Capsule Summary
In the nasopharyngeal microbiome of children with severe bronchiolitis, Proteobacteria, specifically Haemophilus influenzae and Moraxella catarrhalis, were associated with the viral etiology of bronchiolitis, and Moraxella species were more common among children with acute wheezing.
Keywords: bronchiolitis, respiratory syncytial virus, human rhinovirus, microbiome, Proteobacteria, Haemophilus influenzae, Moraxella catarrhalis
To the editor:
Bronchiolitis is the leading cause of hospitalization for US infants1 and is usually caused by respiratory syncytial virus (RSV) or human rhinovirus (HRV).2 These early-life viral infections are associated with the development of recurrent wheezing and asthma.3 While the primary focus of research on the development of wheezing and asthma has been on viruses, there is increasing evidence that bacteria also play a role in asthma pathogenesis.4
Bisgaard and colleagues, in a prospective study of 321 healthy neonates, found an increased risk of recurrent wheezing and asthma in infants who had hypopharyngeal bacterial colonization by Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis.4 Moreover, Hilty and colleagues found that the pathogenic Proteobacteria phylum (e.g. Haemophilus and Moraxella species) was significantly more common in children with asthma than in controls. 5 It is unknown, however, if specific bacteria colonizing the respiratory tract (i.e. respiratory microbiome) contribute to recurrent wheezing and the development of asthma in children with severe bronchiolitis (e.g. children hospitalized with bronchiolitis).
Since children with severe bronchiolitis are at high risk for later recurrent wheezing and asthma,3 we examined if these children would have similar microbial perturbations as those observed in older children with asthma..5 As part of a prospective, multicenter, multiyear study of >2,000 children hospitalized with bronchiolitis, we used 16S rRNA gene pyrosequencing to analyze 100 nasopharyngeal aspirates (NPAs) from children age <2 years hospitalized with bronchiolitis at one participating hospital. Since we did not have healthy controls, we hypothesized that the Proteobacteria phylum would be associated with the viral etiology of the child's bronchiolitis (RSV, HRV, both) and with acute wheezing status (present/absent). Detailed methods, including study inclusion/ exclusion criteria and patient demographics, may be found in the online supplement.
Site teams gathered detailed clinical data, collected NPAs, and performed short-term patient follow-up.2 As described previously,2 every child in this cohort had a NPA tested for 16 viruses by real-time RT-PCR.2 16S rRNA gene (n=100) and whole genome shotgun (WGS, n=10) sequencing and analysis were performed on bacterial DNA isolated from each sample (details in the online supplement). Statistical analyses and supervised machine learning (see online supplement for more details) were used to identify bacterial taxa associated with viral etiology and acute wheezing status. Covariates examined but not associated with microbiome differences were: age, exposure to cigarette smoke, antibiotic treatment, and history of breastfeeding (data not shown). In addition, restricting the following analyses to children with a more stringent definition of bronchiolitis (e.g. age <1 year and no prior history of wheezing) did not materially change the results (data not shown).
We found that an increase in H. influenzae and M. catarrhalis discriminated between children with RSV/HRV co-infection and those children with a single virus infection (Table 1). The RSV/HRV co-infection finding is of interest given that in a separate multivariable analysis from the >2000 children in this cohort, those with RSV/HRV co-infection had a significantly longer length-of-stay when compared with children with RSV only infections.2 In WGS analysis, H. influenzae was also detected in most RSV only infected and co-infected samples, but not in samples from children infected with HRV only. These results suggest that in the context of bronchiolitis, it is possible that specific viruses may promote the presence of specific bacterial species or vice versa. Indeed, respiratory viruses disturb the respiratory epithelium, allowing for greater bacterial adherence, and possibly increase the chances of a secondary bacterial infection.6 Interestingly, the opposite (i.e. colonizing bacteria predisposing to viral disease) may also be true since specific bacteria may increase the chance of viral infection.7 Therefore, a virus-only or bacteria-only approach to respiratory conditions may be too simplistic. And more studies with a larger number of samples are needed to confirm these results and determine whether and how bacterial species interact with RSV and HRV or, alternatively, whether the presence of specific bacterial species is a sign of a more global susceptibility state.
