Abstract
Background
Adding telaprevir to pegylated-interferon and ribavirin increased both response rates and side effects of hepatitis C virus (HCV) treatment.
Aims
We identified variables associated with severe anemia during telaprevir-based triple therapy.
Methods
An observational study was performed on 142 HCV-infected patients between June 2011 and March 2012. All subjects completed 12 weeks of telaprevir-based triple therapy or discontinued early due to anemia. Severe anemia was defined by a hemoglobin ≤8.9 g/dL; advanced fibrosis was determined by Fib-4 ≥3.25.
Results
The 47 (33%) patients who developed severe anemia were similar to those who did not in sex, race, and prior response to dual therapy, but they were more likely to have diabetes (23.4% vs. 6.3%, p<0.01), advanced fibrosis (46.8% vs. 29.5%, p=0.04), and a history of anemia during previous dual therapy (29.7% vs. 11.4%, p=0.02). Patients developing severe anemia were older (59 vs. 56 years, p=0.02), had lower baseline platelet counts (134 vs. 163 x109/L, p=0.04), hemoglobin (14.0 vs. 15.0 g/dL, p<0.01), estimated glomerular filtration rate (79 vs. 90 mL/min/1.73m2, p=0.03), and a higher median ribavirin/weight ratio (14.9 vs. 13.2 mg/kg, p<0.01). In multivariable logistic regression, presence of diabetes (OR=5.61, 95%CI: 1.59–19.72), Fib-4 ≥3.25 (OR=3.09, 95%CI: 1.28–7.46), higher ribavirin/weight ratio (OR=1.31 per mg/kg, 95%CI: 1.13–1.52), and lower baseline hemoglobin (OR=0.57 per g/dL, 95%CI, 0.41–0.80) were independently associated with developing severe anemia.
Conclusions
Severe anemia occurred in one-third of patients receiving telaprevir-based triple therapy. Risk was greater in patients with diabetes, advanced liver fibrosis, higher ribavirin/weight ratio and lower baseline hemoglobin.
Keywords: hepatitis C virus, telaprevir, pegylated-interferon, ribavirin, triple therapy, anemia, diabetes mellitus
INTRODUCTION
The addition of telaprevir (TVR) to dual therapy with pegylated-interferon (PEG-IFN) and ribavirin (RBV) improved sustained virologic response (SVR) rates in patients with chronic hepatitis C virus infection (HCV) (1). While generally well tolerated by patients in the registration trials, TVR has significant side effects, including severe rash that has led to fatalities in patients outside the registration trials (1–4). Pruritus, gastrointestinal side effects, and anemia also occur more frequently in patients on TVR-based triple therapy than in patients on PEG-IFN/RBV dual therapy.
Anemia contributes to a variety of symptoms including fatigue, shortness of breath, and palpitations, and occurs about twice as frequently during TVR-based triple therapy as during dual therapy (3). Treatment-related anemia can reduce health-related quality of life (5). Responding to reports of severe anemia, the FDA recently modified the safety labeling instructions on TVR to recommend that hemoglobin measurements be made at baseline and at weeks 2, 4, 8, and 12 during treatment (4). Several strategies are available to manage anemia. The first adjustment is usually a reduction in the dose of RBV, a modification that does not appear to reduce SVR rates (6). While this may be sufficient to mitigate mild anemia, severe anemia may require more intensive management (7). Erythrocyte-stimulating agents (ESAs) can be used, although they are not FDA approved for this indication, and their use was not allowed during the initial trials with TVR (8). Further, ESAs have been associated with a number of adverse events, including worsened hypertension, increased incidence of venous thromboembolism, and in patients with malignancy, shortened survival time and reduced time to cancer progression (9). Blood transfusion is a final option for treating severe anemia, but it is reserved for intractable cases because of its risks (10). If anemia cannot be controlled, HCV treatment must be discontinued, which greatly increases the risk of treatment failure.
Previous studies of patients on dual therapy identified older age, lower baseline platelet count, lower creatinine clearance, and increased rate of hemoglobin decline in the first two weeks of treatment as potential risk factors for the development of anemia (4, 11, 12). In post-hoc analyses of patients in the TVR registration trials, older age, lower body mass index (BMI), lower baseline hemoglobin levels, advanced liver fibrosis, and a history of anemia during prior dual therapy were identified as predictors of anemia of any degree (13). No prior study, however, has examined risk factors specifically for severe, clinically significant grade 3 or 4 anemia during TVR-based triple therapy outside the registration trials. Herein, we investigated the characteristics of patients who developed severe anemia on TVR-based triple therapy. Information about these risk factors may enable more timely interventions, potentially reducing the need for transfusion, hospitalization, and/or cessation of therapy.
