Histology and Cytology. 2001;64(2):191–201. doi: 10.1679/aohc.64.191. [DOI] [PubMed] [Google Scholar]
- 31.Hebrok M, Reichardt LF. Brain meets pancreas: netrin, an axon guidance molecule, controls epithelial cell migration. Trends in Cell Biology. 2004;14(4):153–155. doi: 10.1016/j.tcb.2004.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langley OK, Aletseeufrecht MC, Grant NJ, Gratzl M. Expression of the Neural Cell-Adhesion Molecule Ncam in Endocrine-Cells. Journal of Histochemistry & Cytochemistry. 1989;37(6):781–791. doi: 10.1177/37.6.2723399. [DOI] [PubMed] [Google Scholar]
- 33.van Arensbergen J, Garcia-Hurtado J, Moran I, Maestro MA, Xu XB, Van de Casteele M, et al. Derepression of Polycomb targets during pancreatic organogenesis allows insulin-producing beta-cells to adopt a neural gene activity program. Genome Research. 2010;20(6):722–732. doi: 10.1101/gr.101709.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philipson LH, Kusnetsov A, Larson T, Zeng YJ, Westermark G. Human, Rodent, and Canine Pancreatic Beta-Cells Express a Sodium-Channel Alpha-1-Subunit Related to a Fetal Brain Isoform. Diabetes. 1993;42(9):1372–1377. doi: 10.2337/diab.42.9.1372. [DOI] [PubMed] [Google Scholar]
- 35.Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells - Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. Journal of Biological Chemistry. 1997;272(3):1929–1934. doi: 10.1074/jbc.272.3.1929. [DOI] [PubMed] [Google Scholar]
- 36.Cruz SA, Tseng YC, Kaiya H, Hwang PP. Ghrelin affects carbohydrate-glycogen metabolism via insulin inhibition and glucagon stimulation in the zebrafish (Danio rerio) brain. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(2):190–200. doi: 10.1016/j.cbpa.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin Gene-Expression and Insulin Synthesis in Mammalian Neuronal Cells. Journal of Biological Chemistry. 1994;269(11):8445–8454. [PubMed] [Google Scholar]
- 38.Leloup C, Arluison M, Lepetit N, Cartier N, Marfaingjallat P, Ferre P, Penicaud L. Glucose-Transporter 2 (Glut-2) - Expression in Specific Brain Nuclei. Brain Research. 1994;638(1–2):221–226. doi: 10.1016/0006-8993(94)90653-x. [DOI] [PubMed] [Google Scholar]
- 39.Thor S, Ericson J, Brannstrom T, Edlund T. The Homeodomain Lim Protein Isl-1 Is Expressed in Subsets of Neurons and Endocrine-Cells in the Adult-Rat. Neuron. 1991;7(6):881–889. doi: 10.1016/0896-6273(91)90334-v. [DOI] [PubMed] [Google Scholar]
- 40.Jensen J. Gene regulatory factors in pancreatic development. Developmental Dynamics. 2004;229(1):176–200. doi: 10.1002/dvdy.10460. [DOI] [PubMed] [Google Scholar]
- 41.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Current Opinion in Neurobiology. 2002;12(1):26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 42.Muller M, Jabs N, Lorke DE, Fritzsch B, Sander M. Nkx6.1 controls migration and axon pathfinding of cranial branchio-motoneurons. Development. 2003;130(23):5815–5826. doi: 10.1242/dev.00815. [DOI] [PubMed] [Google Scholar]
- 43.Vult von Steyern F, Martinov V, Rabben I, Nja A, de Lapeyriere O, Lomo T. The homeodomain transcription factors Islet 1 and HB9 are expressed in adult alpha and gamma motoneurons identified by selective retrograde tracing. Eur J Neurosci. 1999;11(6):2093–2102. doi: 10.1046/j.1460-9568.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 44.Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48(11):628–634. doi: 10.1002/dvg.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas-Reetz AC, De Camilli P. A role for synaptic vesicles in non-neuronal cells: clues from pancreatic beta cells and from chromaffin cells. FASEB J. 1994;8(2):209–216. doi: 10.1096/fasebj.8.2.7907072. [DOI] [PubMed] [Google Scholar]
- 46.Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, et al. Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature. 1989;341(6239):233–236. doi: 10.1038/341233a0. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nature Medicine. 2011;17(7):888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, et al. Friedreich's ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271(5254):1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 49.Koutnikova H, Campuzano V, Foury F, Dolle P, Cazzalini O, Koenig M. Studies of human, mouse and yeast homologues indicate a mitochondrial function for frataxin. Nature Genetics. 1997;16(4):345–351. doi: 10.1038/ng0897-345. [DOI] [PubMed] [Google Scholar]
