Abstract
A spinal cord injury (SCI) clearly results in greater cardiovascular risk, however, accompanying changes in peripheral vascular structure below the lesion, mean the real impact of a SCI on vascular function is unclear. Therefore, utilizing passive leg movement-induced (PLM) hyperemia, an index of nitric oxide (NO)-dependent vascular function, and the central hemodynamic response to this intervention, we studied 8 individuals with a SCI, and 8 age-matched controls (CTRL). Specifically, we assessed heart rate (HR), stroke volume (SV), cardiac output (CO), mean arterial pressure (MAP), leg blood flow (LBF), and thigh composition. In CTRL, passive movement, transiently decreased MAP, and increased HR and CO from baseline by 2.5±1 mmHg, 7±2 bpm, and 0.5±0.1 l/min respectively. In SCI, HR and CO responses were unidentifiable. LBF increased to a greater extent in CTRL (515±41 Δml/min) compared to SCI, (126±25 Δml/min) (p<0.05). There was a strong relationship between ΔLBF and thigh muscle volume (r = 0.95). After normalizing ΔLBF for this strong relationship (ΔLBF/muscle volume), there was evidence of preserved vascular function in SCI (CTRL: 120±9; SCI 104±11 ml/min/l). A comparison of ΔLBF in the passively moved and stationary leg, to partition the contribution of the blood flow response, implied that 35% of the hyperemia resulted from cardioacceleration in the CTRL, whereas all the hyperemia appeared peripheral in origin in the SCI. Thus, utilizing PLM-induced hyperemia as marker of vascular function, it is evident that peripheral vascular impairment is not an obligatory accompaniment to a SCI.
Keywords: blood flow, vascular dysfunction, spinal cord injury
INTRODUCTION
Due to forced immobility of the lower limbs in humans with a chronic spinal cord injury (SCI), this population is both at greater risk of cardiovascular disease (Myers et al., 2007) and likely to experience significant adaptations to the structure and function of the leg vasculature. Indeed, previous studies have documented a 30% decrease in arterial diameter below the spinal lesion (de Groot et al., 2006, De Groot et al., 2003). Whereas findings related to the effect of a SCI on the circulation, itself, have been equivocal, with some studies reporting a decrease in resting blood flow (Hopman et al., 1996, Huonker et al., 2003), while others found no difference between subjects with a SCI and controls (de Groot et al., 2006, Olive et al., 2003, de Groot et al., 2004). Additionally, based upon the assessment of flow-mediated dilation in the superficial femoral artery, research has suggested preserved endothelial function in SCI, however, such studies, and their conclusions, are complicated by the accompanying reduction in vessel size that bias this assessment (Kooijman et al., 2008a, Thijssen et al., 2008). In contrast, the assessment of passive limb movement (PLM)-induced hyperemia is a novel approach to determine NO-dependent vascular function in vivo that is independent of changes in conduit vessel size (Trinity et al., 2012), however, to date, this approach has not been utilized in the SCI population.
Previous investigations have recognized that the hyperemic response to PLM is not solely a consequence of peripheral vascular factors. Indeed studies have highlighted the role of cardioacceleration (Crow and Kushmerick, 1982, Nobrega and Araujo, 1993, Wray et al., 2005) and therefore cardiac output (CO) in this hyperemic response (McDaniel et al., 2010a). Recently, to better understand the integration of the central and peripheral factors that contribute to the immediate increase in blood flow to a moving limb, we studied heart transplant recipients with denervated hearts (Hayman et al., 2010) and young subjects with pharmacologically attenuated feedback from mechano-receptors in the moving limb (Trinity et al., 2010). These studies revealed that the magnitude of femoral blood flow response to PLM is, in fact, augmented by the concomitant chronotropic response. These findings suggest that heart rate (HR) and ultimately CO contribute significantly to the movement-induced hyperemia, likely by minimizing the fall in blood pressure that is a consequence of the PLM-induced vasodilation in the leg. Passive movement studies examining subjects with a SCI have yielded conflicting results, with some studies revealing significant cardiac output and peripheral blood flow responses (Ballaz et al., 2007, Muraki et al., 2000), while others have reported no LBF response in this population (Ter Woerds et al., 2006).
As, paraplegics are at an increased risk of developing cardiovascular disease (Myers et al., 2007), the accurate evaluation of vascular function in individuals with a SCI is of significant clinical importance. Therefore, using PLM to induce hyperemia, the primary goal of this study was to assess leg vascular function in subjects with a SCI and compare their responses with CTRL. Additionally, with the recognition that individuals with a SCI are typically deprived of afferent feedback from the passively moved limb, this study sought to determine the impact of limb movement on HR, SV, CO, and MAP responses in a sample of this population with a complete lesion compared to CTRL. Specifically we hypothesized that a combination of altered HR and CO responses and diminished vasodilatation, as assessed by PLM-induced hyperemia, will be greatly attenuated in subjects with a SCI compared to their able bodied counterparts.
METHODS
Subjects
8 individuals with a SCI (6 men and 2 women, 42 ± 8 yrs, 74 ± 10 kg, 180 ± 12 cm) and 8 able-bodied subjects (CTRL) (6 men and 2 women, 40 ± 7 yrs, 68 ± 6 kg, 180 ± 7 cm) participated in this study. All SCI subjects had clinically confirmed paraplegia with complete lesions between the 6th (T-6) and 12th (T-12) thoracic vertebrae (American Spinal Injury Association class A) (Maynard et al., 1997). Neurological examinations were carefully performed in all SCI subjects by a neurologist and spinal lesions were diagnosed. Time post injury was 9 ± 3 yrs (4 – 16 yrs), and, based on the Ashworth test (Ashworth, 1964), at the time of evaluation, none of the SCI subjects exhibited muscle spasticity, or experienced patellar and ankle clonus triggered by PLM. None of the study participants were smokers and most were physically active, with an average time spent performing endurance-type exercise of 7.0 ± 3.5 and 5.5 ± 2.5 hours a week in the SCI and CTRL, respectively. Descriptive characteristics of the subjects are reported in Table 1. All procedures conformed to the standards set by the Declaration of Helsinki, and approved by the Institutional Review Boards of the University of Utah, and the Salt Lake City VA Medical Center. Subjects gave written informed consent prior to their participation. Subjects reported to the laboratory in a fasted state and had not exercised for the past 24 hrs.
