Abstract
The goal of this study was to determine the molecular identity of a small conductance (~5-pS) background K+ channel expressed in trigeminal ganglion neurons. We tested the hypothesis that the 5-pS channel is a K2P channel by comparing the pharmacological and single-channel properties of THIK-1 expressed in HEK293 cells. As reported earlier, whole-cell THIK-1 current was inhibited by halothane and activated by arachidonic acid. Among 25 additional modulators tested, bupivacaine (100 μM), quinidine (50 μM) and Ba2+ (3 mM) and cold (10°C) were most effective inhibitors of THIK-1 current (>50% inhibition). In cell-attached patches with high KCl in the pipette and bath solutions, THIK-1 produced a small conductance (~5-pS) channel with a weak inwardly rectifying current-voltage relationship. Halothane, bupivacaine and cold inhibited the single-channel activities of both THIK-1 and the 5-pS channel in TG neurons, whereas arachidonic acid augmented them. THIK-1 expressed in HEK293 cells and the 5-pS channels in TG neurons were insensitive to hypoxia. Reverse transcriptase-PCR, western blot and immunocytochemical analyses suggested that THIK-1 mRNA and protein were expressed in TG neurons. These results show that THIK-1 is functionally expressed in TG neurons and contributes to the background K+ conductance.
Keywords: arachidonic acid, background K+ channel, cold, halothane, hypoxia, two-pore domain
Introduction
Background or leak K+ channels help to set the resting membrane potential and thus regulate cell excitability. The background K+ channels are also targets of many intracellular and extracellular biological signals, and thus regulate cell function under various physiological and pathophysiological conditions. For example, TASK-1 and TASK-3 are oxygen-sensing background K+ channels in chemoreceptor cells of the carotid body and regulate ventilation [1-3]. TASK-1 and TASK-3 are also expressed in the adrenal gland and regulate aldosterone secretion in response to angiotensin II [4]. TASK-2 is expressed in retrotrapezoid neurons and may serve as a pH sensor in central chemoreception [5, 6]. TASK-2 is also highly expressed in proximal renal tubules and regulates bicarbonate reabsorption and cell volume regulation [7, 8]. Studies in knockout mice suggest that TREKs are involved in pain perception and neural activity such as depression and anesthetic response [9, 10]. TRESK is believed to be involved in sensory transduction, as it is highly expressed in sensory ganglion neurons [11, 12]. Therefore, identifying the background K+ channels and their molecular counterpart are crucial for understanding how a cell regulates its excitability.
Recent studies show that sensory neurons such as dorsal root ganglion (DRG) and trigeminal ganglion (TG) neurons express background K+ channels such as TRESK and TREK [11, 12]. These K+ channels are believed to be involved in neuroprotection, nociception and mechanotransduction in these neurons [9, 13, 14]. In addition to TRESK and TREK, we found a small conductance (~5-pS) K+ channel with properties of a background K+ channel in TG neurons. The 5-pS K+ channel is observed frequently and therefore may contribute significantly to the background K+ conductance and to setting the resting membrane potential. Because most functional K2P channels show properties of a background K+ channel, it is possible that the 5-pS channel is a member of the K2P channel family. However, most K2P channels that have been analyzed at the single-channel level show conductance that are greater than ~15-pS. Functional K2P channels whose single-channel properties are still undefined are TWIK and THIK. Therefore, the 5-pS channel could be TWIK, THIK, or possibly a non-K2P channel, as some voltage-gated K+ channels may be active near the resting membrane potential.
TWIK-1 produces a functional current that is small [15], whereas THIK-1 produces a large sustained K+ current when expressed in mammalian cell lines [16]. In this study, therefore, we tested the possibility that the small conductance K+ channel in TG neurons is THIK-1. We studied the response of THIK-1 to pharmacological agents that were not tested previously, and recorded single-channel currents from HEK293 cells expressing THIK-1 to identify the general kinetics of this channel. We also determined the expression of THIK-1 at the mRNA and protein levels in TG neurons. The results from these studies indicate that the small conductance background K+ channel present in trigeminal ganglion neurons is THIK-1.
Materials and Methods
Trigeminal ganglion neuronal culture
Rats were used in accordance with the guidelines of Rosalind Franklin University Animal Committee. Trigeminal ganglia (TG) were dissected from the brain of 2-week-old rats (n = 6) and collected in culture medium (DMEM/F-12). Ganglia were incubated for 1.5 h at 37°C in the F-12 medium containing 0.125% collagenase (Type II). The ganglia were then incubated in F-12 medium containing 0.25% trypsin for 30 min at 37°C. The tissue pieces were then placed in DMEM medium containing FBS (10%) and streptomycin/penicillin (0.1%) and gently triturated with a polished glass pipette tip. The suspended cells were plated on poly-L-lysine-coated glass coverslips in a culture dish. Cells were incubated at 37°C in a 95% air-5% CO2 gas mixture, and used 1 day after plating.
Transfection in HEK293 cells
Full lengths human THIK-1 and THIK-2 were cloned by RT-PCR, inserted into the pcDNA3.1 vector and sequenced for confirmation. HEK293 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). HEK293 cells were co-transfected with plasmids (pCDNA3.1) containing full open reading frame of THIK-1 or THIK-2 DNA and green fluorescent protein (GFP) using LipofectAMINE2000 and OPTI-MEM I Reduced Serum Medium (Life technologies, Grand Island, NY). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon microscope equipped with a mercury lamp light source. Cells were used 2 days after transfection.
Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200B, Molecular Devices). Thick-walled borosilicate patch pipettes coated with sylgard were used to minimize background noise. Channel current was filtered at 2 kHz and transferred to a computer using the Digidata 1320 interface at a sampling rate of 20 kHz. Single-channel currents were analyzed with the pCLAMP program (Version 10). For analysis of single-channels, the amplitude of each channel was set at ~0.5 pA (see dotted lines on the current tracings in Fig. 6 and Fig. 8) and the minimum duration was set at 0.05 ms. Using the 50% detection threshold, we determined channel activity (NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open) from ~15 sec of current recording. In halothane and cold-treated patches in which the current amplitude was reduced, the amplitude for single-channel analysis was set at ~0.3 pA. Total current (I=NPoi) was calculated by multiplying NPo and the mean single-channel amplitude for each experiment. Those patches in which we could not clearly identify THIK-1 single-channel open levels were removed from analysis. Single-channel current tracings shown in the figures were filtered at 1 kHz. In experiments using cell-attached and inside-out patches, pipette and bath solutions contained (mM): 150 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3). For whole-cell current recording, bath perfusion solution contained 117 mM NaCl, 23 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2 and 11 mM glucose (pH 7.3). All experiments were performed at ~25°C unless indicated otherwise (hypoxia and cold studies). Cooling of the perfusion solution was done using a temperature controller with in-line cooler placed next to the recording chamber. The temperature of the solution perfusing the cells during recording was monitored with a thermistor (Warner Instruments).
Figure 6. Effects of halothane, AA and cold on THIK-1 and TASK-3 single-channel currents.
