Abstract
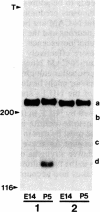
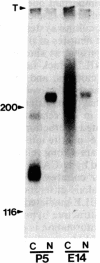
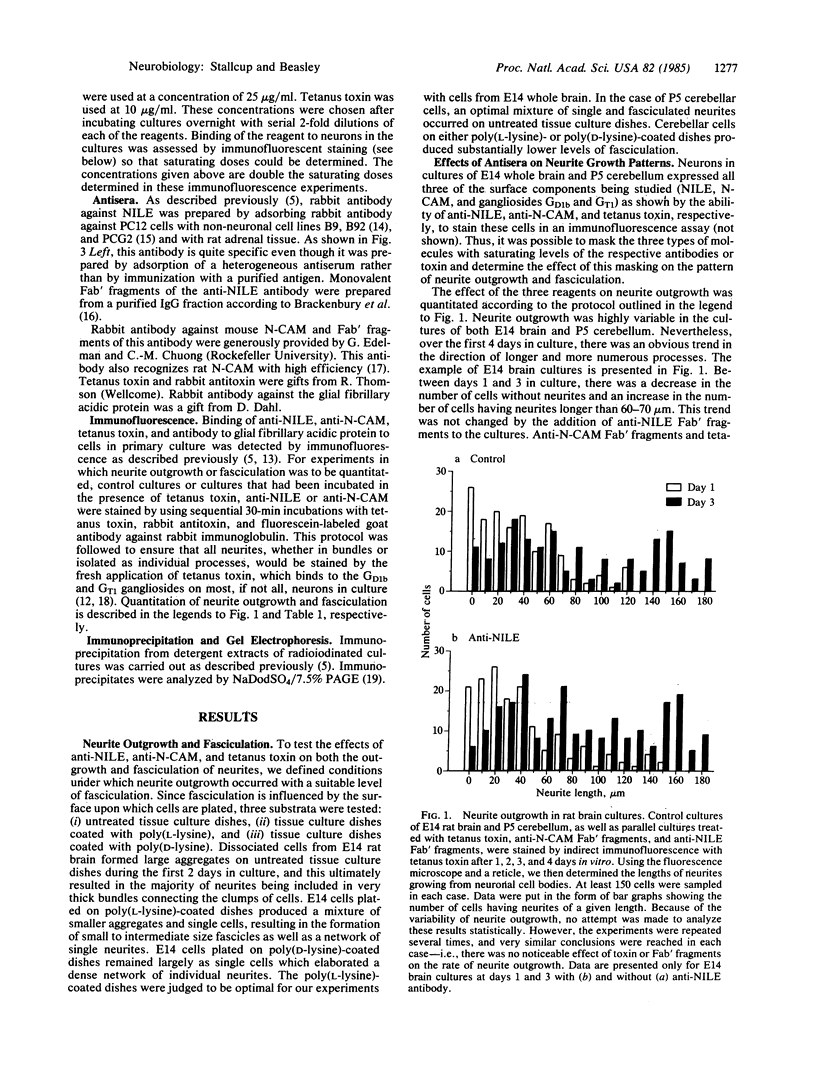
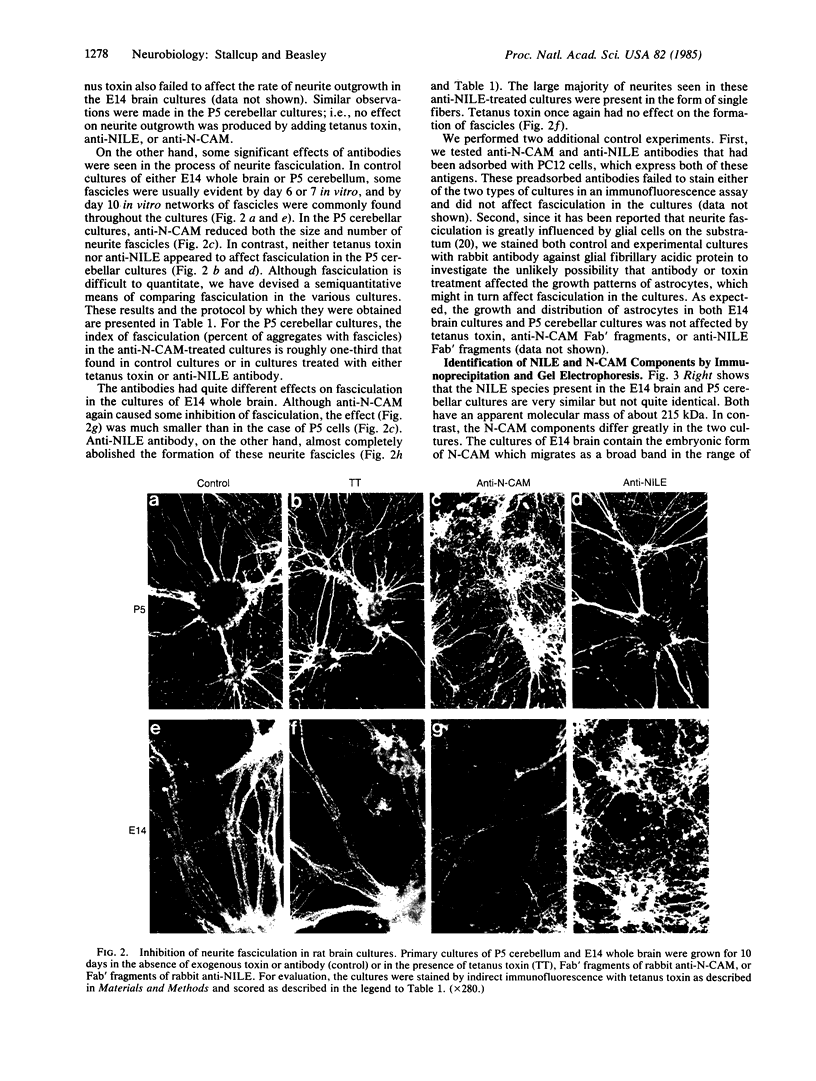
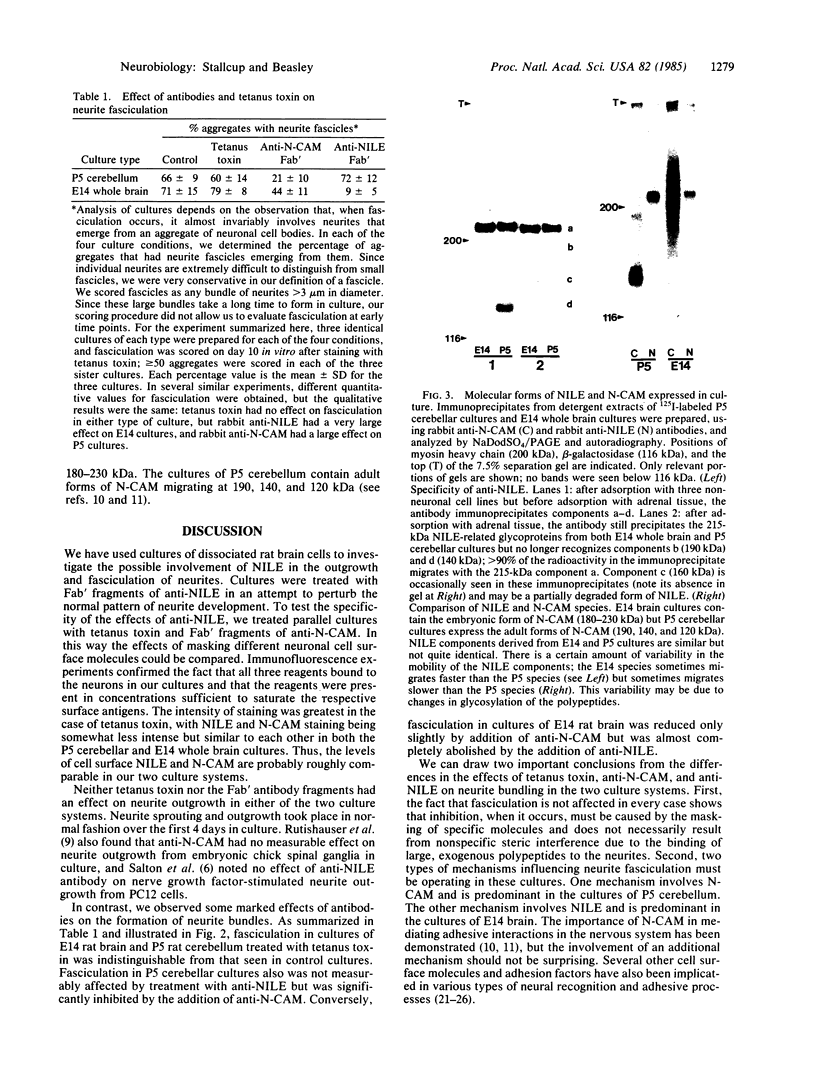
The nerve growth factor-inducible large external glycoprotein (NILE) has been found only on the surface of neuronal cells and Schwann cells. Since NILE seems to be concentrated on neurites, we have investigated its possible role in the development of neurites in primary cultures of rat brain. Cultures of embryonic day 14 (E14) whole brain and cultures of postnatal day 5 (P5) cerebellum were grown in the presence of Fab' fragments of antibody against NILE in an attempt to perturb the normal pattern of neurite development. For comparison, cultures were treated with two other reagents that recognize neuronal cell surface molecules: tetanus toxin, which binds to the GD1b and GT1 gangliosides, and Fab' fragments of antibody against neural cell adhesion molecule (N-CAM). Under the conditions used, none of the exogenous reagents affected neurite outgrowth, but specific effects on neurite fasciculation were observed. Anti-NILE inhibited fasciculation in cultures of E14 whole brain but had no effect on fasciculation in cultures of P5 cerebellum. Conversely, anti-N-CAM inhibited fasciculation in cultures of P5 cerebellum, which contain the adult form of N-CAM, but had little effect on fasciculation in cultures of E14 whole brain, which contain the embryonic form of N-CAM. Tetanus toxin had no effect on fasciculation in either culture system. Our results imply that NILE-mediated neurite-neurite interactions are stronger than N-CAM (embryonic)-mediated interactions in the E14 brain cultures, whereas N-CAM (adult)-mediated interactions are stronger than NILE-mediated interactions in the P5 cerebellar cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson R., Hsu W. C. Identification of a high molecular weight nervous system specific cell surface glycoprotein on murine neuroblastoma cells. Exp Cell Res. 1978 Sep;115(2):367–377. doi: 10.1016/0014-4827(78)90290-2. [DOI] [PubMed] [Google Scholar]