Table 1.
Subject characteristics
| SCI | CTRL | |
|---|---|---|
| Subjects | Mean ± SEM | Mean ± SEM |
| Age (yrs) | 42±8 | 40±7 |
| Mass (kg) | 74±10 | 68±6 |
| Height (m) | 1.8±0.12 | 1.8±0.07 |
| MRI thigh muscle volume (I) | 1.16±0.38 | 3.89±0.16 * |
| Common femoral artery diameter (cm) | 0.66±0.04 | 0.93±0.07 * |
| ASIA grade | A | - |
| Years post injury | 9±3 | - |
| Quadriceps spasticity Ashworth scale (0-4) | 0 | - |
| Glucose (mg/dL) | 97±11 | 62±10 |
| Cholesterol (mg/dL) | 160±8 | 184±6 * |
| HDL (mg/dL) | 43±5 | 65±2 * |
| LDL (mg/dL) | 98±5 | 108±6 |
| Triglycerides (mg/dL) | 96±15 | 78±19 |
| Hemoglobin (g/dL) | 14.6±0.3 | 14.8±0.8 |
| Hematocrit (%) | 47±2 | 46±3 |
| WBC (K/uL) | 4.4±0.7 | 5.1±0.6 |
| Neutrophil (K/uL) | 2.6±0.3 | 2.9±0.4 |
| Lymphocyte (K/uL) | 1.7±0.2 | 1.6±0.2 |
| Monocyte (K/uL) | 0.43±0.2 | 0.41±0.03 |
Data are presented as mean ± SEM. All SCI subjects had a spinal lesion between T-6 and T12. American Spinal Injury Association (ASIA) (Maynard et al., 1997) score was used to classify the severity of the lesion: A = sensory and motor complete. The quadriceps spasticity Ashworth scale (Ashworth, 1964) is used to classify the severity of muscular spasms during passive movements in SCI subjects: 0 = no spasms. HDL = high-density lipoprotein; LDL = low-density lipoprotein; WBC = white blood cells.
represents values significantly different between groups.
PLM protocol
Subjects rested in the upright-seated position for 20 min before the start of data collection and remained in this position throughout the study. The protocol consisted of 60 sec of resting baseline followed by 2 min of passive knee-extension. PLM was performed by a member of the research team, who moved the subject's lower leg through a 90° range of motion (from full extension to 90° knee joint angle) at 1 Hz.
Knee angle
During each protocol, knee joint angle of the passive leg was continuously recorded using a Vishay Spectrol 360-degree Smart Position Sensor (Vashay Intertechnology, Malvern, PA) mounted on a BREG X2K knee brace (BREG, Vista, CA) worn by each subject.
Leg blood flow
Measurements of arterial blood velocity and vessel diameter were performed in both legs, distal to the inguinal ligament and proximal to the deep, superficial femoral bifurcation with Logiq-7 and Logiq-e ultrasound systems (General Electric Medical Systems, Milwaukee, WI, USA). The ultrasound systems were equipped with 12-14 MHz linear array transducers. Artery diameter was determined at a 90° angle along the central axis of the scanned area, with the depth of the measured vessels falling between 2.5 and 4.5 cm. Blood velocity (V) was measured using the same probes at a frequency of 5 MHz. Measurements of blood velocity were obtained with the probe positioned to maintain an insonation angle of 60° or less and the sample volume was centered and maximized according to vessel size. Arterial diameter was measured, and second-by-second blood velocity was automatically calculated using the Logiq-7 and Logiqe software. Utilizing arterial diameter and Vmean, LBF flow was calculated as:
where LBF is in milliliters per minute. Both, anterograde and retrograde LBF were also calculated on a second-by-second basis for both the control and passively moved leg. All scanning and blinded analyses were performed by experienced and skilled sonographers.
HR, SV, CO and MAP
These data were determined using a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The photoplethysmographic cuff was placed on the third finger of the left hand. The subject's arm was supported by an armrest, to avoid movement and compression of the finger. The Finometer signal was calibrated utilizing the procedure indicated by the manufacturer. The height adjustment sensor and reference were positioned according to the manufacturer's instructions. SV was estimated using the Modelflow algorithm (Beatscope version 1.1a; Finapres Medical Systems) (Bogert and van Lieshout, 2005). CO was then calculated as the product of HR and SV. The same method has been documented to accurately track CO during exercise (Azabji Kenfack et al., 2004, Tam et al., 2004).
Thigh muscle volume
MRI was performed using a clinical 3T MRI system (Tim-Trio, Siemens Medical Solutions, Erlangen, Germany). T1-weighted images were acquired at rest using a turbo spin echo sequence with 15 axial slices covering the region from the greater trochanter to the knee (slice thickness = 1 cm, gap thickness = 12-16 mm, turbo factor = 3, TE = 12 ms; TR = 700 ms; turbo factor = 3; concatenation = 2, field of view = 20×20 cm; matrix size = 256×256; acquisition time = 2 min 03 sec). The cross-sectional area of each slice was determined using image J software 1.46r (National Institute of Health, Bethesda, Maryland, USA). On the basis of a signal-intensity threshold, muscle was distinguished from other tissues and quantified. Muscle volume was calculated by summing the areas of all the slices, taking into account the slice thickness and the inter-slice space (Tonson et al., 2008).