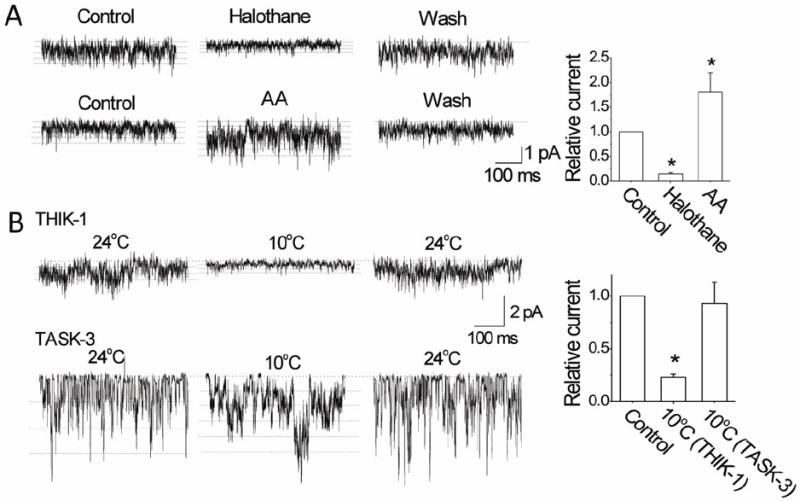
A. Tracings show single-channel currents recorded from outside-out patches before, during and after perfusion with halothane (2 mM) and AA (10 μM) in cells expressing THIK-1. Multiple openings are indicated by dotted lines. Halothane reduced the single-channel amplitude. Graph plots the relative currents (NPoi) produced by halothane and AA. Each bar is the mean±SD of 7 determinations.
B. Tracings show single-channel currents recorded from outside-out patches before, during and after perfusion with cold solution in cells expressing THIK-1 or TASK-3. Multiple openings are indicated by dotted lines. Cold reduced the single-channel amplitude. Graph plots the current (NPoi) change produced by cold. Each bar is the mean±SD of 10 determinations. Asterisk indicates a significant difference from control (p<0.05).
Figure 8. Properties and expression of THIK-1 in TG neurons.
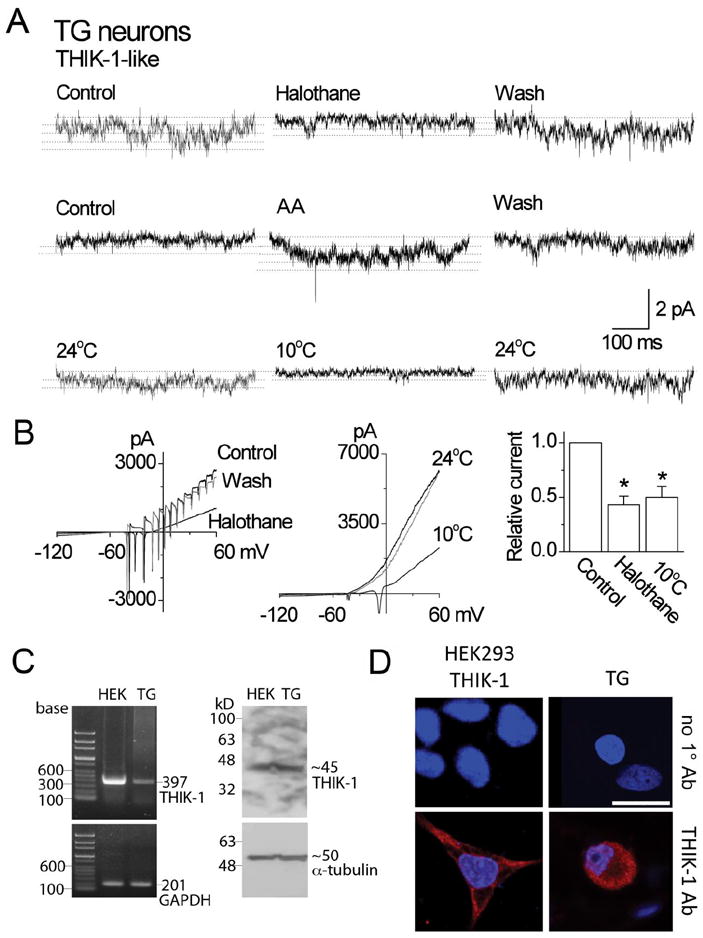
A. Tracings show single-channel currents in cell-attached patches of TG neurons. Pipette potential was +100 mV. Effects of halothane, arachidonic acid and cold are shown. Dotted lines indicate the open levels.
B. Whole-cell current tracings show the effect of halothane and cold in TG neurons. The bar graph shows the relative inhibition of the current by halothane and cold (mean±SD). Asterisks indicate a significant difference (p<0.05; n=5 each).
C. RT-PCR shows an expected 397-bp band from both HEK293 cells expressing THIK-1 and rat TG neurons (left panel). GADPH was used as control (201-bp). Western blot analysis shows ~45 kD band when immunoblotted with THIK-1 antibody (right panel). α-tubulin was used as a loading control (50 kD). The exposure time for α-tubulin was much shorter than that for THIK-1.
D. Immunocytochemistry shows positive staining (red) in both HEK293 cells expressing THIK-1 and rat TG neurons. Cells were stained with DAPI to identify nucleus (blue). In the absence of THIK-1 primary antibody (1°Ab), only nuclear staining (blue) was observed. Scale bar=20 μm
Hypoxia studies
Cell-attached patches were formed on HEK cells transfected with THIK-1 and perfused with a bicarbonate-buffered solution containing 117 mM NaCl, 23 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2 and 11 mM glucose bubbled with 5% CO2/95% air mixture (normoxia) for at least 60 min. After steady-state channel activity was obtained, the perfusion solution was switched to solution gassed (for ~60 min) with 5% CO2/95% N2 mixture (hypoxia) for ~5 min. The pipette solution contained (mM) 150 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3). The temperature of the perfusion solutions was kept at ~35°C. O2 pressure of the solutions was checked using an oxygen meter (ISO2, WPI, Sarasota, FL) that was calibrated to 0% with solution gassed with pure nitrogen for 60 min and to 21% with solution gassed with air for 60 min at 37°C. The O2 partial pressure as judged by the reading on the meter for the hypoxic solution inside the recording chamber used in this study ranged from ~1.7% to 2.3% (~15 mmHg O2).
Reverse transcriptase (RT)-PCR
TGs were isolated from the brain of three 2-week-old rats. Total RNA was extracted using QIAzol Lysis Reagent (Qiagen). First-strand cDNA was synthesized from total RNA isolated from the TG and HEK cells transfected with THIK-1 and using oligo (dT) (DiaStar™ RT Kit; SolGent, Daejeon, South Korea) and was then used as a template for PCR amplification. HEK cells transfected with THIK-1 was used as a positive control. Specific primers for THIK-1 (GenBank accession number, AF287301: forward 5’-CTGCTGCATCTACTCCATGTT-3’ and reverse 5’-GGTCTCTGCCAACCTGTTATT-3’) were used in PCR reactions with Taq polymerase (G-Taq™; Cosmo Genetech, Seoul, South Korea). PCR was conducted in a final reaction volume of 20 μl under the following PCR conditions: initial denaturation at 94°C for 5 min, followed by 32 cycles of 94°C for 45 sec, 57°C for 45 sec, and 72°C for 45 sec and a final extension step at 72°C for 10 min. The products were electrophoresed on a 1.2% (w/v) agarose gel to check product size (397 bp). The PCR products were directly sequenced with a PRISM® 3100-Avant genetic analyzer (Applied Biosystems, Foster City, CA).
Western blot analysis
TGs were homogenized in PRO-PREP™ protein extraction solution (iNtRON Biotechnology, Seongnam, South Korea) containing 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM dithiothreitol (DTT), 0.5% NP-40, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM EGTA, 1 g/ml leupeptin, 1 g/ml pepstatin A, 1 mM phenylmethylsulfonyl fluoride and 10.5 g/ml aprotinin. The mixture was incubated for 60 min on ice with intermittent vortexing. Extracts were clarified by centrifugation at 13,000 rpm (16,609 g) for 20 min at 4°C. The resulting supernatant was separated by 10% SDS-polyacrylamide gel electrophoresis, and the proteins resolved in the gel were transferred to a polyvinylidene fluoride membrane using a semi-dry transfer (Bio-Rad, Hercules, CA). Equal amounts (50 μg) of total protein were loaded. The membranes were blocked with 5% fat-free dry milk and then incubated with 1:500 dilution of anti-THIK-1 antibody (Abcam®, Cambridge, MA) and with 1:1000 dilution of anti-α-tubulin antibody. These were followed by incubation with a 1:10 000 dilution of horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (Assay Designs, Ann Arbor, MI). Immuno-positive bands were visualized by enhanced chemiluminescence (ECL Plus kit; ELPIS, Taejon, South Korea), following the manufacturer’s instructions.