- Bizzini B. Tetanus toxin. Microbiol Rev. 1979 Jun;43(2):224–240. doi: 10.1128/mr.43.2.224-240.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury R., Thiery J. P., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. I. An immunological assay for molecules involved in cell-cell binding. J Biol Chem. 1977 Oct 10;252(19):6835–6840. [PubMed] [Google Scholar]
- Chuong C. M., McClain D. A., Streit P., Edelman G. M. Neural cell adhesion molecules in rodent brains isolated by monoclonal antibodies with cross-species reactivity. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4234–4238. doi: 10.1073/pnas.79.13.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. J., Glaser L. Inhibition of embryonic neural retina cell-substratum adhesion with a monoclonal antibody. J Biol Chem. 1984 Apr 10;259(7):4031–4034. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Goodman R., Herschman H. R. Nerve growth factor-mediated induction of tyrosine hydroxylase in a clonal pheochromocytoma cell line. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4587–4590. doi: 10.1073/pnas.75.9.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Hoffman S., Edelman G. M. Two antigenically related neuronal cell adhesion molecules of different specificities mediate neuron-neuron and neuron-glia adhesion. Proc Natl Acad Sci U S A. 1984 Jan;81(1):267–271. doi: 10.1073/pnas.81.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald G. B., Pratt R. S., Lilien J. Enzymic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retina cells. J Cell Sci. 1982 Jun;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- Hausman R. E., Moscona A. A. Isolation of retina-specific cell-aggregating factor from membranes of embryonic neural retina tissue. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3594–3598. doi: 10.1073/pnas.73.10.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Greene L., Shelanski M. L. Identification of neural and adrenal medullary surface membrane glycoproteins recognized by antisera to cultured rat sympathetic neurons and PC12 pheochromocytoma cells. Neuroscience. 1981;6(12):2773–2786. doi: 10.1016/0306-4522(81)90119-6. [DOI] [PubMed] [Google Scholar]