Calculation of central and peripheral contributors to leg blood flow
The difference in ΔLBF response between the passively moved leg and the non-moving leg was used as an indicator of the peripheral contribution to the hyperemic response. Specifically, the percent difference in ΔLBF between the passively-moved leg and the non-moving leg represents an index of the contribution of peripheral factors to the hyperemic response.
Data collection and analysis
HR, SV, CO, MAP, ECG, and knee joint angle underwent A/D conversion and were simultaneously acquired (200 Hz) by commercially available data acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA, USA). The data acquisition software allowed second-by-second analysis of HR, SV, CO and MAP throughout the PLM. Mean blood velocity was analyzed with 1Hz resolution on the Doppler ultrasound systems (GE Logiq-7 and Logiq-e) for 60 sec at rest and the first 60 sec of passive movement, 12 sec averages were then employed from 60 to 120 sec of the movement. A 3-second rolling average was applied to the HR, SV, CO, MAP, LBF, and vascular conductance data. Two-way ANOVA was used to establish differences between groups at rest and during passive limb movement. Following these analyses, where indicated, a Tukey post hoc was used to define which of the trials was different. A Pearson correlation was used to examine the correlation between ΔLBF, thigh muscle volume, and time form SCI. Significance was set at an α level of 0.05 and data are presented as mean ± SEM throughout the manuscript.
RESULTS
Subject characteristics
All participants, SCI and CTRL, took part in this experimental protocol without incident or discomfort and none of the SCI experienced spastic muscle contractions during the passive movement (Table 1). Neurological tests confirmed that, in contrast to the CTRL, all SCI subjects were insensate below the level of the thoracic cord lesion, while sensation was fully intact and therefore similar to the CTRL, in the upper torso and arms. Results from the blood analyses were similar between groups with the exception of cholesterol and HDL which were lower in the SCI compared to the CTRL.
LBF, HR, SV, CO, and MAP at rest
All resting hemodynamic variables are summarized in Table 2. LBF at rest was significantly reduced in SCI compared to CTRL (145 ± 28 ml/min Vs 195 ± 32 ml/min; p < 0.05). Within the SCI subjects the relationship between resting LBF and thigh muscle volume was significantly correlated (r = 0.91, p < 0.01), while, in contrast, there was no such relationship in the CTRL subjects (p < 0.6). The SCI subjects tended to exhibit higher, HR, SV, CO, and MAP at rest, but this did not achieve statistical significance.