Immunocytochemistry
TG neurons cultured on round cover slips coated with poly-L-lysine were fixed with 4% paraformaldehyde in 0.1 M PBS for 30 min, washed and incubated in a blocking buffer containing 1% normal goat serum and 0.1% Triton X-100 for 2 h at room temperature under gentle rotation. The neurons were incubated with a 1:100 dilution of anti-THIK-1 antibody in PBS overnight at 4°C. After incubation, the neurons were washed with PBS three times and then incubated in the dark for 1.5 h with a 1:100 dilution of cyanine 3 (Cy3)-conjugated anti-rabbit IgG for THIK-1. Stained cells were wet-mounted on glass slides and observed using a confocal laser-scanning microscope (Olympus, Tokyo, Japan). Negative and positive controls were checked by omitting the primary antibody and by transfection of the cDNA, respectively. Cells were also stained with DAPI to identify the nucleus of each cell. No red fluorescence was observed on the any of negative control, while only DAPI (4,6-diamidino-2-phenylindole, dihydrochloride, Molecular Probes, Inc., Eugene, OR) nuclear stain was seen.
Chemicals
AICAR (5-aminoimidazole-4-carboxamide riboside) and tetraethyammonium were purchased from Tocris bioscience (Bristol, UK). Verapamil was purchased from Calbiochem (La Jolla, CA). All other chemicals were from Sigma Aldrich. Saturated halothane solution (~18 mM; [17]) was prepared by dissolving halothane in recording solution in a glass container for several hours, and diluted to desired concentrations for immediate use.
Statistical analysis
Student’s t-test (for comparison of two sets of data) and one-way analysis of variance with Bonferroni correction (for comparison of three data sets) were performed for statistical analysis. Data were analyzed using PRISM software (Graphpad) and represented as mean ± S.D. Post hoc testing was based on unpaired t-test with Bonferroni correction. Significance level was set at p<0.05.
Results
Small conductance background K+ channel in TG neurons
In cultured neurons from trigeminal ganglia, we recorded several K+ channels that were active at rest. In neurons with small- to medium-sized cell bodies, we found TRESK (K2P18.1)-like channels with ~15-pS single-channel conductance similar to those observed in dorsal ganglion neurons [12]. Large conductance TREK (K2P2.1/10.1)-like channels were also observed but were generally inactive at rest in cell-attached patches, although they became more active in inside-out patches (Fig. 1A). In addition to these already well-characterized channels, a small conductance K+ channel with a noisy open state was frequently present in cell-attached patches (Fig. 1B). Although we initially ignored the small conductance channels, close inspection showed channel-like behavior with multiple levels of opening. The channel showed no inactivation or desensitization even in inside-out patches. The single-channel conductance was ~5-pS at +100 mV pipette potential, and the channel showed weak inward rectification (Fig. 1C). Such a channel was not observed when the pipette solution contained NaCl instead of KCl. To test the hypothesis that the 5-pS background K+ channel is a member of the K2P channel family, we first studied the pharmacological and single-channel properties of THIK-1. We then tested the effect of agents that modulated THIK-1 on the 5-pS channel in TG neurons for comparison.
Figure 1. Background K+ channels in TG neurons.
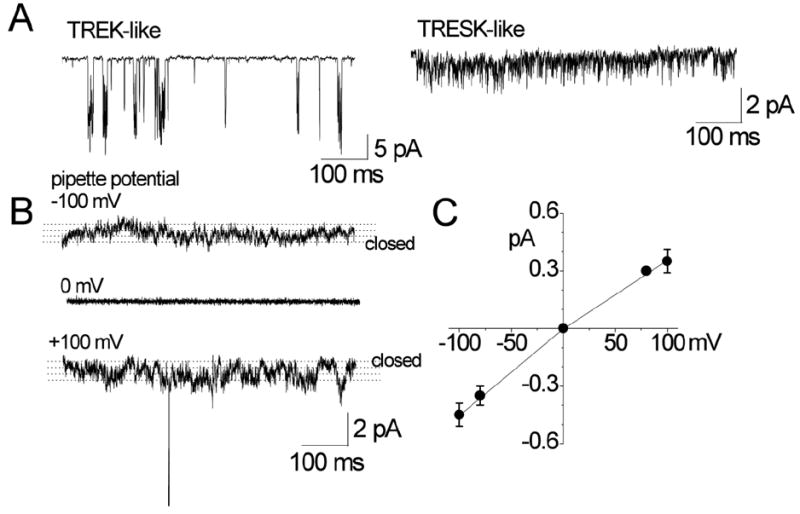
A. Cell-attached patches were formed on TG neurons (small to medium-sized cell bodies) and inward single-channel current recorded at pipette potential of +100 mV in bath solution containing 150 mM KCl. TREK- and TRESK-like channels are shown.
B. Small-conductance channels in cell-attached patches are shown at three pipette potentials (0 and ±100 mV). Dotted lines are drawn by eye to indicate open levels.
C. Amplitude of channel opening was measured at different potentials by drawing lines through open and closed levels, and plotted as a function of membrane potential.
THIK-1 modulation by pharmacological agents
To confirm that THIK-1 is a functional K+ channel, whole-cell K+ current was recorded from HEK293 cells transfected with a plasmid containing THIK-1 cDNA. In external solution containing 5 mM KCl, whole-cell currents of 1-3 nA (at +60 mV) were elicited by voltage ramp from -120 mV to +60 mV (Fig. 2A). The currents reversed between -80 and -90 mV, close to the K+ equilibrium potential. Control HEK293 cells transfected with a plasmid containing only GFP showed very small currents (0.2-0.3 nA at +60 mV). Cells transfected with THIK-2 showed whole-cell currents similar to control cells, confirming that THIK-2 does not form a functional K+ channel at the plasma membrane, as reported earlier [16]. Confocal images of HEK293 cells transfected with a plasmid containing GFP fused to THIK-1 at the C-terminus (THIK-1-GFP) or to THIK-2 at the C-terminus (THIK-1-GFP) showed that THIK-1 was localized at the plasma membrane whereas THIK-2 was present at perinuclear regions (Fig. 2B). Similar localization patterns were observed when THIK-1 and THIK-2 were expressed in COS-7 cells. THIK-1-GFP also produced whole-cell currents of similar magnitude (2.8±0.5 nA at +60 mV) in HEK293 cells, indicating that GFP did not interfere with cell surface expression and function of THIK-1.
Figure 2. THIK-1 whole-cell current and expression patterns in HEK293 cells.