- McGuire J. C., Greene L. A., Furano A. V. NGF stimulates incorporation of fucose or glucosamine into an external glycoprotein in cultured rat PC12 pheochromocytoma cells. Cell. 1978 Oct;15(2):357–365. doi: 10.1016/0092-8674(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Mirsky R., Wendon L. M., Black P., Stolkin C., Bray D. Tetanus toxin: a cell surface marker for neurones in culture. Brain Res. 1978 Jun 9;148(1):251–259. doi: 10.1016/0006-8993(78)90399-2. [DOI] [PubMed] [Google Scholar]
- Noble M., Fok-Seang J., Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci. 1984 Jul;4(7):1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathjen F. G., Schachner M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984 Jan;3(1):1–10. doi: 10.1002/j.1460-2075.1984.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984 Aug 16;310(5978):549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Gall W. E., Edelman G. M. Adhesion among neural cells of the chick embryo. IV. Role of the cell surface molecule CAM in the formation of neurite bundles in cultures of spinal ganglia. J Cell Biol. 1978 Nov;79(2 Pt 1):382–393. doi: 10.1083/jcb.79.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton S. R., Richter-Landsberg C., Greene L. A., Shelanski M. L. Nerve growth factor-inducible large external (NILE) glycoprotein: studies of a central and peripheral neuronal marker. J Neurosci. 1983 Mar;3(3):441–454. doi: 10.1523/JNEUROSCI.03-03-00441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton S. R., Shelanski M. L., Greene L. A. Biochemical properties of the nerve growth factor-inducible large external (NILE) glycoprotein. J Neurosci. 1983 Dec;3(12):2420–2430. doi: 10.1523/JNEUROSCI.03-12-02420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Klier F. G., Birdwell C. A role for adherons in neural retina cell adhesion. J Cell Biol. 1983 Apr;96(4):990–998. doi: 10.1083/jcb.96.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Arner L. S., Levine J. M. An antiserum against the PC12 cell line defines cell surface antigens specific for neurons and Schwann cells. J Neurosci. 1983 Jan;3(1):53–68. doi: 10.1523/JNEUROSCI.03-01-00053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981 Apr 15;83(1):154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Rutishauser U., Edelman G. M. Cell adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]