Table 2.
Resting HR, CO, SV, MAP, and femoral blood flow in subjects with spinal cord injury (SCI), and control (CTRL).
| Rest | ||
|---|---|---|
| Cardiac output | SCI | 6.0±0.8 |
| (l/min) | CTRL | 5.7±0.6 |
| Heart rate | SCI | 59.0±6 |
| (bpm) | CTRL | 58.5±4 |
| Stroke volume | SCI | 99.5±2.8 |
| (ml) | CTRL | 98.6±4.5 |
| Mean arterial pressure | SCI | 95.0±6.4 |
| (mmHg) | CTRL | 93.2±1.7 |
| Passive leg blood flow | SCI | 145±28 |
| (ml/min) | CTRL | 195±32 * |
Data are mean ± SEM.
significant difference between groups.
LBF, HR, SV, CO, and MAP during PLM
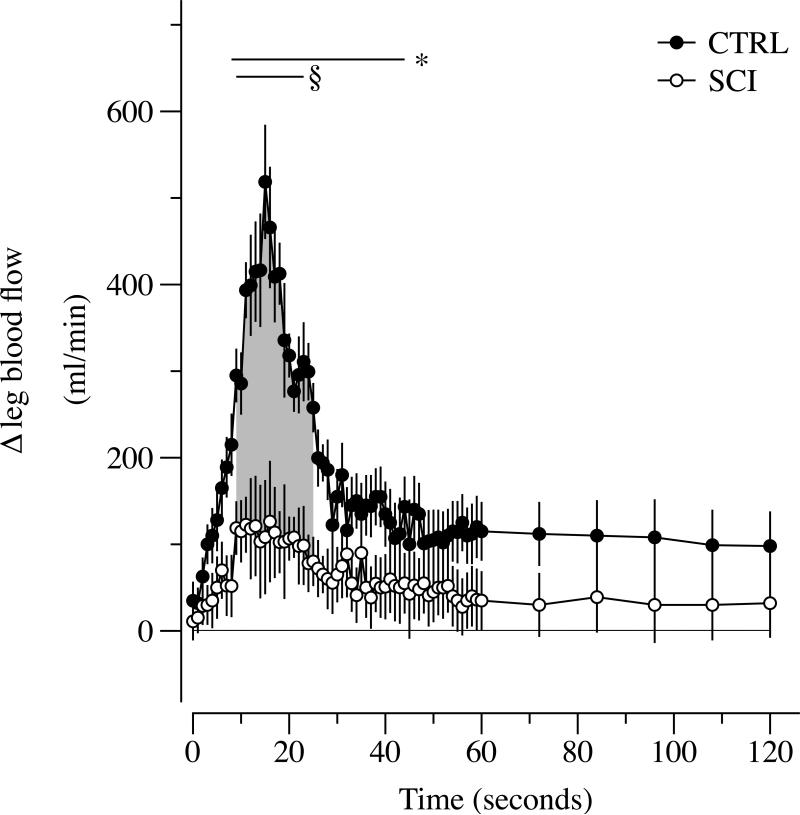
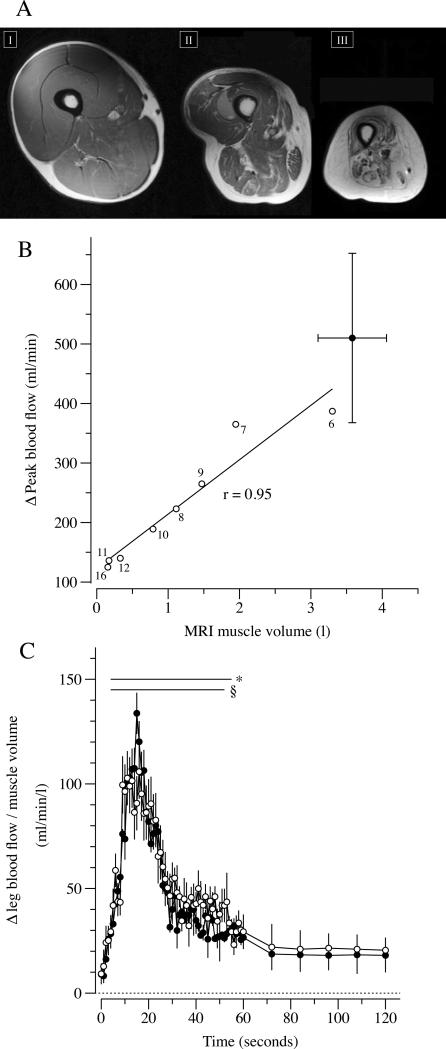
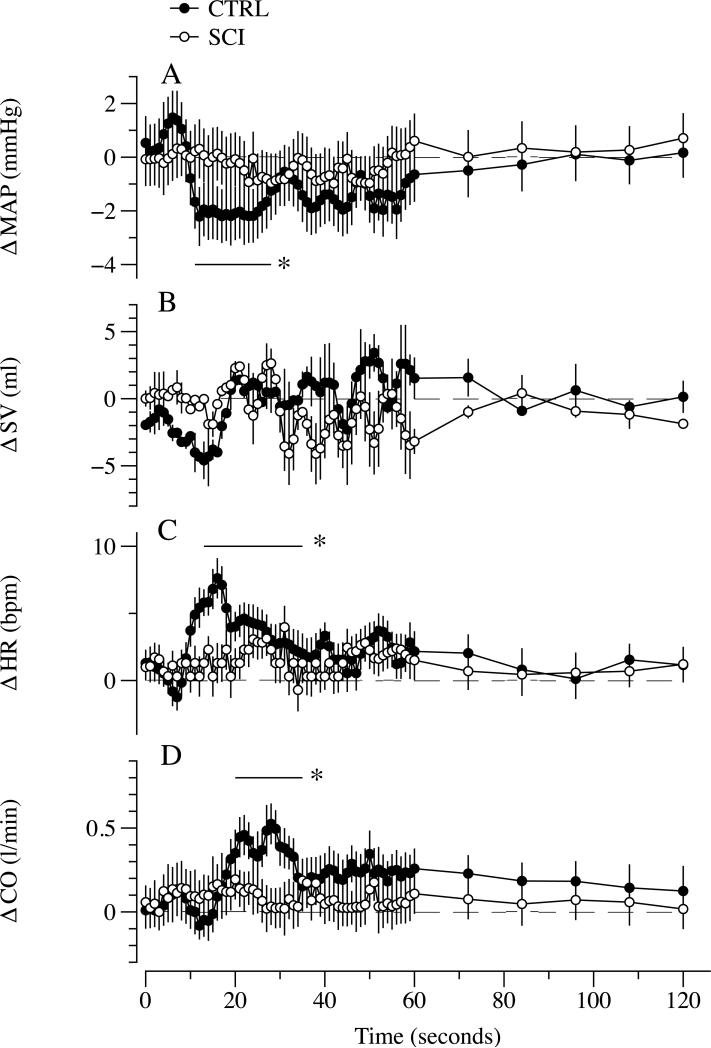
During PLM, ΔLBF in the passively moved leg transiently increased from the 8th to the 46th sec in the CTRL and from the 9th to the 23rd sec in the SCI. The PLM-induced hyperemic response was significantly greater in the CTRL compared to the SCI between the 11th to the 25th sec of PLM (Figure 1). Within SCI subjects the relationship between ΔLBF and thigh muscle volume was significantly correlated (r = 0.95, p < 0.01), while, in contrast, there was no such relationship in the CTRL subjects (p < 0.6) (Figure 2; Panels A, and B). Additionally, ΔLBF was also significantly correlated with the time from the spinal cord lesion (r = 0.84, p < 0.01). When expressed per unit of thigh muscle volume, ΔLBF in the passively moved leg was not different between groups (Figure 2; Panel C). HR, SV, CO and MAP (Figure 3) were unchanged in the SCI as a consequence of passive movement, whereas the CTRL exhibited a transient decline, ~2.5 mmHg, in MAP from the 6th to the 23th sec (Figure 3; Panel A). HR was significantly increased in CTRL from the 9th to the 34th sec of passive movement (Figure 3; Panel C), and there was a subsequent increase in CO from the 18th to the 34th sec (Figure 3; Panel D).
Figure 1. Hyperemic response to passive limb movement (PLM) in subjects with a spinal cord injury (SCI) and able-bodied controls (CTRL).
Data are mean ± SEM; * significantly different from baseline in the CTRL; § significantly different from baseline in SCI group; and gray area indicates significantly lower values in the SCI group compared to CTRL.
Figure 2. Evidence of the impact of a spinal cord injury (SCI) on thigh muscle volume (Panel A), the strong link between thigh muscle volume and passive limb movement (PLM)-induced hyperemia (Panel B), and PLM-induced hyperemia normalized for muscle volume in subjects SCI and able-bodied controls (CTRL).