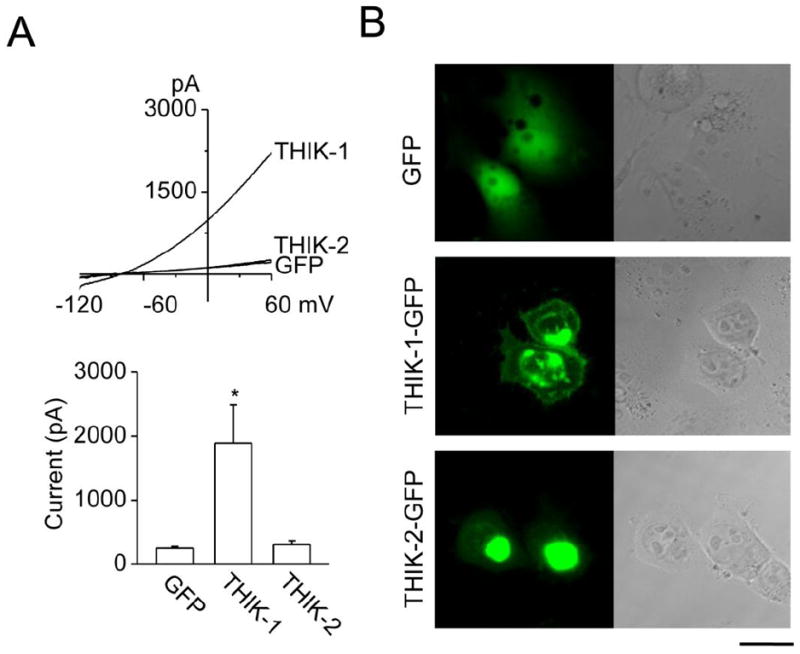
A. Whole-cell currents were recorded from HEK293 cells transfected with THIK-1 and GFP, THIK-2 and GFP or GFP alone (top). Current levels at +60 mV were determined and plotted (bottom). Each bar is the mean±SD of 6 determinations. Asterisk indicates a significant difference from the control value (GFP alone, p<0.05).
B. Expression patterns of THIK-1-GFP and THIK-2-GFP in HEK293 cells are shown. GFP is joined to the C terminus of THIK. Left and right panels show green fluorescent and phase contrast images, respectively. Scale bar=20 μm
Halothane has been identified as an inhibitor and arachidonic acid as an activator of THIK-1 [16]. Consistent with these findings, halothane (2 mM) inhibited THIK-1 current by 73±8% and arachidonic acid (10 μM) augmented it by 30±10% (Fig. 3A). To identify additional inhibitors and activators of THIK-1, effects of known modulators of ion channels were tested in HEK293 cells expressing THIK-1. Of 25 additional agents tested, bupivacaine (100 μM), quinidine (50 μM) and Ba2+ (3 mM) were the only three that produced ~50% or greater inhibition of THIK-1 current at the concentrations used (Fig. 3B). Lidocaine (100 μM) inhibited THIK-1 current by 33±3%. 4-aminopyridine (1 mM) inhibited THIK-1 by 20±8%. Methanandamide (5 μM), ZnCl2 (100 μM), verapamil (50 μM), NiCl2 (100 μM), glybenclamide (100 μM), and flufenamic acid (100 μM) all produced negligible effects on THIK-1 current (p>0.05; n=5-6). K2P channels are generally insensitive to TEA, but the effect of TEA on cloned THIK-1 has not yet been reported. TEA (1 mM) showed negligible effect on THIK-1 current at potentials between -90 and -20 mV, but a small inhibition was observed at more depolarized potentials such that TEA produced a 23±9% inhibition of the whole-cell current at +60 mV.
Figure 3. Effects of pharmacological agents on THIK-1 current.
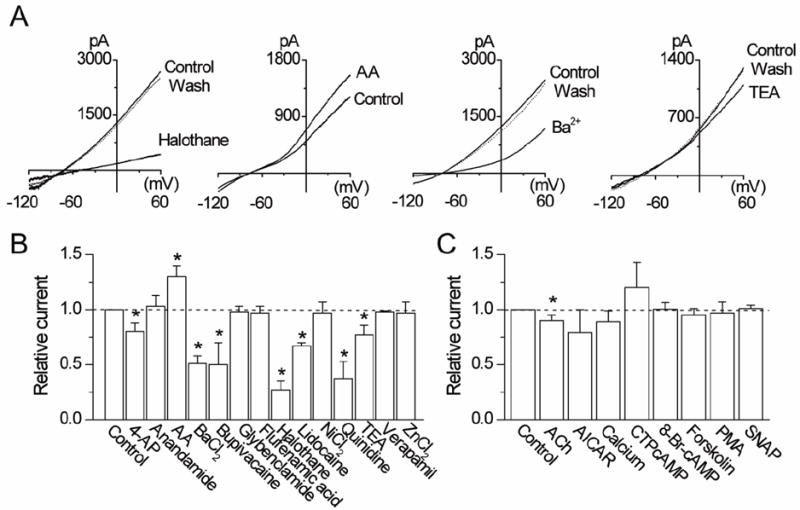
A. Representative tracings show whole-cell currents before, during and after perfusion with halothane (2 mM), arachidonic acid (10 μM), Ba2+ (3 mM) and TEA (1 mM).
B and C. Relative changes in whole-cell currents measured at +60 mV are shown. Control is taken as 1.0. Each bar is the mean±SD of 5-10 determinations. Asterisk indicates a significant difference from the control value (p<0.05).
In an earlier study, genistein, a tyrosine kinase inhibitor, inhibited THIK-1 ([18]). We tested the effects of agents known to modulate the activity of other protein kinases. Treatment of cells for 30 min with 8-Br-cAMP (500 μM), forskolin (10 μM) or CTP-cAMP (10 μM) failed to produce significant changes in whole-cell THIK-1 current (p>0.05; n=5-10; Fig. 3C). Phorbol myristate acetate (PMA, 5 μM) and AICAR (1 mM) showed no effect on THIK-1 current when incubated for 30 min, indicating that protein kinase C and AMP-activated kinase do not modulate THIK-1. THIK-1 was also insensitive to changes in extracellular [Ca2+] from 0 to 1 mM (10±5%, p>0.05; Fig. 3C). ACh (100 μM) showed a small inhibition of THIK-1 current in cells expressing both THIK-1 and M1 receptor (13±7%, p<0.05; n=10).
Temperature change from 22°C to 37°C was found to increase THIK-1 current by 1.6-fold [16], but its sensitivity to cold was not tested previously. To test for cold sensitivity, the temperature of the perfusion solution was switched from 24°C to 10°C while recording the whole-cell current. Cold decreased THIK-1 whole-cell current by ~50% (Fig. 4A). A rapid reversible decrease in whole-cell holding current was also observed when the perfusion temperature was switching from 24°C to 10°C (Fig. 4B). Relative changes in current as a function of temperature is shown in Fig. 4B. Under identical experimental conditions, cold did not significantly affect the TASK-3 whole-cell current, showing that THIK-1 and TASK-3 show different responses to cold (Fig. 4C and 4D). Thus, cool and cold temperatures decreased the THIK-1 current.
Figure 4. Inhibition of THIK-1 current by cold.
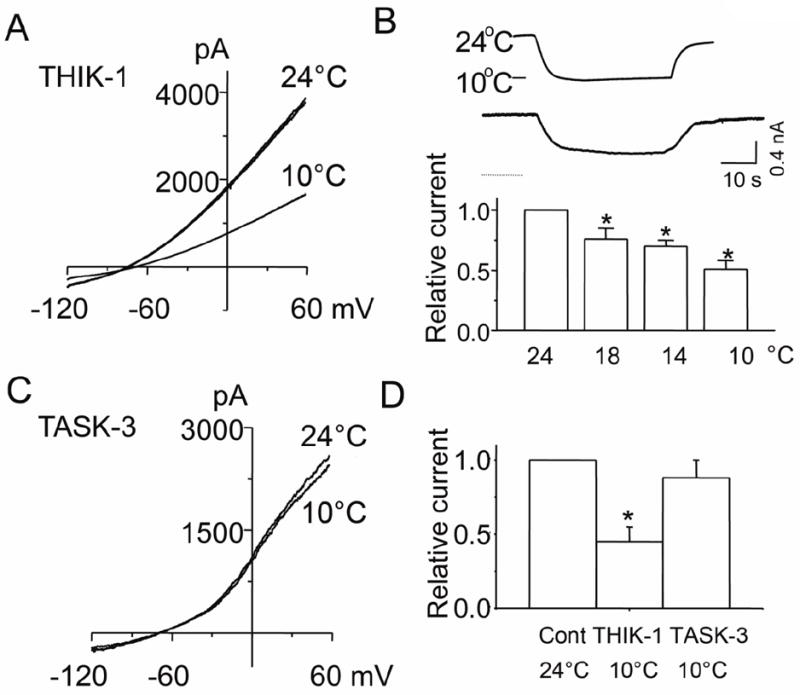
A. Tracing shows THIK-1 whole-cell currents recorded at 24°C and 10°C. Cells were held at -80 mV, and voltage ramps applied from -120 mV to +60 mV.