PATIENTS AND METHODS
The study group was comprised of 142 patients with genotype 1 HCV infection who were treated with TVR-based triple therapy at the Mount Sinai Medical Center between June 2011 and March 2012, who completed at least 12 weeks of treatment or discontinued early due to anemia, and for whom complete records were available. An additional 65 patients who received TVR-based triple therapy were excluded for the following reasons: 25 discontinued TVR prior to 12 weeks because of virologic failure, 20 discontinued early due to side effects other than anemia, 18 were excluded due to missing hemoglobin levels, and two were lost to follow up. Severe anemia was defined as hemoglobin ≤ 8.9 g/dL, which corresponds to grade 3 or 4 anemia by modified DAIDS criteria (14). Of note, the DAIDS definition of severe anemia also includes a decrease in hemoglobin from baseline of ≥4.5 g/dL, but this was not used to grade anemia in this study because anemia in our population was managed primarily based on the absolute value of hemoglobin, rather than on the change from baseline. Mild anemia was defined as a hemoglobin level < 13.5 g/dL for males and < 12.0 g/dL for females. Patients were followed through 24 weeks of treatment. Data were generated during the course of standard clinical care and extracted via chart review. The Mount Sinai IRB approved the study, which was conducted as specified by the Helsinki Accord (GCO# 10-0032).
Data were obtained on patient characteristics, including demographics, comorbid medical conditions, HCV genotype, HCV viral load, prior virologic response to dual therapy, history of anemia with dual therapy, and dosing of TVR, PEG-IFN, and RBV during triple therapy. Laboratory data including complete blood count, chemistry, liver function tests, and HCV viral load were collected at baseline and at weeks 2, 4, 8, 12, and 24 during treatment. HCV viral loads were determined using the Cobas AmpliPrep/Cobas TaqMan HCV test (Roche). Presence of diabetes mellitus (DM) was determined by documented diagnosis and/or by laboratory data (hemoglobin A1c ≥6.5 %, fasting plasma glucose ≥ 6.993 mmol/L, or a plasma glucose ≥11.1 mmol/L after a 2-hour glucose tolerance test). The presence of chronic kidney disease (CKD), human immunodeficiency virus (HIV) infection, and non-alcoholic fatty liver disease (NAFLD) was determined by documented diagnoses. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula (15). Liver fibrosis was estimated using the Fib-4 score (Fib-4=(Age[years] x AST[IU/L])/(Platelet count [x109/L]/√ALT [IU/L]) with a score ≥ 3.25 denoting advanced fibrosis/cirrhosis (16). Information about use and timing of initiation of ESAs and transfusion of packed red blood cells (PRBCs) was also recorded. Ribavirin dosing was weight-based (17). The total ribavirin dose in milligrams per kilogram of body weight was calculated at the start of treatment for each patient. In general, the ribavirin dose was reduced to 600 mg during treatment, if clinically indicated. Because this adjustment was made by on an individual basis by providers and reductions were not always clearly documented in the medical record, dose reductions were excluded from the analysis.
Statistical Analysis
Continuous variables were analyzed with either two-tailed t-tests or Mann-Whitney U-tests, as appropriate. Categorical variables were analyzed with chi-square or Fisher exact tests. A p-value below 0.05 was considered statistically significant. Logistic regression analysis was used to identify variables associated with severe anemia. The independent variables are presented in Table 1. Variables with a p-value below 0.10 in the univariable analysis were retained in the final multivariable model if the p value was below 0.05. To avoid collinearity due to their inclusion as variables in the Fib-4 equation, age and platelet count were excluded from the final model. Weight was excluded due to its inclusion in the RBV/weight ratio. History of anemia with prior dual therapy was excluded from the model as it applied only to those patients who were previously treated with dual therapy. All data were analyzed in SPSS version 20.
Table 1.