- 50.Durr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. Clinical and genetic abnormalities in patients with Friedreich's ataxia. N Engl J Med. 1996;335(16):1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 51.Kim E, Napierala M, Dent SYR. Hyperexpansion of GAA repeats affects post-initiation steps of FXN transcription in Friedreich's ataxia. Nucleic Acids Research. 2011;39(19):8366–8377. doi: 10.1093/nar/gkr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polling S, Hill AF, Hatters DM. Polyglutamine aggregation in Huntington and related diseases. Adv Exp Med Biol. 2012;769:125–140. doi: 10.1007/978-1-4614-5434-2_8. [DOI] [PubMed] [Google Scholar]
- 53.Robertson AL, Bottomley SP. Molecular pathways to polyglutamine aggregation. Adv Exp Med Biol. 2012;769:115–124. doi: 10.1007/978-1-4614-5434-2_7. [DOI] [PubMed] [Google Scholar]
- 54.Bauer PO, Nukina N. The pathogenic mechanisms of polyglutamine diseases and current therapeutic strategies. J Neurochem. 2009;110(6):1737–1765. doi: 10.1111/j.1471-4159.2009.06302.x. [DOI] [PubMed] [Google Scholar]
- 55.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin SR, et al. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nature Structural & Molecular Biology. 2009;16(4):390–396. doi: 10.1038/nsmb.1579. [DOI] [PubMed] [Google Scholar]
- 56.Schmucker S, Martelli A, Colin F, Page A, Wattenhofer-Donze M, Reutenauer L, et al. Mammalian Frataxin: An Essential Function for Cellular Viability through an Interaction with a Preformed ISCU/NFS1/ISD11 Iron-Sulfur Assembly Complex. Plos One. 2011;6(1) doi: 10.1371/journal.pone.0016199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perdomini M, Hick A, Puccio H, Pook MA. Animal and cellular models of Friedreich ataxia. Journal of Neurochemistry. 2013;126:65–79. doi: 10.1111/jnc.12219. [DOI] [PubMed] [Google Scholar]
- 58.Ristow M, Mulder H, Pomplun D, Schulz TJ, Muller-Schmehl K, Krause A, et al. Frataxin deficiency in pancreatic islets causes diabetes due to loss of beta cell mass. J Clin Invest. 2003;112(4):527–534. doi: 10.1172/JCI18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Puccio H, Simon D, Cossee M, Criqui-Filipe P, Tiziano F, Melki J, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet. 2001;27(2):181–186. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 60.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–291. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 61.Hutton M, Hardy J. The presenilins and Alzheimer's disease. Hum Mol Genet. 1997;6(10):1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 62.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, et al. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1(12):1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 63.Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 64.Wang W, Guo Y, Xu M, Huang HH, Novikova L, Larade K, et al. Development of diabetes in lean Ncb5or-null mice is associated with manifestations of endoplasmic reticulum and oxidative stress in beta cells. Biochim Biophys Acta. 2011;1812(11):1532–1541. doi: 10.1016/j.bbadis.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersen G, Wegner L, Rose CS, Xie J, Zhu H, Larade K, et al. Variation in NCB5OR: studies of relationships to type 2 diabetes, maturity-onset diabetes of the young, and gestational diabetes mellitus. Diabetes. 2004;53(11):2992–2997. doi: 10.2337/diabetes.53.11.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, et al. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans. 2008;36(Pt 6):1282–1287. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, et al. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157(2):401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Protter D, Lang C, Cooper AA. alphaSynuclein and Mitochondrial Dysfunction: A Pathogenic Partnership in Parkinson's Disease? Parkinsons Dis. 2012;2012:829207. doi: 10.1155/2012/829207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie JX, Zhu H, Larade K, Ladoux A, Seguritan A, Chu M, et al. Absence of a reductase, NCB50R, causes insulin-deficient diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10750–10755. doi: 10.1073/pnas.0404044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van den Ouweland JM, Lemkes HH, Trembath RC, Ross R, Velho G, Cohen D, et al. Maternally inherited diabetes and deafness is a distinct subtype of diabetes and associates with a single point mutation in the mitochondrial tRNA(Leu(UUR)) gene. Diabetes. 1994;43(6):746–751. doi: 10.2337/diab.43.6.746. [DOI] [PubMed] [Google Scholar]