B. Tracing shows whole-cell holding current while changing the temperature of the perfusion solution from 24°C to 10°C, and back to 24°C. Whole-cells were held at -60 mV. Relative currents at different temperatures are also shown in the bar graph (mean±SD from 10 cells)
C. Tracings show whole-cell currents of TASK-3 recorded at 24°C and 10°C.
D. Summary of the cold effect on THIK-1 and TASK-3 whole-cell relative currents. Each bar is the mean±SD of 10 determinations. Asterisk indicates a significant difference from the control value (p<0.05).
Single-channel properties of THIK-1
A useful method to help identify the presence of THIK-1 in native cells is to show that a channel in the native cell has single-channel kinetic properties similar to those obtained with cloned THIK-1 expressed in a cell line. To characterize THIK-1 at the single-channel level, cell-attached patches were formed on HEK293 cells expressing THIK-1/GFP using pipette and bath solutions containing 150 mM KCl. In most untransfected cells (n>20) and cells transfected with plasmid containing GFP cDNA (n=16), the recorded current was quiet with no opening of ion channels (Fig. 5A). In a few patches, endogenous channels with single-channel conductance of 15 pS or 36 pS were present. In cells transfected with plasmid containing THIK-1/GFP cDNA, channels with short and spiky openings were consistently present in nearly all cell-attached patches (n>50). A record of single-channel openings obtained at the pipette potential of +100 mV is shown in expanded scale in Fig. 5A. Close inspection of the current recording showed two channel openings in this patch, as indicated by dotted lines.
Figure 5. Single-channel properties of THIK-1.
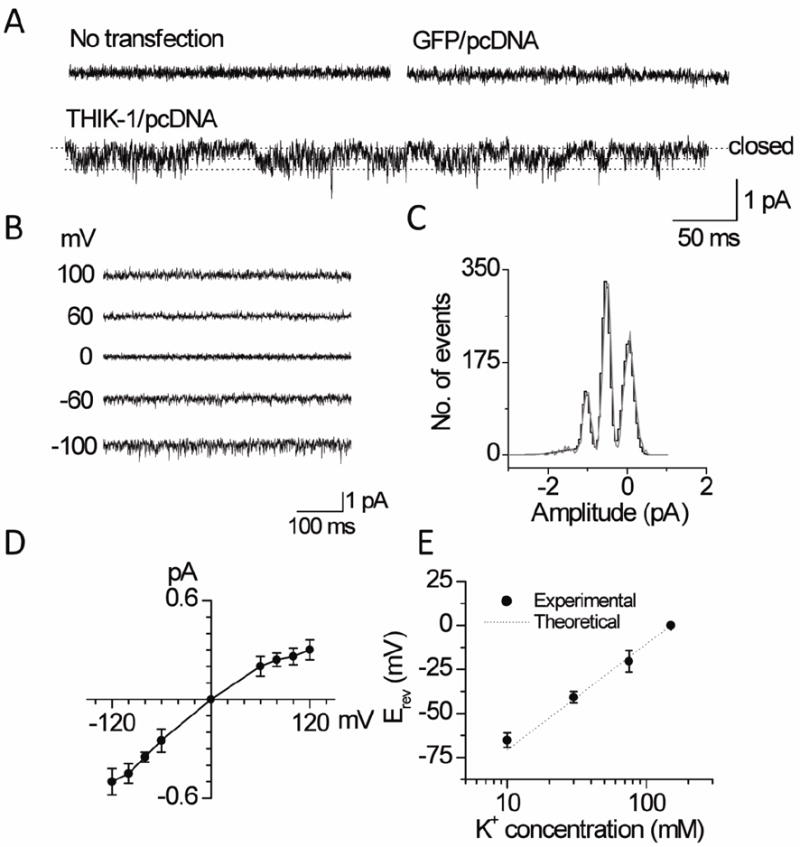
A. Tracings show currents from cell-attached patches from untransfected cells and cells transfected with plasmid containing GFP and/or THIK-1 DNA. Cells transfected with THIK-1 DNA show low amplitude channel openings indicated by dotted lines.
B. Currents were recorded from inside-out patches held at various membrane potentials as indicated.
C. Plot shows an amplitude histogram of channel openings recorded at +100 mV (pipette potential). The histogram was fitted with a Gaussian curve.
D. Plot shows a single-channel current-voltage relationship of THIK-1.
E. Plot shows changes in reversal potential (Erev) at different [K+]o. Experimental and theoretical (Nernst Equation) data are shown.
Single-channel openings at different membrane potentials (-100 mV to +100 mV) are shown in Fig. 5B. An amplitude histogram determined from a channel current recording at the pipette potential of +100 mV is shown in Fig. 5C. The current-voltage relationship, plotted using the mean amplitude values at different potentials, showed that THIK-1 has a weak inward rectification. The calculated single-channel conductance values at -100 mV and +100 mV (pipette potentials) were 3.0±0.6 pS and 4.5±0.6 pS, respectively (n=6). Plot of the reversal potential as a function of pipette K+ concentration in outside-out patches showed a 58±3 mV shift in reversal potential per 10-fold change in [K+]o, consistent with the K+-selectivity of the THIK-1 channel (Fig. 5E, n=5). As predicted, no THIK-1-like channels were present when the pipette contained 150 mM NaCl at the pipette potential of +100 mV. Thus, THIK-1 is a background K+ channel whose single-channel conductance is the smallest of the K2P channels reported so far.
In agreement with whole-cell studies, halothane (2 mM) decreased the relative THIK-1 current (NPoi) by 84±5%, and arachidonic acid (10 μM) increased it by 1.8±0.5-fold in outside-out patches (n=7; Fig. 6A). Formation of inside-out patches did not cause rundown of THIK-1 channel activity (n=5). Application of ATP (2 mM) or CaCl2 (100 μM) to the cytoplasmic side of the membrane produced no significant effect on channel activity (n=4 each). THIK-1 was not affected by application of negative pressure (~60 mmHg) in the inside-out patch mode (n=5). Consistent with the effect of cold on the THIK-1 whole-cell current, lowering the perfusion temperature from 24°C to 10°C produced a reversible inhibition of THIK-1 single-channel current by 77±7% in cell-attached patches (Fig. 6B, n=10). Analysis of single-channel openings indicated that cold reduced the current amplitude by ~40%. For TASK-3, cold reduced the amplitude of single-channel opening by ~40%, but prolonged the open time duration, producing no significant change in total current (I=NPoi; 0.81±0.07 (24°C) and 0.75±0.15 (10°C), Fig. 6B). This is consistent with the lack of cold effect on the whole-cell TASK current. These results confirm at the single-channel level that cold inhibits THIK-1 but not TASK-3.