Baseline characteristics of patients who did and did not develop severe anemia
| Total | Severe Anemia | No Severe Anemia | p-value | Test | |
|---|---|---|---|---|---|
| n | 142 | 47 | 95 | - | |
| Demographics and Anthropometrics | |||||
| Age (year)* | 57 (51–61) | 59 (54–62) | 56 (49.5–60) | 0.02 | t-test |
| Sex, male** | 101 (71.1%) | 30 (63.8%) | 71 (74.7%) | 0.18 | Chi-square |
| Race: Caucasian** | 94 (66.2%) | 30 (61.7%) | 64 (67.0%) | 0.61 | Chi-square |
| BMI, kg/m2* | 26.9 (26.7–29.9) | 26.4 (23.2–28.8) | 27.0 (24.7–30.4) | 0.22 | Mann-Whitney |
| Baseline Laboratory Values | |||||
| Hemoglobin, g/dL* | 14.4 (13.4–15.4) | 14.0 (13.1–14.6) | 15.0 (13.8–15.6) | <0.01 | t-test |
| MCV, fL* | 95.5 (90.5–98.3) | 95.8 (89.2–98.5) | 95.1 (90.6–98.2) | 0.77 | t-test |
| Platelets, 109/L* | 161 (113–202) | 134 (98–188) | 163 (127–205) | 0.04 | t-test |
| eGFR, mL/min/1.73m2* | 88 (74–97.3) | 79 (71–94) | 90 (77–99) | 0.03 | t-test |
| AST, U/L* | 50.5 (34–77.3) | 51 (37–77) | 50 (33–79) | 0.87 | Mann-Whitney |
| ALT, U/L* | 52.5 (34–94.8) | 45 (31–86) | 63 (39–108) | 0.07 | Mann-Whitney |
| HCV Viral Load, log IU/mL* | 6.27 (5.7–6.7) | 6.17 (5.7–6.6) | 6.31 (5.9–6.8) | 0.10 | t-test |
| RBV Dosing | |||||
| RBV/Weight, mg/kg* | 13.6 (11.9–15.4) | 14.9 (12.5–17.3) | 13.2 (11.9–14.5) | <0.01 | Mann-Whitney |
| HCV Treatment History | |||||
| Naïve to HCV therapy** | 35 (24.6%) | 10 (21.3%) | 25 (26.3%) | 0.52 | Chi-square |
| History of anemia with dual therapy** | 19/107 (17.8%) | 11/36 (29.7%) | 8/70 (11.4%) | 0.02 | Chi-Square |
| Medical History | |||||
| Anemia at baseline** | 26 (18.3%) | 13 (27.7%) | 13 (13.7%) | 0.06 | Chi-square |
| Diabetes mellitus** | 17 (12%) | 11 (23.4%) | 6 (6.3%) | <0.01 | Chi-square |
| HIV coinfection** | 27 (19.0%) | 11 (23.4%) | 16 (16.8%) | 0.35 | Chi-square |
| NAFLD** | 29 (20.4%) | 10 (21.3%) | 19 (20%) | 0.86 | Chi-square |
| eGFR <60 mL/min/1.73m2** | 11 (7.7%) | 6 (12.8%) | 5 (5.3%) | 0.18 | Fisher-exact |
| Estimation of Fibrosis | |||||
| Fib-4 score ≥3.25** | 50 (35.2%) | 22 (46.8%) | 28 (29.5%) | 0.04 | Chi-square |
median (IQR);
n (%).
RESULTS
Baseline Characteristics
A total of 207 patients were treated with TVR-based triple therapy during the study period, of whom 142 met the entry criteria and were included in this analysis. Forty-seven (33%) patients developed severe anemia while 95 (67%) patients maintained hemoglobin >8.9 g/dL while on treatment. Demographics, data on prior treatment and baseline laboratory values of patients who did and who did not develop severe anemia are shown in Table 1. At baseline, the two groups did not differ in race, sex, HCV genotype or viral load, previous virologic response to treatment with dual therapy, BMI, mean corpuscular volume (MCV), white blood cell count, or presence of anemia prior to treatment.
Patients who developed severe anemia were older and were more likely to have a history of anemia during prior dual therapy. Baseline hemoglobin, platelet count, and eGFR were lower in patients who developed severe anemia. Patients who developed severe anemia began treatment at a higher RBV dose for their body weight (RBV/weight ratio). A greater percentage of them had diabetes mellitus and/or advanced liver fibrosis or cirrhosis as determined by a Fib-4 score ≥3.25.
Logistic Regression Model for Severe Anemia
In multivariable logistic regression with severe anemia as the outcome (Table 2), presence of diabetes mellitus, Fib-4 ≥3.25, a higher RBV/weight ratio at the start of treatment, and lower baseline hemoglobin were independently associated with developing severe anemia. Baseline eGFR also approached statistical significance and was retained in the final model.
Table 2.
Factors associated with severe anemia in a logistic regression model.