Panel A: 3 mid-thigh magnetic resonance images of a CTRL (I) and 2 subjects with a complete SCI 6 (II) and 16 yrs (II) after the injury. Panel B: Significant correlation between changes in blood flow and thigh muscle volume in SCI, (r = 0.95; p < 0.05). Labels near open circles of indicate time from the injury in subjects with a SCI. Data in panel B, filled square (CTRL), and C are mean ± SEM; * significantly different from baseline in the CTRL; § significantly different from baseline in SCI group.
Figure 3. HR, SV, CO and MAP responses to passive limb movement (PLM) in subjects with a spinal cord injury (SCI) and able-bodied controls (CTRL).
Panels A, B, C, and D illustrate mean arterial pressure (MAP), stroke volume (SV), heart rate (HR), and cardiac output (CO) over time, respectively. Data are mean ± SEM; * significantly different from baseline in the CTRL group.
Thigh muscle volume
Thigh muscle volume measured with MRI was significantly smaller in the SCI compared to CTRL (~70%; Table 1), with the subjects with a SCI exhibiting a marked and progressive thigh muscle atrophy and a shift to a greater proportion of fat and connective tissue. The time from the SCI and these thigh composition changes were strongly correlated (e.g. time and thigh muscle volume: r = 0.83; p = 0.01) (Figure 2; Panels A and B).
DISCUSSION
In agreement with our hypothesis, subjects with a SCI exhibited an attenuated PLM-induced hyperemia compared to their able-bodied counterparts and no HR and CO responses. However, when the significantly attenuated ΔLBF in the SCI subjects was normalized by their greatly reduced thigh muscle volume, the difference in the hyperemic response between the SCI and CTRL was ablated. Therefore, despite the absence of a HR and CO response in the SCI, when muscle volume was taken into account, these data provide evidence of preserved vascular function in subjects with SCI. Thus, utilizing a vascular function assessment that is not directly dependent upon the assessment of vessel diameter change, it is evident that peripheral vascular dysfunction is not an obligatory accompaniment to a SCI. In fact, in combination, these data imply that in subjects with a SCI there is an increased vascular sensitivity, defined as an amplified vasodilatory response to leg movement, likely due to unaccustomed limb motion, which compensates for the absence of cardioacceleration in this population, resulting in a similar muscle volume specific LBF response.
Muscle volume and PLM-induced hyperemia
Previous studies have recognized the importance of muscle volume in terms of the peripheral vascular adaptation and vasoreactivity subsequent to a SCI. Indeed, the 30% difference in femoral artery diameter between SCI subjects and CTRL is associated with alterations in limb muscle mass (De Groot et al., 2003, de Groot et al., 2006). Additionally, Ichinose et al. (Ichinose et al., 2008) revealed in an animal model, that after denervation and subsequent muscle atrophy, the correlation between capillary length-to-fiber ratio and muscle fiber radius is preserved, thus the structural changes in the vasculature of denervated muscle is highly correlated with changes in muscle mass. A novel observation in the current study, germane to this link between muscle volume and the peripheral vasculature is that, in subjects with SCI, there was a strong and statistically significant correlation between changes in LBF induced by PLM and thigh muscle volume (r = 0.95; p < 0.01) (Figure 2; Panel C). This finding suggests that during PLM the volume of muscle manipulated is an important factor in determining the amplitude of the hyperemic response. This is a factor that was not highlighted in our recent work, which revealed the importance of NO in the passive movement-induced hyperemia in able bodied subjects, because this study only involved a single group of participants with relatively homogenous thigh volumes (Trinity et al., 2012). This issue may only be of major importance in the PLM model when muscle volume varies greatly between subjects, as in the current study.
The positive correlation between peak ΔLBF and thigh muscle volume is likely due to the nature of the muscle volume-dependent peripheral factors that contribute to the PLM-induced hyperemia. Specifically, mechanically induced vasodilation (Clifford et al., 2006, Kirby et al., 2007, Tschakovsky and Sheriff, 2004), mechanical distortion of arterioles (Segal, 2000), and flow-mediated dilation (Kooijman et al., 2008b, Pohl et al., 1986), all of which contribute to the hyperemic response and the magnitude of each could be effected by muscle volume. Thus, it is probable that movement-induced hyperemia is mediated by the sum of the microcirculatory responses per unit of muscle volume. Again, this highlights the need to take muscle volume into account when assessing vascular function with the passive movement technique when large differences in muscle volume exist between subjects. Therefore, for the evaluation of vascular function by PLM in individuals that have very large differences in limb volumes, it is likely important to express ΔLBF per unit of muscle volume.
Skeletal muscle atrophy below the spinal lesion is a common adaptation following SCI resulting from the loss of central activation and subsequent unloading (Dudley et al., 1999). For example, the cross-sectional area of the lower limbs has been reported to be reduced by 45% after just 6 weeks following a clinically complete spinal cord lesion (Castro et al., 1999). Moreover, this progressive skeletal muscle atrophy is associated with an accumulation of intramuscular fat (Gorgey and Dudley, 2007). Different factors contribute to the extension and severity of these adaptations, such as the level of injury, presence of spasticity, and medications (Gorgey and Dudley, 2008). It is important to note that the process of skeletal muscle atrophy has adverse consequences on cardiovascular, metabolic, and musculoskeletal profiles in individuals with a SCI (Bauman and Spungen, 2001, Kocina, 1997). The current data reveal that in subjects with a complete SCI, and in the absence of spasticity, the skeletal muscle atrophy in the lower limbs is significantly correlated with the time from the spinal cord lesion (r = 0.83; p = 0.01) Figure 2, Panels 1, and 2. Additionally, it is interesting to note that PLM-induced hyperemia was highly correlated with both thigh muscle volume and time from the injury.