Negligible effects of hypoxia and mitochondrial inhibitors on THIK-1 current
Hypoxia has been reported to produce a small decrease in whole-cell current (~10%) in HEK293 cells expressing THIK-1 [19]. In our own experiments, hypoxia failed to significantly inhibit the THIK-1 whole-cell current in HEK293 cells (p>0.05; n=10, Fig. 7A). Mitochondrial inhibitors of oxidative phosphorylation such as sodium cyanide (NaCN; 1 mM) and sodium hydrogen sulfide (NaHS; 100 μM) inhibited TASK-1/3 activity in carotid body chemoreceptor cells by 78±5 and 84±6%, respectively [20, 21]. The same agents used under identical experimental conditions showed no inhibition of the THIK-1 whole-cell current (Fig. 7A). Only trifluorocarbonylcyanide phenylhydrazone (FCCP; 1 μM, a mitochondrial uncoupler) produced a small inhibition of THIK-1 current (19 ± 11% at +60 mV; p<0.05). Fig. 7B summarizes the results of whole-cell studies. In cell-attached patches, hypoxia did not significantly affect THIK-1 single-channel activity (NPo: 1.7±0.3 and 1.6±0.4), consistent with the whole-cell current data (n=10, Fig. 7C). In TG neurons, hypoxia showed no effect on THIK-1-like channel activity (p>0.05; n=6, Fig. 7D), but reversibly inhibited TASK activity in carotid body glomus cells by 76±6% (p<0.05; n=6; Fig. 7D). In 5 cells, hypoxia did not affect the whole-cell outward current in TG neurons (Fig. 7E). These results show that THIK-1 is not an O2-sensitive channel in these cell types.
Figure 7. Lack of effect of hypoxia and mitochondrial inhibitors/uncoupler on THIK-1 current.
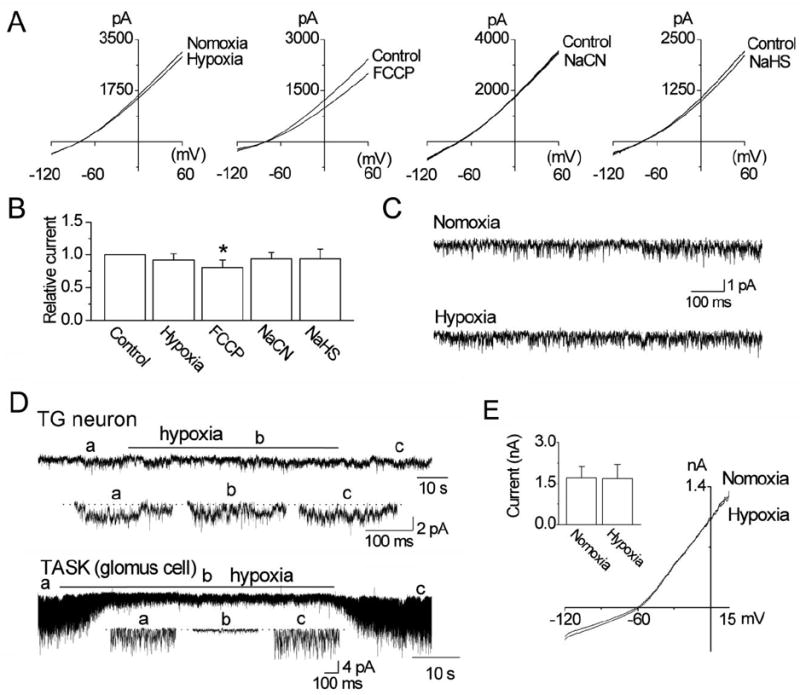
A. Graphs show whole-cell currents before and after exposure to hypoxia, FCCP (1 μM), NaCN (1 mM) and NaHS (100 μM) in HEK293 cells.
B. Summary of the experiments in A. Each bar is the mean±SD of 10 determinations. Asterisk indicates a significant difference from the control value (p<0.05).
C. Tracings show THIK-1 channels before and during exposure to hypoxia. No significant difference was found between the two (p>0.05).
D. Current tracings are from cell-attached patches from a TG neuron and a carotid body glomus cell perfused with normoxic and hypoxic solutions. Expanded tracings are also shown as indicated below each tracing.
E. Whole-cell currents from a TG neuron before and after hypoxia. The bar graph shows current measured at +15 mV. No significance was present (p>0.05).
THIK-1-like channel in the trigeminal ganglion (TG) neuron
Comparison of the THIK-1 single-channel kinetics with those of the 5-pS channel in TG neurons suggested that they could be the same channel. To further confirm that the 5-pS channel is THIK-1, we next tested the effect of several agents that modulated THIK-1 at the single-channel level. In outside-out patches from TG neurons, halothane (2 mM) inhibited the small conductance channels by 74±15% (n=6 each; Fig. 8A). Arachidonic acid increased THIK-1-like channel by 2.1±0.6-fold (n=6). Bupivacaine (100 μM) inhibited the small conductance channels by 23 ± 8% (n=4). In cell-attached patches, cold (10°C) inhibited the channel by 69±13% (n=6). Thus, these effects of halothane, arachidonic acid and cold are similar to those observed with THIK-1.
To assess how much THIK-1 contributes to the whole-cell current, we recorded whole-cell currents from TG neurons before and after application of halothane and cold. In control conditions, we observed two types of whole-cell currents in response to ramp voltage pulses: one type showing spontaneous depolarizations occurring during depolarization and the other type showing no depolarizations, as shown in Fig. 8B. In both examples, halothane and cold inhibited the whole-cell current by ~50%, and therefore the results from both types of cells were averaged and plotted (Fig. 8B). Although these results suggest that THIK-1 contributes ~50% of the whole-cell outward current, they need to be interpreted with caution, as halothane and cold are likely to have effects on other ion channels. For example, in TG neurons, both halothane and cold reduced the number of spontaneous depolarizations when they were present, suggesting that Na+ current may be inhibited [22].
RT-PCR analysis showed expression of THIK-1 mRNA in TG neurons as well as in HEK293 cells expressing THIK-1 (Fig. 8C). Western blot analysis showed expression of THIK-1 protein in HEK293 cells transfected with THIK-1 DNA, and also in TG neurons (Fig. 8C). Immunocytochemistry in cultured TG neurons showed a circular layer of red stain (THIK-1). Although somewhat indistinct, the outer circle of the red stain overlapped with the plasma membrane, suggesting that THIK-1 is expressed at the plasma membrane as well as below the plasma membrane (Fig. 8D). In control cells that were not treated with the primary antibody, only the nuclear DAPI signal was present. Thus, single-channel characteristics, sensitivity to pharmacological agents and cold, and biochemical analyses of mRNA and protein expression provide evidence that the 5-pS channel that is active in TG neurons represents THIK-1.
Discussion
In sensory neurons such as DRG and TG neurons, TRESK and TREK have been identified as background K+ channels [11, 12, 23]. In addition to these K2P channels, we found the presence of a small conductance K+ channel in TG neurons. The goal of this study was therefore to determine the molecular identity of this small conductance K+ channel by testing the hypothesis that it is also a K2P channel. Our measurement of single-channel conductance levels of all functional K2P channels suggested that if the small conductance K+ channel in TG neurons were a K2P channel, it would be THIK-1 or TWIK-1. Both THIK-1 and TWIK-1 expressed in heterologous expression systems produce K+ currents that do not inactivate, consistent with their role as a background K+ channel. We were able to record single-channel currents from cells transfected with THIK-1 but not TWIK-1. Therefore, we characterized the single-channel kinetics of THIK-1 expressed in HEK293 cells, and also tested the effects of pharmacological agents that were previously not used. The results of these studies, together with the positive expression of THIK-1 mRNA and protein, indicate that the small conductance background K+ channels present in TG neurons is most likely THIK-1.