Table I.
Taxa that discriminate between viral etiology of children with severe bronchiolitis
| RSV only vs. HRV only | ||
|---|---|---|
| OTU ID# | Taxonomic Classification | Change |
| 122 | Porphyromonas | Increased in HRV only |
| 350 | Prevotella | Increased in HRV only |
| 1337 | Prevotella | Increased in HRV only |
| 232 | Fusobacterium | Increased in HRV only |
| 297 | Leptotrichia | Increased in HRV only |
| Single (RSV or HRV) vs. Co-infection | ||
|---|---|---|
| OTU ID# | Taxonomic Classification | Change |
| 1878 | Haemophilus | Increased in co-infection |
| 1558 | Haemophilus | Increased in co-infection |
| 1944 | Actinomyces | Increased in co-infection |
| RSV only vs. Co-infection | ||
|---|---|---|
| OTU ID# | Taxonomic classification | Change |
| 1878 | Haemophilus | Increased in co-infection |
| 1558 | Haemophilus | Increased in co-infection |
| 1337 | Prevotella | Increased in co-infection |
| 350 | Prevotella | Increased in co-infection |
| HRV only vs. Co-infection | ||
|---|---|---|
| OTU ID# | Taxonomic Classification | Change |
| 1878 | Haemophilus | Increased in co-infection |
| 1558 | Haemophilus | Increased in co-infection |
| 213 | Propionibacterium | Increased in HRV only |
| 252 | Moraxella | Increased in co-infection |
| Acute Wheeze on Admission vs. Absence of Acute Wheeze on Admission | ||
|---|---|---|
| OTU ID# | Taxonomic Classification | Change |
| 122 | Porphyromonas | Increased in acute wheeze |
| 350 | Prevotella | Increased in acute wheeze |
| 1558 | Haemophilus | Increased in acute wheeze |
| 296 | Moraxella | Increased in acute wheeze |
| 834 | Staphylococcus | Increased in no acute wheeze |
| 240 | Staphylococcus | Increased in no acute wheeze |
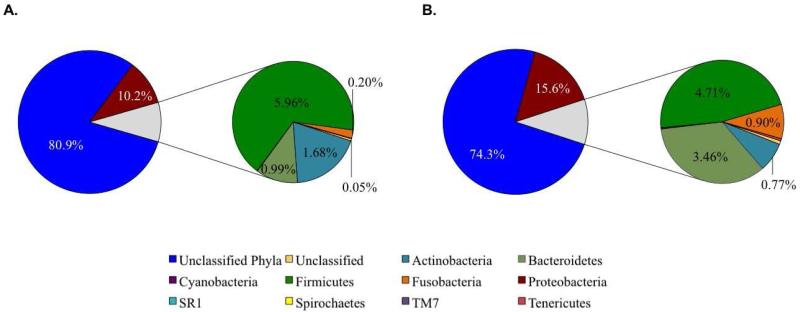
Another means of confirming the potential importance of Proteobacteria in severe bronchiolitis is to examine whether perturbations in the microbiome are associated with acute wheezing at admission. Wheezing is not required to diagnose a child with bronchiolitis,8 but infants who wheeze at certain times of year are more likely to develop asthma.3 In the present study, children who wheezed on admission had an insignificant increase in the mean relative abundance of Proteobacteria (15.6% vs. 10.2%, P=0.58) compared with children without wheezing (Figure 1). Examining this phyla at the genus level demonstrated that Moraxella were significantly more common in children who were wheezing upon admission than those who were not wheezing (10.3% vs. 3.64%, P=0.009, Table I and Online Table II).
Figure 1. Average abundances of phyla present by patient wheeze status.