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age, years | 1.05 | 1.01 – 1.10 | 0.03 | . | . | . |
| RBV/Weight, mg/kg | 1.2 | 1.06 – 1.36 | <0.01 | 1.31* | 1.13–1.52 | <0.01 |
| Fib-4 ≥3.25 | 2.11 | 1.02 – 4.34 | 0.04 | 3.09 | 1.28–7.46 | 0.01 |
| Diabetes | 4.53 | 1.56 – 13.18 | 0.01 | 5.61 | 1.59–19.72 | 0.01 |
| Hemoglobin, g/dL | 0.61 | 0.46 – 0.82 | <0.01 | 0.57** | 0.41 – 0.80 | <0.01 |
| eGFR, mL/min/1.73m2 | 0.98 | 0.96 – 0.99 | 0.03 | 0.98*** | 0.95–1.0 | 0.051 |
NB: Variables with a p-value <0.1 were included in the model for multivariable analysis, and are shown here; all others were excluded from the model. Please note, age, platelets, ALT, and AST were excluded from the final model as these factors are used to calculate the Fib-4 score.
per mg/kg;
per g/dL;
per mL/min/1.73m2
On-Treatment Changes in Hemoglobin and Development of Severe Anemia
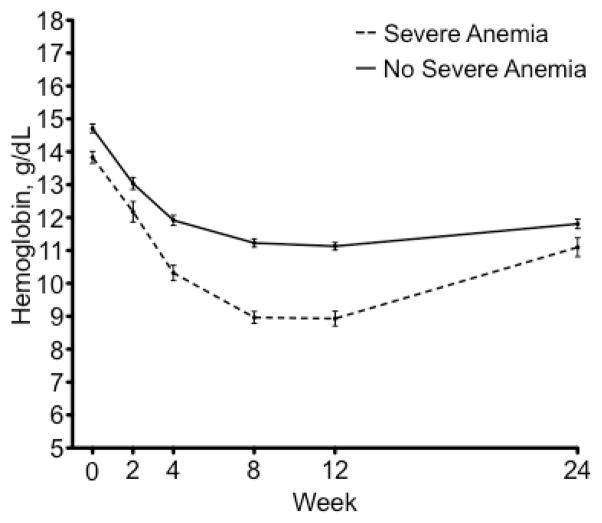
The change in hemoglobin in patients who did and who did not develop severe anemia can be seen in Figure 1. All patients developed mild anemia or severe anemia while on treatment. The median time to the hemoglobin nadir was 10 weeks [interquartile range (IQR)=8–13]. The median time to the development of severe anemia was 8 weeks (IQR=4–10). The median hemoglobin nadir was 8.1 g/dL (IQR=7.3–8.7) in patients with severe anemia. Throughout treatment, hemoglobin remained significantly lower in the patients who developed severe anemia. While hemoglobin values began to increase after TVR was stopped at week 12, they had not returned to baseline by week 24 of treatment when follow up ended.
Figure 1.
Hemoglobin levels throughout treatment in patients who developed severe anemia and those who did not. Standard error is shown. Patients with severe anemia are shown in a dotted line and those without are shown in a solid line. The nadir for the entire group occurred at week 10.
On-Treatment Changes in Kidney Function
Estimated GFR declined in the majority (72%) of patients by week 4 (Figure 2). As noted above, median baseline eGFR was lower in patients who developed severe anemia, and it remained lower at week 8 (75 vs. 81 mL/min/1.73m2, p=0.03), week 12 (76 vs. 83, p=0.02), and week 24 (84 vs. 95, p=0.01). The magnitude of the eGFR decrease did not differ significantly between the two groups (p=0.118). The median eGFR calculated at time of nadir hemoglobin was significantly lower in patients with severe anemia than in those without (76 vs. 84.5 mL/min/1.73m2, p=0.02). Median eGFR began to increase after week 4 in both groups, and returned to baseline by week 24.
Figure 2.
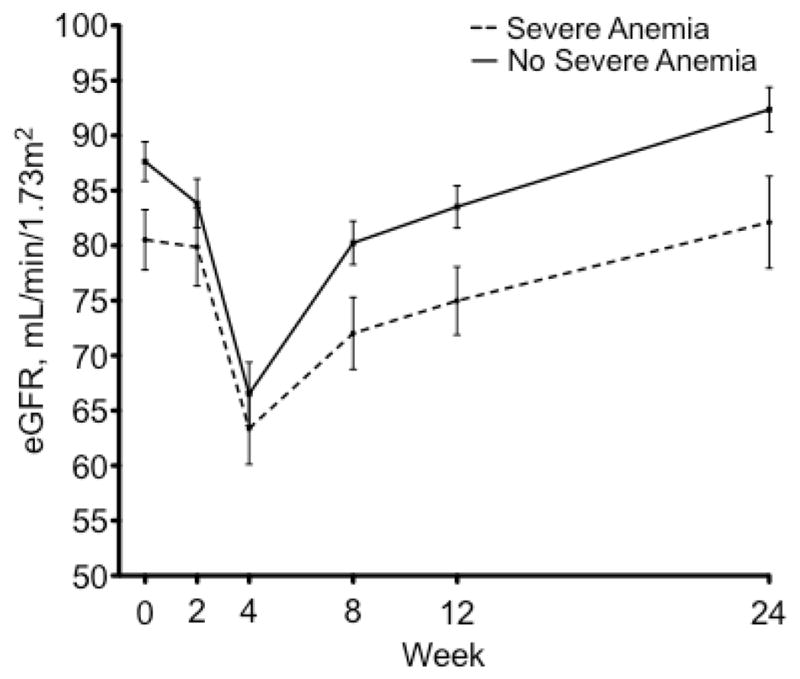
Estimated glomerular filtration rate throughout treatment is plotted here for patients who developed severe anemia and those who did not. Standard error is shown. Those who developed severe anemia are shown in a dotted line and those who did not are in a solid line. The nadir for both groups occurred at week 4.