Studies using Xenopus oocytes have shown that THIK-1 activity is inhibited by halothane, and stimulated by arachidonic acid [16]. This property of THIK-1 has been used to demonstrate the presence of a THIK-1-like current in native cells. Based on these properties, the background K+ currents in rat glossopharyngeal neurons [24], mouse cerebellar Purkinje neurons [25], and mouse central chemoreceptor neurons from the retrotrapezoid nucleus [26] were assumed to be THIK-1. While it may be true that a halothane-inhibited and arachidonic acid-activated current is indeed THIK-1 or THIK-1-like, it is possible that the native K+ current is another unrelated K+ channel with a similar pharmacological profile. For example, the intermediate conductance Ca2+-activated K+ channel and human ether-a-go-go-related (HERG) channels are also inhibited by halothane and activated by arachidonic acid [27-29]. Small conductance K+ channels in human alveolar epithelial cells are also inhibited by halothane and activated by arachidonic acid [30]. Ca2+-activated K+ channels and HERG can be active near the resting membrane potential and contribute to the background K+ conductance, similar to THIK-1. Halothane also inhibits the transient outward K+ current in rat cardiac myocyte [31], whereas arachidonic acid activates the Aplysia S-K channels [32]. For these reasons, additional characteristics of THIK-1, such as single-channel kinetics, would help to more clearly identify the native THIK-1-like current.
Single-channel properties of THIK-1
Unlike most other K2P channels whose single-channel conductance levels range from ~15-pS (TASK-1, TRESK) to 50-220-pS (TREK-1, TREK-2), THIK-1 showed a very small single-channel conductance of ~5-pS. For this reason, thick-walled glass pipettes were used to minimize the baseline membrane noise and observe opening of THIK-1 channels. THIK-1 also showed very short open time duration (<0.5 ms). Due to the flickery nature of THIK-1 and its small conductance, its openings were initially difficult to identify, particularly if the current recording has some basal membrane noise. More careful examination showed that these low amplitude openings were real channel openings with rapid transitions between closed and open states. The channel activity was always larger in one current direction when the pipette potential was held away from the reversal potential. If it were simply membrane noise, the current fluctuations would be similar in both inward and outward directions. The characterization of single-channel kinetics and pharmacological profile reported here should help to identify a native channel current as THIK-1. The recording of single-channels of THIK-1 should be possible in native tissues if THIK-1 is the major component of the background K+ current. If other background channels are also highly active, it may be difficult to clearly isolate and identify THIK-1.
Studies in native cells indicate that only one or two types of K2P channels provide the major background K+ current for each cell type, although mRNA transcripts of many K2P channel may be present. For example, in cerebellar granule cells, mostly TASK-1/3 and Type 4 K+ channels are active at rest [33]. In DRG neurons, opening of TRESK single-channels can be observed clearly, because the activity of other background K+ channels are low [34]. In carotid body glomus cells, TASK-1/3 is the main K+ channel that is active at rest [3]. Therefore, it is possible that THIK-1 is a predominant background K+ channel in certain cell types, and in these cells, single-channel recording would prove useful to confirm the molecular identity of the channel as THIK-1. Based on sensitivity to halothane and arachidonic acid, THIK-1-like K+ currents have been recorded from mouse cerebellar Purkinje neurons, nitric oxide synthase-positive neurons of the glossopharyngeal nerve and central chemoreceptor respiratory chemoreceptor neurons [24-26]. It would be interesting to know whether single-channels with kinetic properties of THIK-1 can be recorded in these cells. Clear identification of the current as THIK-1 would be important for further studies to define the physiological role of THIK-1 in such cell types.
Pharmacology of THIK-1
Earlier studies have shown that halothane inhibits and arachidonic acid activates THIK-1 expressed in Xenopus oocytes and HEK293 cells [16]. THIK-1 was found to show low sensitivity to changes in intracellular or extracellular pH, temperature and lysophosphatidylcholine [16]. However, the effects of many other agents known to block K+ channels have not yet been reported. Our own tests in HEK293 show that THIK-1 is partially inhibited by quinidine and bupivacaine. THIK-1 was insensitive to direct application of signaling molecules that regulate protein kinases A and C, and AMP-activated kinase. ACh showed a very small effect on THIK-1 current via M1 receptor. In mouse cerebellar Purkinje cells, the non-inactivating K+ current was inhibited by halothane and Ba2+, and activated by arachidonic acid, and therefore shares properties similar to THIK-1 [25]. In these Purkinje cells, baclofen and DAMGO increased the outward current by ~30-40%, and glutamate and DHPG inhibited the outward current by ~40-50%. These results suggest that receptor agonists regulate THIK-1 expressed in native cells but not in HEK293 cells. This aspect of THIK-1 regulation needs further study, perhaps at the level of the single-channel, to be certain that only THIK-1 current is being studied.
Cold sensitivity of THIK-1
Our finding that THIK-1 is inhibited by cold is anticipated because of the general thermodynamic effect of low temperature on protein function. Nevertheless, the ~80% decrease in THIK-1 activity produced by cold (10°C) recorded at the single-channel level may be physiologically significant. The non-selective cation channel TRPM8 is believed to be the major determinant of cold sensing in TG neurons, as cold activates TRPM8 and TRPM8-/- mice fails to respond to cold [35-37]. In addition to cation-selective channels, K+ channels such as TREK-1 and Kv1 have also been suggested as possible candidates for sensing cold [38, 39]. We believe that TREK-1 may not sense cold because it is mostly in the closed state at room temperature [40, 41]. Our data suggest that THIK-1 could very well participate in sensing of noxious cold, because it is active at rest and is inhibited by cold in TG neurons. The expression of TRPM8 is high in subset of TG neurons that sense cool temperature (~30°C) and low in subset of neurons that sense cold temperature (<20°C) [42]. THIK-1 could potentially contribute more to cold sensing in those neurons that are sensitive to cold temperature. A more detailed study of the expression and function of THIK-1 in different subsets of TG neurons as well as in other sensory neurons should help to better understand the role of THIK-1 in the excitability of cold-sensitive neurons.
Oxygen sensitivity of THIK-1
THIK-1 current was reported to be slightly inhibited (~10%) by hypoxia in HEK293 cells, and this was associated with ~2 mV depolarization [43]. The small inhibition of THIK-1 by hypoxia was not due to mitochondrial inhibition, because it still occurred in the presence of sodium cyanide and myxothiazole. Also, DPI, an inhibitor of NADHP oxidase, did not block the hypoxia-induced inhibition of THIK-1 [43]. Under our own experimental conditions, hypoxia that usually inhibits ~75% of TASK current in carotid body glomus cells showed very little inhibition (~7%) of THIK-1 in HEK293 cells. Although our hypoxia experiment was performed at 35°C, whereas earlier experiments were done at 22°C, this temperature difference seems unlikely to account for the small difference in the hypoxic response of THIK-1. If anything, hypoxia is expected to have a stronger effect at high than at a low temperature. Hypoxia showed essentially no significant change in THIK-1 single-channel activity in cell-attached patches in our study. Overall, our data suggest that THIK-1 is not sensitive to hypoxia and mitochondrial inhibitors when expressed in HEK293 cells.