- 72.Wang HY, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4 alpha regulates the expression of pancreatic beta-cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. Journal of Biological Chemistry. 2000;275(46):35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 73.Wang HY, Antinozzi PA, Hagenfeldt KA, Maechler P, Wollheim CB. Molecular targets of a human HNF1 alpha mutation responsible for pancreatic beta-cell dysfunction. Embo Journal. 2000;19(16):4257–4264. doi: 10.1093/emboj/19.16.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brehm MA, Powers AC, Shultz LD, Greiner DL. Advancing Animal Models of Human Type 1 Diabetes by Engraftment of Functional Human Tissues in Immunodeficient Mice. Cold Spring Harbor Perspectives in Medicine. 2012;2(5) doi: 10.1101/cshperspect.a007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larade K, Jiang ZG, Zhang YZ, Wang WF, Bonner-Weir S, Zhu H, et al. Loss of Ncb5or Results in Impaired Fatty Acid Desaturation, Lipoatrophy, and Diabetes. Journal of Biological Chemistry. 2008;283(43):29285–29291. doi: 10.1074/jbc.M804645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalman FS, Lizak B, Nagy SK, Meszaros T, Zambo V, Mandl J, et al. Natural mutations lead to enhanced proteasomal degradation of human Ncb5or, a novel flavoheme reductase. Biochimie. 2013;95(7):1403–1410. doi: 10.1016/j.biochi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Zhu H, Wang WF, Wang HP, Xu MEL, Swerdlow RH. Impaired iron metabolism in monogenic Ncb5or diabetes. Diabetes. 2013;62:556–587. (Abstract). [Google Scholar]
- 78.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population - Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 79.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reiman EM, Chen KW, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, et al. Reduced Posterior Cingulate Mitochondrial Activity in Expired Young Adult Carriers of the APOE epsilon 4 Allele, the Major Late-Onset Alzheimer's Susceptibility Gene. Journal of Alzheimers Disease. 2010;22(1):307–313. doi: 10.3233/JAD-2010-100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer's disease. Lancet. 1994;344(8926):895. doi: 10.1016/s0140-6736(94)92871-1. [DOI] [PubMed] [Google Scholar]
- 84.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 85.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer's Disease Treatment Studies. Am J Psychiatry. 2002;159(5):738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 87.Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21(13):4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, et al. Alzheimer's disease is associated with reduced expression of energy in posterior cingulate metabolism genes neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bloss CS, Delis DC, Salmon DP, Bondi MW. Decreased Cognition in Children with Risk Factors for Alzheimer's Disease. Biological Psychiatry. 2008;64(10):904–906. doi: 10.1016/j.biopsych.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makris A, Piperopoulos A, Karmaniolou I. Multiple sclerosis: basic knowledge and new insights in perioperative management. J Anesth. 2013 doi: 10.1007/s00540-013-1697-2. [DOI] [PubMed] [Google Scholar]
- 91.Ponka P. Hereditary causes of disturbed iron homeostasis in the central nervous system. Ann N Y Acad Sci. 2004;1012:267–281. doi: 10.1196/annals.1306.022. [DOI] [PubMed] [Google Scholar]
- 92.Vlassenko AG, Mintun MA, Xiong CJ, Sheline YI, Goate AM, Benzinger TLS, et al. Amyloid-beta plaque growth in cognitively normal adults: Longitudinal [11C]Pittsburgh compound B data. Annals of Neurology. 2011;70(5):857–861. doi: 10.1002/ana.22608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer's disease. Int J Biochem Cell Biol. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trimmer PA, Swerdlow RH, Parks JK, Keeney P, Bennett JP, Jr, Miller SW, et al. Abnormal mitochondrial morphology in sporadic Parkinson's and Alzheimer's disease cybrid cell lines. Exp Neurol. 2000;162(1):37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 95.Belin AC, Bjork BF, Westerlund M, Galter D, Sydow O, Lind C, et al. Association study of two genetic variants in mitochondrial transcription factor A (TFAM) in Alzheimer's and Parkinson's disease. Neurosci Lett. 2007;420(3):257–262. doi: 10.1016/j.neulet.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Swerdlow RH, Khan SM. A "mitochondrial cascade hypothesis" for sporadic Alzheimer's disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 97.Swerdlow RH, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swerdlow RH, Burns JM, Khan SM. The Alzheimer's Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH. Coenzyme A-acetylating enzymes in Alzheimer's disease: possible cholinergic 'compartment' of pyruvate dehydrogenase. Neurosci Lett. 1980;18(1):105–110. doi: 10.1016/0304-3940(80)90220-7. [DOI] [PubMed] [Google Scholar]