Treatment of Anemia during Triple Therapy
Nineteen (40%) of the 47 patients who developed severe anemia required blood transfusion, compared to 1 (1%) of the 95 who did not develop severe anemia (p<0.01). Therapy with an ESA was initiated in 40 (82.9%) of the patients who developed severe anemia, compared to 35 (36.8%) of the patients who did not (p<0.01). ESA therapy was initiated earlier in patients who developed severe anemia than in those who did not (median week 4 vs. week 8, p<0.01).
DISCUSSION
In this study, which investigated side effects outside of a registration trial, severe anemia occurred in about one-third of patients receiving TVR-based triple therapy. Future management of severe anemia will depend on the availability of information about risk factors. We found that patients who developed severe anemia were older, had a history of anemia during prior dual therapy, and had lower hemoglobin and a higher RBV/weight ratio at the start of treatment. Notably, patients who developed severe anemia were more frequently diabetic and more frequently had advanced liver fibrosis as determined by a Fib-4 score ≥3.25. Although of marginal statistical significance in the multivariable analysis, baseline eGFR was also lower in patients who developed severe anemia. ESA use and blood transfusion was required more frequently in the group that developed severe anemia.
In the TVR registration trials, including ADVANCE and REALIZE, anemia occurred more frequently and with more severity in the TVR arms than in the dual therapy arms. In ADVANCE, 9% of the patients receiving TVR-based triple therapy developed severe anemia (hemoglobin <8.5 g/dL) compared with 2% in the PEG-IFN/RBV dual therapy group. In REALIZE, 11% of patients in the TVR arm developed severe anemia versus 5% in the control arm (1, 18). In our experience, which was outside the registration trials, all patients developed at least mild anemia while on TVR-based triple therapy. The higher incidence of anemia we observed is most likely a consequence of including patients who were older, had more comorbidities and more advanced liver disease than those in the randomized trials. Indeed, advanced fibrosis and cirrhosis have been shown previously to be associated with development of severe anemia while undergoing TVR-based triple therapy (13). Severe anemia occurred in 33% of the patients in our study, which defined severe anemia according to DAIDS definitions as hemoglobin ≤8.9 g/dL. The registration trials used a cut off of 8.5 g/dL. Using that threshold value, 22% of our patients developed severe anemia.
Anemia was also managed differently among the patients in this study than it was in the early TVR trials. In ADVANCE and REALIZE, ESA use was not permitted, and anemia was managed strictly with RBV dose reduction. In contrast, we often initiated ESAs early to forestall a RBV dose reduction. While ESAs have been shown to be useful in maintaining RBV dose and improving health-related quality of life for patients undergoing treatment for chronic HCV, they are not FDA approved for this indication (19). In addition to ESAs, approximately 40% of the patients with severe anemia required blood transfusion, which usually necessitated an emergency department visit or hospitalization. Along with increased resource utilization, blood transfusion itself has well-known health risks.
The mechanism of worsened anemia in patients undergoing TVR-based triple therapy is multifactorial. PEG-IFN leads to anemia via bone marrow suppression, while RBV produces hemolysis via depletion of erythrocyte adenosine triphosphate (ATP) stores (20). The mechanism by which TVR potentiates this effect, however, remains unclear. Pre-clinical pharmacokinetic studies revealed that there was a relationship between the occurrence and severity of hemoglobin abnormalities and the exposure (area under the curve) to TVR, as well as PEG-IFN and RBV concentration at week 4. In the initial trials, while decreases in mean hemoglobin values were greater in the TVR groups compared to placebo groups, the corresponding increase in mean reticulocyte production appeared to be blunted relative to the observed changes in hemoglobin. This is reflective of an inadequate bone marrow response in the TVR groups (11). While TVR may have a direct effect on red blood cell production, it may also worsen anemia indirectly by leading to increased RBV exposure. Patients on TVR-based triple therapy have an increased incidence of impaired kidney function while on treatment compared to patients treated with dual therapy (21). Acute kidney injury may be caused in part by volume depletion owing to the gastrointestinal side effects of TVR, including nausea, vomiting, and diarrhea (22). Given that RBV is cleared primarily by the kidneys, and that increased serum levels of RBV are correlated with a decrease in hemoglobin (23), it is likely that any decrease in eGFR while on TVR may lead to increased blood levels of RBV, and increased RBV-induced anemia.