Exploded pie charts illustrate the abundance (as a percentage) of phyla present across all samples in A) patients without wheeze (n=36), and B) patients with wheeze (n=59) upon admission.
Data regarding the association of Proteobacteria with wheezing and asthma seems to be accumulating. Directly building on the data from Bisgaard 4 and Hilty5, Marri and colleagues found that Proteobacteria were present in higher proportions of adults with mild asthma compared with controls (37% vs. 15%; P<0.001). Our data also support the hypothesis that Proteobacteria may play a role in wheezing respiratory illnesses.
We believe this study is the first to examine the nasopharyngeal microbiome in children with severe bronchiolitis. Our data suggest that the microbial perturbations previously reported in children with asthma extend to younger children with severe bronchiolitis. Of particular interest are the Proteobacteria, specifically Haemophilus influenzae and Moraxella catarrhalis, since our data show that Proteobacteria were associated with RSV and RSV/HRV infections and acute wheezing. However, studies following children with severe bronchiolitis until the development of recurrent wheezing or asthma are necessary to fully understand the relationship between the microbiome, the viral causes of bronchiolitis, and the risk of developing wheezing respiratory illnesses. As future respiratory microbiome studies are conducted, researchers will need to address the challenges of sampling the lung microbiome by including both upper and lower airway samples.9 Further studies in this area may support and inform the development of new therapeutic strategies, including probiotics, for children with severe bronchiolitis to prevent the development of recurrent wheezing and childhood asthma.
Online Methods
Study Design
One hundred nasopharyngeal aspirates (NPAs) were analyzed from one hospital participating in a 3-year, prospective cohort study that was part of the Multicenter Airway Research Collaboration (MARC), a program of the Emergency Medicine Network (EMNet) (www.emnet-usa.org). Inclusion criteria were an attending physician's diagnosis of bronchiolitis among hospitalized children age <2 years and the ability of the parent/guardian to give informed consent. The exclusion criterion was previous enrollment. All patients were treated at the discretion of the treating physician. The institutional review board at each participating hospital approved the study.
Sample processing and 16S pyrosequencing
Consistent with protocols benchmarked as part of the NIH Human Microbiome Project, bacterial genomic DNA was extracted from nasopharyngeal samples using the PowerSoil DNA Isolation Kit (MoBio; Carlsbad, CA). The V3-V5 hypervariable regions of the 16S rRNA gene were amplified from genomic DNA using primer 357F (5’-CCTACGGGAGGCAGCAG-3’) and barcoded primer 926R (5’-CCGTCAATTCMTTTRAGT-3’). The forward primer also contained 454 B Adaptor sequence (5’ CCTATCCCCTGTGTGCCTTGGCAGTCTCAG 3’) and the reverse primer contained 454 A Adaptor sequence (5’CCATCTCATCCCTGCGTGTCTCCGACTCAG-3’) for 454 Titanium pyrosequencing, which was performed in one multiplexed run at the Human Genome Sequencing Center at Baylor College of Medicine (Houston, TX).
Sequence processing and analysis with QIIME
Sequence processing and analysis was performed with QIIME version 1.5. Sequences were de-multiplexed and associated with their sample of origin based on individual barcodes. Quality trimming was performed according to the following parameters: minimum/maximum sequence length of 200/1000bp, minimum average quality score of 25 over a sliding 50bp window, no ambiguous bases allowed, only two primer mismatches allowed, only one barcode mismatch allowed, and a maximum homopolymer length of 8bp. After quality trimming, three samples had less than 1,000 sequences associated with them; thus, these samples were not included in downstream analyses. Quality trimming was followed by de novo and reference-based chimera checking and removal and binning of sequences into operational taxonomic units (OTUs) based on 97% identity (equivalent of species) using USEARCH. Taxonomic classification was performed using the RDP classifier retrained with the GreenGenes database. Prior to alpha and beta diversity analyses, singletons were removed and the number of sequences per sample was normalized to 1,007 (the smallest number of sequences associated with any one sample).