- 101.Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13(1):72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- 102.Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol. 1988;45(8):836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 103.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 104.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer's disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 106.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, et al. Brain cytochrome oxidase in Alzheimer's disease. J Neurochem. 1992;59(2):776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- 107.Mutisya EM, Bowling AC, Beal MF. Cortical Cytochrome-Oxidase Activity Is Reduced in Alzheimers-Disease. Journal of Neurochemistry. 1994;63(6):2179–2184. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 108.Gasparini L, Racchi M, Benussi L, Curti D, Binetti G, Bianchetti A, et al. Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett. 1997;231(2):113–117. doi: 10.1016/s0304-3940(97)00536-3. [DOI] [PubMed] [Google Scholar]
- 109.Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem. 1994;269(18):13623–13628. [PubMed] [Google Scholar]
- 110.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, et al. Alzheimer's disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48(2):148–155. [PubMed] [Google Scholar]
- 111.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85(15):3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- 112.Schneider SA, Zorzi G, Nardocci N. Pathophysiology and treatment of neurodegeneration with brain iron accumulation in the pediatric population. Curr Treat Options Neurol. 2013;15(5):652–667. doi: 10.1007/s11940-013-0254-5. [DOI] [PubMed] [Google Scholar]
- 113.Lee E, Eom JE, Kim HL, Baek KH, Jun KY, Kim HJ, et al. Effect of conjugated linoleic acid, mu-calpain inhibitor, on pathogenesis of Alzheimer's disease. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2013;1831(4):709–718. doi: 10.1016/j.bbalip.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Briggs CA, Schneider C, Richardson JC, Stutzmann GE. Beta amyloid peptide plaques fail to alter evoked neuronal calcium signals in APP/PS1 Alzheimer's disease mice. Neurobiol Aging. 2013;34(6):1632–1643. doi: 10.1016/j.neurobiolaging.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141(6):1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757(5–6):553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 117.Ansari MA, Scheff SW. Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex. Journal of Neuropathology and Experimental Neurology. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1(1):304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer's disease cases: evidence for maternal inheritance. Neurology. 1996;47(1):254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- 120.Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, de Leon M. Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Hum Genomics. 2010;4(3):170–193. doi: 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Duara R, Lopez-Alberola RF, Barker WW, Loewenstein DA, Zatinsky M, Eisdorfer CE, et al. A comparison of familial and sporadic Alzheimer's disease. Neurology. 1993;43(7):1377–1384. doi: 10.1212/wnl.43.7.1377. [DOI] [PubMed] [Google Scholar]
- 122.Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gotz ME, Double K, Gerlach M, Youdim MBH, Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. Redox-Active Metals in Neurological Disorders. 2004;1012:193–208. doi: 10.1196/annals.1306.017. [DOI] [PubMed] [Google Scholar]
- 124.Atwood CS, Huang XD, Moir RD, Tanzi RE, Bush AI. Role of free radicals and metal ions in the pathogenesis of Alzheimer's disease. Metal Ions in Biological Systems, Vol 36. 1999;36:309–364. [PubMed] [Google Scholar]
- 125.Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT. Redox-active metals, oxidative stress, and Alzheimer's disease pathology. Ann N Y Acad Sci. 2004;1012:153–163. doi: 10.1196/annals.1306.012. [DOI] [PubMed] [Google Scholar]