Vascular function and SCI
As a consequence of many unavoidable lifestyle changes following the injury, individuals with SCI are at an increased risk of developing cardiovascular disease and often at an earlier age (Myers et al., 2007). The assessment of endothelial function via flow mediated vasodilation following cuff occlusion (Harris et al., 2010) has been proposed to represent a functional bioassay for endothelium-derived nitric oxide (NO) bioavailability in humans (Joannides et al., 1995, Doshi et al., 2001) which is recognized to anti atherogenic. Based upon the assessment of flow-mediated dilation in the superficial femoral artery, studies have reported preserved endothelial function in SCI, however, such studies, and their conclusions, are complicated by the accompanying reduction in vessel size that bias this assessment (Kooijman et al., 2008a, Thijssen et al., 2008). These concerns combined with new evidence challenging the view that FMD is a reliable and selective index of endothelial NO function (Pyke et al., 2010, Wray et al., 2013) call for further efforts to characterize the vascular function of the large vessel beds distal to the lesion in subjects with a SCI.
Recently, Trinity et al. (Trinity et al., 2012) documented that in healthy subjects the inhibition of eNOS during PLM reduced leg vascular conductance and vasodilation by ~80%, indicating that NO is the primary mechanism responsible for the PLM-induced vasodilation. In accordance with this study, in a previous investigation, our group also determined that with age, a condition typically associated with reductions in vascular function, the LBF response to PLM was also reduced (McDaniel et al., 2010b). Therefore, a primary aim of the current study was to evaluate vascular function in the lower limbs of subjects with a SCI in comparison with healthy age-matched CTRL utilizing the PLM model. Interestingly, the current assessments revealed that subjects with a SCI exhibited a 70% attenuation of their PLM-induced hyperemic response compared to CTRL. However, as already noted, when the ΔLBF response was expressed per unit of thigh muscle volume, which was markedly different between groups and well correlated with the hyperemic response, there was no longer a difference between the subjects with SCI and CTRL. Consequently, it appears that vascular function, likely dependent upon NO, was not affected by the SCI, per-se, but rather vasoreactivity was strongly related to the SCI-induced changes in muscle volume, which when appropriately taken into account reveals that vascular function, per unit of thigh muscle volume was preserved.
Interestingly, although not by experimental design, it should be noted that, while the current subjects with SCI were well matched for activity with the CTRL, they were, as a group, on the active end of the spectrum for this population. Based upon the current literature this may (Wray et al., 2009, DeSouza et al., 2000), or may not (Thijssen et al., 2005) have had an impact upon the current assessment of vascular function in the inactive limbs of these subjects, but does not alter the conclusion that vascular dysfunction distal to the lesion is not an obligatory accompaniment to a SCI.
Impact of PLM on cardiac output and blood flow responses in SCI and CRTL
In able-bodied subjects afferent signals from muscle and joint mechanoreceptors increase HR and CO, which likely contribute and sustain the hyperemic response during PLM (Crow and Kushmerick, 1982, Nobrega and Araujo, 1993, McDaniel et al., 2010a, Wray et al., 2005, Adreani et al., 1997, Adreani and Kaufman, 1998, Hayes et al., 2005, Trinity et al., 2011). In previous studies the central and peripheral hemodynamic effects of passive movement in subjects with SCI have been equivocal. Muraki et al. (Muraki et al., 2000) and more recently Ballaz et al. (Ballaz et al., 2007) reported that passive cycle exercise enhances both cardiac output and blood flow, but Ter Woerds et al. (Ter Woerds et al., 2006) failed to document a change in LBF during passive movement. The reason for these disparate results is likely due to the different passive movement protocols employed and the collection of central and peripheral variables with low time resolution (every 60 seconds). In the current study, high temporal resolution and simultaneous collection of central and blood flow responses revealed a clear movement-induced hyperemia in the subjects with SCI and no measurable increase in HR and CO.
The comprehensive approach adopted in the current study, including the documentation of the blood flow responses in both the passively moved limb and the non moved limb allowed the determination of which factors were most likely responsible for the hyperemia at the onset of PLM in both able-bodied subjects and the subjects with a SCI. In healthy able-bodied individuals, growing evidence suggests that, PLM-induced hyperemia is the consequence of a two component model, with both central and peripheral mechanisms playing a significant role (McDaniel et al., 2010a, Crow and Kushmerick, 1982, Nobrega and Araujo, 1993, Hayman et al., 2010, Trinity et al., 2010) (Figure 4, Figure 5). The difference between ΔLBF in the passively-moved leg and the non-moving, control, leg represents an index of the contribution of peripheral factors to the hyperemic response (Figure 4, Panel B and C). In the CTRL, the transient drop in MAP and the subsequent increase in HR and CO resulted in a gradual increase in ΔLBF in the control leg (Figure 4, Panel A). Thus, in the able-bodied subjects, two components (central and peripheral) contributed to the hyperemic response to the passive limb movement with the central factors accounting for ~35% of the total hyperemic response, with the remaining ~65% mediated by peripheral factors (Figure 4, Panel C; Figure 5).
Figure 4. Partitioning the central and peripheral contributors to passive leg movement (PLM)-induced hyperemia in subjects with a spinal cord injury (SCI) and able-bodied controls (CTRL).
Panels A, and B illustrate leg blood flow in the control leg, and the difference between leg blood flow in the passive and control legs, respectively. Panel C illustrates the % contribution of central and peripheral factors based upon these assessments. Data in Panels A, and B are mean ± SEM; * significantly different from baseline in the CTRL, § significantly different from baseline in SCI group, and gray area in Panel A indicates significantly lower values in the SCI group compared to CTRL.
Figure 5. Schematic illustration of the differing responses to passive leg movement (PLM) in subjects with a spinal cord injury (SCI) and able-bodied controls (CTRL).