In addition to RBV exposure, RBV-induced anemia is also influenced by host polymorphisms in the inosine triphosphatase (ITPA) gene. Patients possessing functional ITPA variants rs1127354 and rs7270101 have variably reduced functionality of ITPA (24). Decreased ITPA activity leads to accumulation of ITP within erythrocytes, which is thought to be protective against RBV-induced hemolytic anemia (25). While the influence of these polymorphisms has been studied extensively in PEG-IFN/RBV dual therapy, there is limited data in TVR-based triple therapy. One study in a Japanese cohort demonstrated a greater reduction in hemoglobin in patients with wild-type rs1127354 at both week 1, week 4, and at the end of treatment, suggesting that genetic polymorphisms influence the degree of anemia that develops in patients undergoing TVR-based triple therapy as well (26). Given the extensive variability in ITPA polymorphisms among different ethnic groups (27), further studies will need to be done to examine the effects of these polymorphisms in a more diverse population.
We found that diabetes mellitus predisposed to the development of severe anemia during TVR-based triple therapy, even after adjusting for baseline eGFR. Prior studies suggest that diabetics have a higher prevalence of anemia than the general population (28). Several potential mechanisms have been proposed. Diabetic neuropathy can lead to disrupted splanchnic innervation of the kidney, which may alter endogenous erythropoietin production (29). Further, several commonly prescribed medications may increase risk of anemia in diabetics. Inhibition of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers may potentiate anemia by interfering with the erythropoietic effects of angiotensin II (30). Thiazolidinediones, such as pioglitazone, may lead to increased fluid retention, leading to a dilutional decrease in hemoglobin concentration (31). Metformin, in rare case reports, has been linked to the development of severe hemolytic anemia (32). A combination of these factors may promote the development of anemia in patients on TVR-based triple therapy who have pre-existing diabetes.
The strengths of our study include its timeliness and its focus on patients receiving a newly-introduced therapy for HCV outside of registration trials. Our population, in general, was older and had more non-whites and patients with advanced fibrosis/cirrhosis than the registration trials. We demonstrated a relationship between the development of severe anemia and the presence of DM at baseline that has not been described previously. Further, our conclusions are based on the types of clinical data that are readily available to health care providers. Limitations include our lack of complete data on RBV dose reductions and our inclusion of patients who received treatment at a single tertiary care center. We also did not have complete biopsy data for all patients, and therefore relied on noninvasive measures of liver fibrosis to determine the extent of liver damage. Finally, we lacked ITPA polymorphism data for our patients; however, this information is not commonly available for clinicians outside of a research setting and is rarely used in clinical decision making.
CONCLUSIONS
This study confirms that severe, clinically relevant anemia is an important adverse event associated with TVR-based triple therapy, and establishes a number of factors that increase the risk of severe anemia, including a history of anemia during dual therapy, older age, lower baseline hemoglobin, and higher RBV/weight ratio. The presence of diabetes as well as advanced liver fibrosis or cirrhosis may also increase risk of severe anemia. In patients with the above risk factors, close monitoring of hemoglobin is essential early in the course of treatment with TVR so that interventions such as RBV dose reduction and administration of ESAs can be initiated prior to the development of severe anemia.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health DA031095 and DK090317 to ADB. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Valérie Martel-Laferrière was supported by the 2011 AMMI Canada/Pfizer Post Residency Fellowship, 2012 Grant of the CHUM Foundation.
Abbreviations
- HCV
hepatitis C virus
- OR
odds ratio
- PEG-IFN
pegylated interferon
- RBV
ribavirin
- ESA
erythropoietin stimulating agents
- PRBC
packed red blood cells
- SVR
sustained virologic response
- TVR
telaprevir
- DM
diabetes mellitus
- HIV
human immunodeficiency virus
- NAFLD
non-alcoholic fatty liver disease
- eGFR
estimated glomerular filtration rate
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Andrea D. Branch, Ph.D.