- 126.Goodman L. Alzheimer's disease: a clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J Nerv Ment Dis. 1953;118(2):97–130. [PubMed] [Google Scholar]
- 127.Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PLR, Siedlak SL, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. Journal of Neurochemistry. 1998;70(5):2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 129.Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, et al. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer's disease. J Neurochem. 2002;82(5):1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- 130.Behl C, Davis JB, Lesley R, Schubert D. Hydrogen-Peroxide Mediates Amyloid-Beta Protein Toxicity. Cell. 1994;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 131.Huang XD, Atwood CS, Hartshorn MA, Multhaup G, Goldstein LE, Scarpa RC, et al. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38(24):7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 132.Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer's beta-amyloid peptide (1–40) in cultured hippocampal neurons. Exp Neurol. 1995;131(2):193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 133.Wang L, Xi G, Keep RF, Hua Y. Iron enhances the neurotoxicity of amyloid beta. Transl Stroke Res. 2012;3(1):107–113. doi: 10.1007/s12975-011-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mueller C, Schrag M, Crofton A, Stolte J, Muckenthaler MU, Magaki S, et al. Altered serum iron and copper homeostasis predicts cognitive decline in mild cognitive impairment. J Alzheimers Dis. 2012;29(2):341–350. doi: 10.3233/JAD-2011-111841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Arai Y, Suzuki A, Mizuguchi M, Takashima S. Developmental and aging changes in the expression of amyloid precursor protein in Down syndrome brains. Brain Dev. 1997;19(4):290–294. doi: 10.1016/s0387-7604(97)00559-7. [DOI] [PubMed] [Google Scholar]
- 136.Furuyama K, Kaneko K, Vargas PD. Heme as a magnificent molecule with multiple missions: Heme determines its own fate and governs cellular homeostasis. Tohoku Journal of Experimental Medicine. 2007;213(1):1–16. doi: 10.1620/tjem.213.1. [DOI] [PubMed] [Google Scholar]
- 137.Horowitz MP, Greenamyre JT. Mitochondrial Iron Metabolism and Its Role in Neurodegeneration. Journal of Alzheimers Disease. 2010;20:S551–S568. doi: 10.3233/JAD-2010-100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barrientos A, Casademont J, Cardellach F, Estivill X, Urbano-Marquez A, Nunes V. Reduced steady-state levels of mitochondrial RNA and increased mitochondrial DNA amount in human brain with aging. Brain Res Mol Brain Res. 1997;52(2):284–289. doi: 10.1016/s0169-328x(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 139.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 140.Swerdlow RH, Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 141.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 142.Jitrapakdee S, Wutthisathapornchai A, Wallace JC, MacDonald MJ. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia. 2010;53(6):1019–1032. doi: 10.1007/s00125-010-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maechler P. Mitochondrial function and insulin secretion. Mol Cell Endocrinol. 2013 doi: 10.1016/j.mce.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 144.Jhun BS, Lee H, Jin ZG, Yoon Y. Glucose stimulation induces dynamic change of mitochondrial morphology to promote insulin secretion in the insulinoma cell line INS-1E. PLoS One. 2013;8(4):e60810. doi: 10.1371/journal.pone.0060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 147.Martin SD, McGee SL. The role of mitochondria in the aetiology of insulin resistance and type 2 diabetes. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbagen.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 148.Sako Y, Grill VE. A 48-Hour Lipid Infusion in the Rat Time-Dependently Inhibits Glucose-Induced Insulin-Secretion and B-Cell Oxidation through a Process Likely Coupled to Fatty-Acid Oxidation. Endocrinology. 1990;127(4):1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- 149.Opie EL. The Relation Oe Diabetes Mellitus to Lesions of the Pancreas. Hyaline Degeneration of the Islands Oe Langerhans. J Exp Med. 1901;5(5):527–540. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Westermark P, Wernstedt C, Wilander E, Sletten K. A Novel Peptide in the Calcitonin Gene Related Peptide Family as an Amyloid Fibril Protein in the Endocrine Pancreas. Biochemical and Biophysical Research Communications. 1986;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 151.Hoppener JW, Ahren B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343(6):411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- 152.Westermark P. Amyloid and Polypeptide Hormones - What Is Their Interrelationship. Amyloid-International Journal of Experimental and Clinical Investigation. 1994;1(1):47–60. [Google Scholar]