In CTRL, neurological feedback from the moving limb triggers central responses (increasing HR and CO) which results in an increase in leg blood flow in the non-moving leg (CONTROL LEG) and the moving leg (PASSIVE LEG), accounting for ~35% of the increase in leg blood flow in the latter. Peripheral vasodilation is responsible for the remaining ~65% increase in leg blood flow in the PASSIVE LEG. In contrast, in the SCI group, there is no feedback from the passively moved limb and so there is no increase in HR and CO, which can contribute to raising blood flow in the CONTROL LEG and the PASSIVE LEG. Hence, in subjects with a SCI, peripheral vasodilation is responsible for all the movement-induced increase in leg blood flow in the PASSIVE LEG.
In the subjects with SCI the absence of an increase in HR and CO resulted in no change in ΔLBF in the control leg (Figure 4; Panel A). Therefore, again subtracting the control leg hyperemia from the passively moved leg response, there was evidence of a solely peripherally-induced hyperemia due to PLM (Figure 4, Panel B and C). Hence, in subjects with SCI, only the peripheral component, of the two component model, was responsible for the PLM-induced hyperemia (Figure 4, Panel C; Figure 5). The observation that the ΔLBF relative to muscle volume in the SCI was similar to that of CTRL, but with no cardioacceleration, which can influence the magnitude of the blood flow response (Trinity et al., 2010), suggests there may even be a vascular hypersensitivity to movement in the vessels below the lesion in this population. It is possible that this response is caused by unaccustomed movement in the paralyzed limb, as previously described in animal models (Carrier and Hester, 1976, Hershman et al., 1993, Bentzer et al., 1997), but this will require additional work to confirm this hypothesis. Moreover, it is important to note that local reflexes related to peripheral sympathetic nervous system activation are preserved following a SCI, and therefore locally mediated responses may play a role in the observed hyperemia. For instance, it has been recognized that the local sympathetic veno-arteriolar reflex can significantly increase the venous transmural pressure, which can influence local blood flow (Henriksen, 1991), and this remains intact with an SCI.
Clinically, the positive effect of PLM as a treatment for spasticity of paralyzed limbs has already been recognized (Dietz and Sinkjaer, 2012). In combination with this prior work, the current findings indicate that PLM has potential as a therapeutic approach to momently increase lower-extremity circulation, possibly preventing peripheral vascular consequences of an SCI such as venous stagnation and thrombosis (Nash et al., 1996, Myers et al., 2007).
CONCLUSIONS
Utilizing PLM to assess vascular function, subjects with a SCI exhibited an attenuated ΔLBF response when compared with their able-bodied counterparts, however, when normalized for muscle volume, vascular function in the leg, appears to be preserved in SCI. It is noteworthy that this occurred with no measurable increase in HR and CO in the SCI, which typically contributes to and sustains the blood flow response. Thus, utilizing a vascular function assessment that is not directly dependent upon the assessment of vessel diameter change, it is evident that peripheral vascular dysfunction is not an obligatory accompaniment to a SCI.
Acknowledgments
FUNDING
This work was financially supported in part by the NIH (PO1 HL, 09830), the VA (Merit Grant E6910R), University of Utah College of Health and Center for Rehabilitative Research Grant CRR-F 2008-04, and Department of VA RR&D CDA2 E7560W.
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–7. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol. 1998;84:1827–33. doi: 10.1152/jappl.1998.84.6.1827. [DOI] [PubMed] [Google Scholar]
- Ashworth B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. Practitioner. 1964;192:540–2. [PubMed] [Google Scholar]
- Azabji Kenfack M, Lador F, Licker M, Moia C, Tam E, Capelli C, Morel D, Ferretti G. Cardiac output by Modelflow method from intra-arterial and fingertip pulse pressure profiles. Clinical science. 2004;106:365–9. doi: 10.1042/CS20030303. [DOI] [PubMed] [Google Scholar]
- Ballaz L, Fusco N, Cretual A, Langella B, Brissot R. Acute peripheral blood flow response induced by passive leg cycle exercise in people with spinal cord injury. Arch Phys Med Rehabil. 2007;88:471–6. doi: 10.1016/j.apmr.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266–77. doi: 10.1080/10790268.2001.11753584. [DOI] [PubMed] [Google Scholar]
- Bentzer P, Nielsen N, Arner M, Danielsen N, Ekblad E, Lundborg G, Arner A. Supersensitivity in rat micro-arteries after short-term denervation. Acta Physiol Scand. 1997;161:125–33. doi: 10.1046/j.1365-201X.1997.00177.x. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Experimental physiology. 2005;90:437–46. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Carrier O, Jr., Hester RK. The relationship between calcium and increased sensitivity of rabbit aortae four hours after reserpine. Br J Pharmacol. 1976;56:449–55. doi: 10.1111/j.1476-5381.1976.tb07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MJ, Apple DF, Jr., Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–8. doi: 10.1007/s004210050606. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol. 2006;572:561–7. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982;79:147–66. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PC, Bleeker MW, van Kuppevelt DH, van der Woude LH, Hopman MT. Rapid and extensive arterial adaptations after spinal cord injury. Archives of physical medicine and rehabilitation. 2006;87:688–96. doi: 10.1016/j.apmr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- de Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. American journal of physiology. Heart and circulatory physiology. 2004;287:H374–80. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- De Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT. Time course of arterial vascular adaptations to inactivity and paralyses in humans. Medicine and science in sports and exercise. 2003;35:1977–85. doi: 10.1249/01.MSS.0000099088.21547.67. [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–7. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spasticity. Handb Clin Neurol. 2012;109:197–211. doi: 10.1016/B978-0-444-52137-8.00012-7. [DOI] [PubMed] [Google Scholar]
- Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–35. [PubMed] [Google Scholar]