Specific Author Contributions: Study conception and design, data collection, drafting of the manuscript: James F. Crismale. Study conception and design, data collection and analysis, critical revision of the manuscript: Valérie Martel-Laferrière. Data collection and analysis, critical revision of the manuscript: Kian Bichoupan. Data collection: Emily Schoenfeld. Data Collection: Alexis Pappas. Data collection and study supervision: Joseph A. Odin; data collection and study supervision: Lawrence L. Liu; data collection and study supervision: Thomas Schiano; data collection and study supervision: Ponni Perumalswami; data collection and study supervision: Meena Bansal; study conception and design, study supervision, critical revision of the manuscript: Christina Wyatt; study conception and design, study supervision, critical revision of the manuscript: Douglas T. Dieterich; study conception and design, study supervision, data collection and analysis, critical revision of the manuscript: Andrea D. Branch. All authors approved the final draft of this manuscript.
Potential Competing Interests: Dr. Douglas Dieterich serves as a paid lecturer, consultant, and is a member on scientific advisory boards of companies which either develop or assess medicines used for the treatment of viral hepatitis. These companies include Gilead Sciences, Boehringer Ingelheim, Novartis, Vertex Pharmaceuticals, Achillion, Tibotec, Idenix, Merck, Kadmon, Bayer Healthcare, Genentech and Hoffman-La Roche, Inc. and Bristol-Myers Squibb.
Dr. Thomas Schiano is a paid lecturer, consultant, and a participant in the DSMB of companies that include Bristol-Myers Squibb/Sanofi-Aventis Partnership; Novartis; Pfizer Inc., and Salix Pharmaceuticals, Inc.
Dr. Andrea D. Branch is a paid consultant for Kadmon.
James F. Crismale, Valérie Martel-Laferrière, Kian Bichoupan, Christina Wyatt, Joseph A. Odin, Lawrence U. Liu, Ponni V. Perumalswami, Meena Bansal, Emily Schoenfeld, and Alexis Pappas have no relevant disclosures.
References
- 1.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for Previously Untreated Chronic Hepatitis C Virus Infection. New England Journal of Medicine. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 2.Burney T, Dusheiko G. Overview of the PROVE Studies Evaluating the Use of Telaprevir in Chronic Hepatitis C Genotype 1 Patients. Expert Review of Anti-infective Therapy. 2010;9:151–160. doi: 10.1586/eri.10.153. [DOI] [PubMed] [Google Scholar]
- 3.Aghemo A, Degasperi E, Colombo M. Directly Acting Antivirals for the Treatment of Chronic Hepatitis C: Unresolved Topics from Registration Trials. Digestive and Liver Disease. 2013;45:1–7. doi: 10.1016/j.dld.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.FDA. Highlights of Prescribing Information for Incivek. 2012 [cited 2013 March 29]; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/201917s007lbl.pdf.
- 5.Bernstein D, Kleinman L, Barker CM, et al. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski M, Reddy R, Afdhal N, et al. Anemia had no Effect on Efficacy Outcomes in Treatment-naive Patients who Received Telaprevir-based Regimen in the ADVANCE and ILLUMINATE Phase 3 Studies. Journal of Hepatology. 2011;54 (Suppl 1) [Google Scholar]
- 7.Hynicka LM, Heil EL. Anemia Management in Patients with Chronic Viral Hepatitis C. The Annals of Pharmacotherapy. 2013;47:228–236. doi: 10.1345/aph.1R513. [DOI] [PubMed] [Google Scholar]
- 8.Ghany MG, Nelson DR, Strader DB, et al. An Update on Treatment of Genotype 1 Chronic Hepatitis C Virus Infection: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett C, Silver S, Djulbegovic B, et al. Venous Thromboembolism and Mortality Associated with Recombinant Erythropoietin and Darbepoetin Administration for the Treatment of Cancer-associated Anemia. JAMA: The Journal of the American Medical Association. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 10.Squires JE. Risks of Transfusion. Southern Medical Journal. 2011;104:762–769. doi: 10.1097/SMJ.0b013e31823213b6. [DOI] [PubMed] [Google Scholar]
- 11.FDA. Antiviral Drugs Advisory Committee Breifing Document: Telaprevir 375-mg Film-Coated Tablet for the Treatment of Genotype 1 Chronic Hepatitis C. 2011 [cited 2012 November 20]; Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252562.pdf.
- 12.Butt AA, Umbleja T, Andersen JW, et al. The Incidence, Predictors and Management of Anaemia and its Association with Virological Response in HCV/HIV Coinfected Persons Treated with Long-term Pegylated Interferon alfa 2a and Ribavirin. Alimentary Pharmacology & Therapeutics. 2011;33:1234–1244. doi: 10.1111/j.1365-2036.2011.04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson IM, Kowdley KV, Kwo PY. Anemia Management in the Era of Triple Combination Therapy for Chronic HCV. Gastroenterology and Hepatology. 2012;8:1–16. [PMC free article] [PubMed] [Google Scholar]
- 14.DAIDS. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. 2009 [cited 2013 March 29]; Available from: http://rsc.tech-res.com/document/safetyandpharmacovigilance/table_for_grading_severity_of_adult_pediatric_adverse_events.doc.