- 153.Li XL, Chen T, Wong YS, Xu G, Fan RR, Zhao HL, et al. Involvement of mitochondrial dysfunction in human islet amyloid polypeptide-induced apoptosis in INS-1E pancreatic beta cells: An effect attenuated by phycocyanin. Int J Biochem Cell Biol. 2011;43(4):525–534. doi: 10.1016/j.biocel.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 154.Xiong H, Zheng C, Wang J, Song J, Zhao G, Shen H, et al. The neuroprotection of liraglutide on Alzheimer-like learning and memory impairment by modulating the hyperphosphorylation of tau and neurofilament proteins and insulin signaling pathways in mice. J Alzheimers Dis. 2013;37(3):623–635. doi: 10.3233/JAD-130584. [DOI] [PubMed] [Google Scholar]
- 155.Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2013 doi: 10.1530/JOE-13-0221. [DOI] [PubMed] [Google Scholar]
- 156.Fan R, Li X, Gu X, Chan JC, Xu G. Exendin-4 protects pancreatic beta cells from human islet amyloid polypeptide-induced cell damage: potential involvement of AKT and mitochondria biogenesis. Diabetes Obes Metab. 2010;12(9):815–824. doi: 10.1111/j.1463-1326.2010.01238.x. [DOI] [PubMed] [Google Scholar]
- 157.Kan M, Guo G, Singh B, Singh V, Zochodne DW. Glucagon-like peptide 1, insulin, sensory neurons, and diabetic neuropathy. J Neuropathol Exp Neurol. 2012;71(6):494–510. doi: 10.1097/NEN.0b013e3182580673. [DOI] [PubMed] [Google Scholar]
- 158.Dandona P, Hussain MAM, Varghese Z, Politis D, Flynn DM, Hoffbrand AV. Insulin Resistance and Iron Overload. Annals of Clinical Biochemistry. 1983 Mar;20:77–79. doi: 10.1177/000456328302000203. [DOI] [PubMed] [Google Scholar]
- 159.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, et al. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117(5):1155–1163. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 160.Thomas MC, MacIsaac RJ, Tsalamandris C, Jerums G. Elevated iron indices in patients with diabetes. Diabet Med. 2004;21(7):798–802. doi: 10.1111/j.1464-5491.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- 161.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among US adults. Diabetes Care. 1999;22(12):1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 162.Aregbesola A, Voutilainen S, Virtanen JK, Mursu J, Tuomainen TP. Body iron stores and the risk of type 2 diabetes in middle-aged men. Eur J Endocrinol. 2013;169(2):247–253. doi: 10.1530/EJE-13-0145. [DOI] [PubMed] [Google Scholar]
- 163.Cheung CL, Cheung TT, Lam KS, Cheung BM. High ferritin and low transferrin saturation are associated with pre-diabetes among a national representative sample of U.S. adults. Clin Nutr. 2012 doi: 10.1016/j.clnu.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 164.Rong Y, Bao W, Rong S, Fang M, Wang D, Yao P, et al. Hemochromatosis gene (HFE) polymorphisms and risk of type 2 diabetes mellitus: a meta-analysis. Am J Epidemiol. 2012;176(6):461–472. doi: 10.1093/aje/kws126. [DOI] [PubMed] [Google Scholar]
- 165.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. American Journal of Clinical Nutrition. 2004;79(1):70–75. doi: 10.1093/ajcn/79.1.70. [DOI] [PubMed] [Google Scholar]
- 166.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clinical Chemistry. 2005;51(7):1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- 167.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 168.Chowdhury SK, Smith DR, Fernyhough P. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 2013;51:56–65. doi: 10.1016/j.nbd.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 169.Chowdhury SK, Dobrowsky RT, Fernyhough P. Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes. Mitochondrion. 2011;11(6):845–854. doi: 10.1016/j.mito.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saleh A, Chowdhury SKR, Smith DR, Balakrishnan S, Tessler L, Martens C, et al. Ciliary neurotrophic factor activates NF-kappa B to enhance mitochondrial bioenergetics and prevent neuropathy in sensory neurons of streptozotocin-induced diabetic rodents. Neuropharmacology. 