Statistics
All statistics for 16S data were performed using Metastats (http://metastats.cbcb.umd.edu), which uses a non-parametric two-sided t-test to effectively handle non-parametric datasets. Additionally, Metastats employs the false discovery rate to improve specificity in complex environments and uses the Fisher's exact test to handle sparsely sampled or “rare” features separately. We set the significance threshold to p=0.05 and the number of permutations to 1,000.
Supervised machine learning
To identify bacterial taxa that were predictive of disease severity or viral agent of bronchiolitis, we performed supervised machine learning using the Genboree machine learning pipeline, implemented as part of the Genboree Microbiome Toolset (www.genboree.org). The pipeline runs the randomForest algorithm and the Boruta package to identify discriminatory OTUs that can act as predictors of specific groups (i.e. biomarkers for specific disease or health states). Together, the randomForest algorithm, which determines the robustness of group clustering, and the Boruta package for feature selection are implemented to identify the most important OTUs involved in discriminating each group of samples from other groups. If one OTU is removed from the dataset and classification of the sample into the correct group becomes impossible, that OTU is identified as discriminatory. The Boruta package calculates the amount of “background noise;” if the decrease in classification accuracy is not above this background, that OTU is not confirmed as discriminatory. We compared groups of samples based on virus present, and on patient's acute wheeze status.
Whole genome shotgun sequencing and analysis
Based on the results of the 16S rRNA gene pyrosequencing and analysis, we chose ten representative samples (ten percent of the cohort; 3 RSV-only infected samples, 4 HRV-only infected samples, and 3 RSV-HRV co-infected samples), and performed whole genome shotgun sequencing (WGS) and analysis. Bacterial genomic DNA isolated from each sample as described above was sequenced on one lane of the Illumina HiSeq 2000 platform at the HGSC at BCM. FASTQ sequencing files (read one and read two) were then quality trimmed and aligned against the human genome (hg19) and PhiX to filter out known contaminants. The trimmed, filtered FASTQ files were interleaved into one FASTQ file, which was converted to FASTA format using a perl script. To obtain species-level identification of the bacterial taxa present in each sample, we passed the WGS FASTA file for each sample through MetaPhlAn, a computational tool that relies on clade specific marker genes for taxonomic assignment. The output table, which lists taxonomic assignment and percent abundance, identified the species present and their abundance in each sample.
Supplementary Material
Acknowledgements
The authors thank Ashley F. Sullivan, MS, MPH, for coordinating the project.
Sources of funding: This study was supported by the Alkek Foundation, the Boston Children's Hospital Research Faculty Council Pilot Award and by the grants U01 AI-67693 and K23 AI-77801 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Abbreviations
- RSV
respiratory syncytial virus
- HRV
human rhinovirus
- NPA
nasopharyngeal aspirate
- WGS
whole genome shotgun
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008 Feb;121(2):244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- 2.Mansbach JM, Piedra PA, Teach SJ, Sullivan AF, Forgey T, Clark S, et al. Prospective Multicenter Study of Viral Etiology and Hospital Length of Stay in Children With Severe Bronchiolitis. Arch. Pediatr. Adolesc. Med. 2012 Apr 2;166(8):700–706. doi: 10.1001/archpediatrics.2011.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr., Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am. J. Respir. Crit. Care Med. 2007 Jan 15;175(2):108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 2007 Oct 11;357(15):1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 5.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallia P, Footitt J, Sotero R, Jepson A, Contoli M, Trujillo-Torralbo, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012 Dec 1;186(11):1117–1124. doi: 10.1164/rccm.201205-0806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 2004 Aug;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberthal AS, Bauchner H, Hall CB, Johnson DW, Kotagal U, Light MJ, et al. Diagnosis and Management of Bronchiolitis. Pediatrics. 2006 Oct;118(4):1774–1793. doi: 10.1542/peds.2006-2223. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011 Oct 15;184(8):957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.