- 15.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Annals of Internal Medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh YY, Tung SY, Lee K, et al. Routine Blood Tests to Predict Liver Fibrosis in Chronic Hepatitis C. World Journal of Gastroenterology. 2012;18:746–753. doi: 10.3748/wjg.v18.i8.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA. Highlights of Prescribing Information for COPEGUS. 2011 [cited 2013 August 27]; Available from: www.accessdata.fda.gov/drugsatfda_docs/label/.../021511s023lbl.pdf.
- 18.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for Retreatment of HCV Infection. New England Journal of Medicine. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 19.Pockros PJ, Shiffman ML, Schiff ER, et al. Epoetin alfa Improves Quality of Life in Anemic HCV-infected Patients Receiving Combination Therapy. Hepatology. 2004;40:1450–1458. doi: 10.1002/hep.20482. [DOI] [PubMed] [Google Scholar]
- 20.McHutchison JG, Manns MP, Longo DL. Definition and Management of Anemia in Patients Infected with Hepatitis C Virus. Liver International. 2006;26:389–398. doi: 10.1111/j.1478-3231.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 21.Mauss S. The International Liver Congress 2013. Amsterdam, The Netherlands: European Association for the Study of the Liver; 2013. Substantial Renal Impairment is not Infrequent in HCV Patients Under Triple Therapy with Telaprevir or Boceprevir. [Google Scholar]
- 22.Cunningham M, Foster G. Efficacy and Safety of Telaprevir in Patients with Genotype 1 Hepatitis C Infection. Therap Adv Gastroenterol. 2012;5(2):139–151. doi: 10.1177/1756283X11426895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu C-C, Weng C-H, Lin C-L, et al. Predictors of Changes in Hemoglobin Levels in Patients with Chronic Hepatitis C Treated with Ribavirin Plus Pegylated Interferon-α. Renal Failure. 2012;34:429–434. doi: 10.3109/0886022X.2011.650562. [DOI] [PubMed] [Google Scholar]
- 24.Fellay J, Thompson AJ, Ge D, et al. ITPA Gene Variants Protect Against Anaemia in Patients Treated for Chronic Hepatitis C. Nature. 2010;464:405–408. doi: 10.1038/nature08825. [DOI] [PubMed] [Google Scholar]
- 25.Hitomi Y, Cirulli ET, Fellay J, et al. Inosine Triphosphate Protects Against Ribavirin-Induced Adenosine Triphosphate Loss by Adenylosuccinate Synthase Function. Gastroenterology. 2011;140:1314–1321. doi: 10.1053/j.gastro.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki F, Suzuki Y, Akuta N, et al. Influence of ITPA Polymorphisms on Decreases of Hemoglobin during Treatment with Pegylated Interferon, Ribavirin, and Telaprevir. Hepatology. 2011;53:415–421. doi: 10.1002/hep.24058. [DOI] [PubMed] [Google Scholar]
- 27.Thompson AJ, Fellay J, Patel K, et al. Variants in the ITPA Gene Protect Against Ribavirin-Induced Hemolytic Anemia and Decrease the Need for Ribavirin Dose Reduction. Gastroenterology. 2010;139:1181–1189. e2. doi: 10.1053/j.gastro.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Unrecognized Anemia in Patients With Diabetes: A cross-sectional survey. Diabetes Care. 2003;26:1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 29.Deray G, Heurtier A, Grimaldi A, et al. Anemia and Diabetes. American Journal of Nephrology. 2004;24:522–526. doi: 10.1159/000081058. [DOI] [PubMed] [Google Scholar]
- 30.Vlahakos DV, Marathias KP, Madias NE. The Role of the Renin-Angiotensin System in the Regulation of Erythropoiesis. American Journal of Kidney Diseases. 2010;56:558–565. doi: 10.1053/j.ajkd.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 31.Raptis AE, Bacharaki D, Mazioti M, et al. Anemia due to Coadministration of Renin-Angiotensin-System Inhibitors and PPARγ Agonists in Uncomplicated Diabetic Patients. Exp Clin Endocrinol Diabetes. 2012;120:416–419. doi: 10.1055/s-0032-1306286. [DOI] [PubMed] [Google Scholar]
- 32.Kashyap A, Kashyap S. Haemolytic Anaemia due to Metformin. Postgrad Med J. 2000;76:125–126. doi: 10.1136/pmj.76.892.125. [DOI] [PMC free article] [PubMed] [Google Scholar]