2013;65:65–73. doi: 10.1016/j.neuropharm.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 172.Beisswenger PJ, Howell SK, Russell GB, Miller ME, Rich SS, Mauer M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care. 2013;36(10):3234–3239. doi: 10.2337/dc12-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18(6):926–933. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 174.Deng Y, Zhang Y, Li Y, Xiao S, Song D, Qing H, et al. Occurrence and distribution of salsolinol-like compound 1-acetyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (ADTIQ) in parkinsonian brains. J Neural Transm. 2012;119(4):435–441. doi: 10.1007/s00702-011-0724-4. [DOI] [PubMed] [Google Scholar]
- 175.Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E. Type 1 diabetes exaggerates features of Alzheimer's disease in APP transgenic mice. Exp Neurol. 2010;223(2):422–431. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Currais A, Prior M, Lo D, Jolivalt C, Schubert D, Maher P. Diabetes exacerbates amyloid and neurovascular pathology in aging-accelerated mice. Aging Cell. 2012;11(6):1017–1026. doi: 10.1111/acel.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Belin C, Gatt MT. Pain and dementia. Psychol Neuropsychiatr Vieil. 2006;4(4):247–254. [PubMed] [Google Scholar]
- 178.Jolivalt CG, Calcutt NA, Masliah E. Similar Pattern of Peripheral Neuropathy in Mouse Models of Type 1 Diabetes and Alzheimer's Disease. Neuroscience. 2012;202:405–412. doi: 10.1016/j.neuroscience.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sima AA, Li ZG. Diabetes and Alzheimer's disease - is there a connection? Rev Diabet Stud. 2006;3(4):161–168. doi: 10.1900/RDS.2006.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 181.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 182.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 183.Aisen PS, Vellas B. Passive immunotherapy for Alzheimer's disease: what have we learned, and where are we headed? J Nutr Health Aging. 2013;17(1):49–50. doi: 10.1007/s12603-013-0001-3. [DOI] [PubMed] [Google Scholar]
- 184.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 185.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9(7):702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 186.Joseph-Mathurin N, Dorieux O, Trouche SG, Boutajangout A, Kraska A, Fontes P, et al. Amyloid beta immunization worsens iron deposits in the choroid plexus and cerebral microbleeds. Neurobiol Aging. 2013;34(11):2613–2622. doi: 10.1016/j.neurobiolaging.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Swerdlow RH. Role and Treatment of Mitochondrial DNA-Related Mitochondrial Dysfunction in Sporadic Neurodegenerative Diseases. Current Pharmaceutical Design. 2011;17(31):3356–3373. doi: 10.2174/138161211798072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Swerdlow RH. Bioenergetic Medicine. Br J Pharmacol. 2013 doi: 10.1111/bph.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yoneda S, Uno S, Iwahashi H, Fujita Y, Yoshikawa A, Kozawa J, et al. Predominance of beta-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab. 2013;98(5):2053–2061. doi: 10.1210/jc.2012-3832. [DOI] [PubMed] [Google Scholar]
- 190.Greenbaum CJ, Buckingham B, Chase HP, Krischer J. Diabetes Prevention Trial TDSG: Metabolic tests to determine risk for type 1 diabetes in clinical trials. Diabetes Metab Res Rev. 2011;27(6):584–589. doi: 10.1002/dmrr.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sysi-Aho M, Ermolov A, Gopalacharyulu PV, Tripathi A, Seppanen-Laakso T, Maukonen J, et al. Metabolic regulation in progression to autoimmune diabetes. PLoS Comput Biol. 2011;7(10):e1002257. doi: 10.1371/journal.pcbi.1002257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49(2):261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- 193.Beck-Nielsen H, Groop LC. Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(5):1714–1721. doi: 10.1172/JCI117518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu M, Wang W, Frontera JR, Neely MC, Lu J, Aires D, et al. Ncb5or deficiency increases fatty acid catabolism and oxidative stress. The Journal of biological chemistry. 2011;286(13):11141–11154. doi: 10.1074/jbc.M110.196543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aiyar L, Shuman C, Hayeems R, Dupuis A, Pu S, Wodak S, et al. Risk estimates for complex disorders: comparing personal genome testing and family history. Genet Med. 2013 doi: 10.1038/gim.2013.115. 10.1038/gim.2013.115. [DOI] [PubMed] [Google Scholar]