- Dudley GA, Castro MJ, Rogers S, Apple DF., Jr. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80:394–6. doi: 10.1007/s004210050609. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45:304–9. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46:96–102. doi: 10.1038/sj.sc.3102087. [DOI] [PubMed] [Google Scholar]
- Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol. 2005;99:1891–6. doi: 10.1152/japplphysiol.00629.2005. [DOI] [PubMed] [Google Scholar]
- Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Wray DW, Bader F, Gilbert EM, Richardson RS. Understanding Exercise-induced Hyperemia: Central and Peripheral Hemodynamic Responses to Passive Limb Movement in Heart Transplant Recipients. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen O. Sympathetic reflex control of blood flow in human peripheral tissues. Acta Physiol Scand Suppl. 1991;603:33–9. [PubMed] [Google Scholar]
- Hershman KM, Taylor DA, Fleming WW. Adaptive supersensitivity in the guinea pig vas deferens is associated with a reduction in the abundance of the alpha 2 subunit isoform of Na+/K(+)-ATPase. Mol Pharmacol. 1993;43:833–7. [PubMed] [Google Scholar]
- Hopman MT, van Asten WN, Oeseburg B. Changes in blood flow in the common femoral artery related to inactivity and muscle atrophy in individuals with long-standing paraplegia. Advances in experimental medicine and biology. 1996;388:379–83. doi: 10.1007/978-1-4613-0333-6_50. [DOI] [PubMed] [Google Scholar]
- Huonker M, Schmid A, Schmidt-Trucksass A, Grathwohl D, Keul J. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. Journal of applied physiology. 2003;95:685–91. doi: 10.1152/japplphysiol.00710.2001. [DOI] [PubMed] [Google Scholar]
- Ichinose E, Kurose T, Daitoku D, Kawamata S. The skeletal muscle vascular supply closely correlates with the muscle fiber surface area in the rat. Arch Histol Cytol. 2008;71:45–57. doi: 10.1679/aohc.71.45. [DOI] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vacular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol. 2007;583:861–74. doi: 10.1113/jphysiol.2007.131250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23:48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. The Journal of physiology. 2008a;586:1137–45. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol. 2008b;586:1137–45. doi: 10.1113/jphysiol.2007.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard FM, Jr., Bracken MB, Creasey G, Ditunno JF, Jr., Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–74. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol. 2010a;108:76–84. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J, Hayman MA, Ives SJ, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated Exercise Induced Hyperemia with Age: Mechanistic Insight From Passive Limb Movement. J Physiol. 2010b doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraki S, Ehara Y, Yamasaki M. Cardiovascular responses at the onset of passive leg cycle exercise in paraplegics with spinal cord injury. Eur J Appl Physiol. 2000;81:271–4. doi: 10.1007/s004210050042. [DOI] [PubMed] [Google Scholar]
- Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- Nash MS, Montalvo BM, Applegate B. Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons. Arch Phys Med Rehabil. 1996;77:1260–5. doi: 10.1016/s0003-9993(96)90190-2. [DOI] [PubMed] [Google Scholar]
- Nobrega AC, Araujo CG. Heart rate transient at the onset of active and passive dynamic exercise. Med Sci Sports Exerc. 1993;25:37–41. [PubMed] [Google Scholar]
- Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Medicine and science in sports and exercise. 2003;35:901–7. doi: 10.1249/01.MSS.0000069755.40046.96. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Pyke K, Green DJ, Weisbrod C, Best M, Dembo L, O'Driscoll G, Tschakovsky M. Nitric oxide is not obligatory for radial artery flow-mediated dilation following release of 5 or 10 min distal occlusion. Am J Physiol Heart Circ Physiol. 2010;298:H119–26. doi: 10.1152/ajpheart.00571.2009. [DOI] [PubMed] [Google Scholar]
- Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand. 2000;168:511–8. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Tam E, Azabji Kenfack M, Cautero M, Lador F, Antonutto G, di Prampero PE, Ferretti G, Capelli C. Correction of cardiac output obtained by Modelflow from finger pulse pressure profiles with a respiratory method in humans. Clinical science. 2004;106:371–6. doi: 10.1042/CS20030302. [DOI] [PubMed] [Google Scholar]
- Ter Woerds W, De Groot PC, van Kuppevelt DH, Hopman MT. Passive leg movements and passive cycling do not alter arterial leg blood flow in subjects with spinal cord injury. Phys Ther. 2006;86:636–45. [PubMed] [Google Scholar]
- Thijssen DH, Heesterbeek P, van Kuppevelt DJ, Duysens J, Hopman MT. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc. 2005;37:1112–8. doi: 10.1249/01.mss.0000170126.30868.fb. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Kooijman M, de Groot PC, Bleeker MW, Smits P, Green DJ, Hopman MT. Endothelium-dependent and -independent vasodilation of the superficial femoral artery in spinal cord-injured subjects. Journal of applied physiology. 2008;104:1387–93. doi: 10.1152/japplphysiol.01039.2007. [DOI] [PubMed] [Google Scholar]
- Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc. 2008;40:918–25. doi: 10.1249/MSS.0b013e3181641bed. [DOI] [PubMed] [Google Scholar]
- Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barret-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component; Evidence from a neural blockade study. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric Oxide and Passive Limb Movement: A New Approach to Assess Vascular Function. J Physiol. 2012 doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barret-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of Body Position on Central and Peripheral Hemodynamic Contributions to Movement-Induced Hyperemia: Implications for Rehabilitative Medicine. American journal of physiology. Heart and circulatory physiology. 2011 doi: 10.1152/ajpheart.00038.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–47. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol. 2005;565:1053–60. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Uberoi A, Lawrenson L, Bailey DM, Richardson RS. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin Sci (Lond) 2009;116:433–41. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does Brachial Artery Flow-Mediated Vasodilation Provide a Bioassay for NO? Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.113.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]