In carotid body glomus cells that express high levels of TASK-like background K+ channels, hypoxia inhibited TASK activity by ~75%, as reported previously [3]. In our preliminary test, when TASK-1, TASK-3 or TASK-1/3 heteromer was expressed in HEK293 or COS-7 cells, hypoxia showed either very small (<10% for TASK-1) or no effect (for TASK-3 and TASK-1/3) on channel activity. Therefore, it is quite plausible that THIK-1 has higher sensitivity to hypoxia in certain native cells, due perhaps to the presence of hypoxia-sensitive signals that are not present in cloned mammalian cell lines. Although we found no effect of hypoxia on the THIK-1-like current in TG neurons, others found a small inhibition in rat glossopharyngeal neurons, [24]. It would be important to show that the THIK-1-like current in these neurons is indeed THIK-1 by identifying the single-channel properties of the THIK-1-like current.
In summary, we show the single-channel characteristics of THIK-1 and the response of THIK-1 to various pharmacological agents at the whole-cell and single-channel levels. These properties should help to confirm the presence of THIK-1 in those cells that predominantly express THIK-1 as their background K+ current. Using single-channel, pharmacological and biochemical analyses, we show that THIK-l is expressed in TG neurons and probably contribute to the background K+ conductance in these cells. The cold-sensitivity of THIK-1 suggests that THIK-1 may be involved in cold-induced sensory transduction by TG neurons, similar to the cold-sensitive TRPM8 also expressed in TG neurons [38, 42]. The possibility that THIK-1 and TRPM8 work together to transduce the cold signal seems likely and needs to be investigated further.
Acknowledgments
This work was funded in part by National Institute of Health grant to D. Kim and National Research Foundation of Korea-2005-0049415 to D. Kang.
Footnotes
Conflict of interest
We declare no conflict of interest.
Ethical standards
We declare that the experiments comply with the current laws of the United States.
References
- 1.Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 5.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci U S A. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K(2P) channels. Physiology (Bethesda) 2011;26:424–437. doi: 10.1152/physiol.00029.2011. [DOI] [PubMed] [Google Scholar]
- 7.L’Hoste S, Barriere H, Belfodil R, Rubera I, Duranton C, Tauc M, Poujeol C, Barhanin J, Poujeol P. Extracellular pH alkalinization by Cl-/HCO3- exchanger is crucial for TASK2 activation by hypotonic shock in proximal cell lines from mouse kidney. Am J Physiol Renal Physiol. 2007;292:F628–638. doi: 10.1152/ajprenal.00132.2006. [DOI] [PubMed] [Google Scholar]
- 8.L’Hoste S, Poet M, Duranton C, Belfodil R, e Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem. 2007;282:36692–366703. doi: 10.1074/jbc.M703933200. [DOI] [PubMed] [Google Scholar]
- 9.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 11.Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, Wischmeyer E, Doring F. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurones. J Physiol. 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- 13.Callejo G, Giblin JP, Gasull X. Modulation of TRESK Background K(+) Channel by Membrane Stretch. PLoS One. 2013;8:e64471. doi: 10.1371/journal.pone.0064471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K(+) channel involved in neuroprotection and general anesthesia. Embo J. 2004:1–12. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, Warth R, Barhanin J, Lesage F. TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci U S A. 2012;109:5499–5504. doi: 10.1073/pnas.1201132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, Karschin A, Derst C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J Biol Chem. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- 17.Seto T, Mashimo T, Yoshiya I, Kanashiro M, Taniguchi Y. The solubility of volatile anaesthetics in water at 25.0 degrees C using 19F NMR spectroscopy. J Pharm Biomed Anal. 1992;10:1–7. doi: 10.1016/0731-7085(92)80003-6. [DOI] [PubMed] [Google Scholar]
- 18.Gierten J, Ficker E, Bloehs R, Schlomer K, Kathofer S, Scholz E, Zitron E, Kiesecker C, Bauer A, Becker R, Katus HA, Karle CA, Thomas D. Regulation of two-pore-domain (K2P) potassium leak channels by the tyrosine kinase inhibitor genistein. Br J Pharmacol. 2008;154:1680–1690. doi: 10.1038/bjp.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fearon IM, Campanucci VA, Brown ST, Hudasek K, O’Kelly IM, Nurse CA. Acute hypoxic regulation of recombinant THIK-1 stably expressed in HEK293 cells. Adv Exp Med Biol. 2006;580:203–8. doi: 10.1007/0-387-31311-7_31. discussion 351-359. [DOI] [PubMed] [Google Scholar]
- 20.Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012;463:743–754. doi: 10.1007/s00424-012-1089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D. K(+) channels in O(2) sensing and postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2013;185:44–56. doi: 10.1016/j.resp.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirois JE, Pancrazio JJ, Lynch C, 3rd, Bayliss DA. Multiple ionic mechanisms mediate inhibition of rat motoneurones by inhalation anaesthetics. J Physiol. 1998;512(Pt 3):851–862. doi: 10.1111/j.1469-7793.1998.851bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulleuda A, Cokic B, Callejo G, Saiani B, Serra J, Gasull X. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain. 2011;7:30. doi: 10.1186/1744-8069-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campanucci VA, Fearon IM, Nurse CA. A novel O2-sensing mechanism in rat glossopharyngeal neurones mediated by a halothane-inhibitable background K+ conductance. J Physiol. 2003;548:731–743. doi: 10.1113/jphysiol.2002.035998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushell T, Clarke C, Mathie A, Robertson B. Pharmacological characterization of a non-inactivating outward current observed in mouse cerebellar Purkinje neurones. Br J Pharmacol. 2002;135:705–712. doi: 10.1038/sj.bjp.0704518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–93334. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavrilova-Ruch O, Schonherr R, Heinemann SH. Activation of hEAG1 potassium channels by arachidonic acid. Pflugers Arch. 2007;453:891–903. doi: 10.1007/s00424-006-0173-3. [DOI] [PubMed] [Google Scholar]
- 28.Hashiguchi-Ikeda M, Namba T, Ishii TM, Hisano T, Fukuda K. Halothane inhibits an intermediate conductance Ca2+-activated K+ channel by acting at the extracellular side of the ionic pore. Anesthesiology. 2003;99:1340–1345. doi: 10.1097/00000542-200312000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Correa AM. Kinetic modulation of HERG potassium channels by the volatile anesthetic halothane. Anesthesiology. 2002;97:921–930. doi: 10.1097/00000542-200210000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Roch A, Shlyonsky V, Goolaerts A, Mies F, Sariban-Sohraby S. Halothane directly modifies Na+ and K+ channel activities in cultured human alveolar epithelial cells. Mol Pharmacol. 2006;69:1755–1762. doi: 10.1124/mol.105.021485. [DOI] [PubMed] [Google Scholar]
- 31.Davies LA, Hopkins PM, Boyett MR, Harrison SM. Effects of halothane on the transient outward K(+) current in rat ventricular myocytes. Br J Pharmacol. 2000;131:223–230. doi: 10.1038/sj.bjp.0703565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buttner N, Siegelbaum SA, Volterra A. Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature. 1989;342:553–555. doi: 10.1038/342553a0. [DOI] [PubMed] [Google Scholar]
- 33.Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang D, Kim D. TREK-2(K2P10.1) and TRESK (K2P18) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- 35.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 36.Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 38.Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29:3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, Lazdunski M, Honore E. TREK-1 is a heat-activated background K(+) channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D. Fatty acid-sensitive two-pore domain K(+) channels. Trends Pharmacol Sci. 2003;24:648–54. doi: 10.1016/j.tips.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;90:515–520. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- 43.Campanucci VA, Brown ST, Hudasek K, O’Kelly IM, Nurse CA, Fearon IM. O2 sensing by recombinant TWIK-related halothane-inhibitable K+ channel-1 background K+ channels heterologously expressed in human embryonic kidney cells. Neuroscience. 2005;135:1087–1094. doi: 10.1016/j.neuroscience.2005.07.009. [DOI] [PubMed] [Google Scholar]


