Abstract
Objective
Patients with neurogenic bladder are at increased risk of developing upper tract stones. We hypothesized that patients with lower urinary tract stone disease are at greater risk of developing upper tract stones.
Methods
We performed a 10-year retrospective case–control study of patients with neurogenic bladder to determine the association between bladder and upper tract stones. Independent risk factors for upper tract stones were assessed. Cases and controls were matched 1:1. Univariable analysis was performed by Fisher's exact test and the Mann–Whitney U test. Multivariable logistic regression was performed.
Results
52 cases and controls were identified. Cases were significantly more likely to be non-ambulatory, have bowel–urinary tract interposition, thoracic level dysraphism, and history of bladder stones. On multivariable analysis, independent predictors of stone formation were male sex (OR 2.82; p = 0.02), dysraphism involving the thoracic spine (OR 3.37; p = 0.014) bowel–urinary tract interposition (OR 2.611; p = 0.038), and a history of bladder stones (OR 3.57; p = 0.015).
Conclusion
Patients with neurogenic bladder are at increased risk for upper tract stones. The presence of bladder stones may herald the development of upper tract stones. The predictors of stone disease identified should guide prospective studies to better understand the natural history of upper tract stone development in this population.
Keywords: Myelomeningocele, Spinal dysraphism, Nephrolithiasis
Introduction
Patients with congenital neurogenic bladder, from disorders including spina bifida and caudal regression, are high risk for urologic disease. The main urologic objectives for all patients with neurogenic bladder are to protect bladder health and compliance, preserve renal function, and achieve urinary continence, when appropriate. Lower urinary tract reconstruction (LUTR) utilizing various bowel segments has played an important role in protecting the upper urinary tracts and improving bladder continence in these patients [1,2]. LUTR does not come without recognized risks, including metabolic imbalances, augment perforation, tumor formation, and bladder calculi [3].
The incidence of bladder calculi in patients with an augmented bladder varies greatly between studies with reported rates as high as 50% in the early 1990s and between 10% and 15% in more recent analyses [3–6]. Anecdotally, we noted that patients with congenital neurogenic bladder and a history of bladder calculi seemed to have a greater propensity for upper tract stones than those without. We hypothesized that the presence of bladder calculi increases the risk of developing upper urinary tract stones in these patients. Our primary study endpoint was to determine if bladder calculi independently predict upper urinary tract stone development. The secondary study endpoint was twofold: 1) to identify overall risk factors associated with upper urinary tract stone development and; 2) characterize the stone burden seen in patients with congenital neurogenic bladder.
Materials and methods
Following Institutional Review Board approval, a retrospective case–control study was performed. A unique institution-specific database, Synthetic Derivative (SD), was used to identify a population of interest and potential study subjects. The SD is a de-identified image of the Vanderbilt electronic medical record and is fully compliant with the administrative, physical, and technical provisions of the HIPAA Security and Privacy Rules [7]. The SD contains ~2 million total records and incorporates data from multiple sources and includes diagnostic and procedure codes (ICD-9 and CPT), basic demographics (age, gender, race), and text from clinical care. All clinical data are updated regularly, thus providing a suitable resource for mining information relative to disease progression over time. Identification of patients with spina bifida or caudal regression was accomplished searching the SD using 11 unique ICD-9 codes for myelomeningocele (741–741.93) combined with the keyword “stone”. Search results were restricted to the years 2001 through 2011. The population of interest was narrowed to the initial data set using the following inclusion criteria: 1) confirmed diagnosis of spina bifida or caudal regression; 2) outpatient urology visits or in-patient urology consultation in the last 10 years; 3) >24 months of urology-specific follow-up; and 4) age >5 years at last urology visit. Cases were selected from the initial data set and were defined as patients with upper urinary tract stones confirmed radiographically by reviewing reports of computed tomography, renal ultrasound, plain film radiography and/or intravenous pyelogram. Review of actual radiologic imaging was not possible using the SD. Controls were selected from the initial data set randomly and matched in a 1:1 fashion with cases using age at last urologic follow-up as the sole selection criteria. A 1:2 case to control matching was attempted but resulted in significant differences in age at last follow-up. Therefore, an upper limit of age was not set and the 1:1 matching strategy allowed length of follow-up and patient age at last visit to be nearly identical in cases and controls.
Data collection included date of birth, sex, race, level of dysraphism according to radiographic studies and/or neurosurgical clinical documents, ambulatory status at last follow-up, ambulatory status at the time of stone development, and ventriculoperionteal shunt status. Ambulatory status was dichotomized as either non-ambulatory (including wheelchair bound or bedridden) or ambulatory (including walking unassisted or walking with an assist device). Clinical rehabilitation notes were primarily used to make this distinction. Bladder stone formation was defined as a previous documented history of bladder stones and/or a confirmed bladder stone at our center by radiography or cystoscopy. Bladder management was determined at last follow-up in cases and controls and also at the time of stone development in cases. Care was taken to determine the management strategy according to the status of the lower urinary tract (i.e. native unreconstructed bladders vs. bowel–urinary tract interposition versus non-bowel urinary diversion).
Stone burden was characterized by collecting information about stone events in the 52 cases. A stone event was defined as a clinically separate stone(s) requiring evaluation and/or treatment. Stone burden variables included age at first upper tract stone at Vanderbilt, maximal single diameter of entire stone burden at the time of the event, bilaterality of stone disease, and surgical interventions required.
Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. The Vanderbilt Institute for Clinical and Translation Research provided support for REDCap and the Synthetic Derivative (1 UL1 RR024975 from NCRR/NIH) [8].
Statistical analysis was performed using Prism 5.0 (GraphPad Software, La Jolla, CA, USA) and SPSS 21 (IBM, New York, NY, USA). Descriptive statistics were generated using Fisher's exact test for categorical data and the Student's t test for continuous data. Odds ratios for potential risk factors for upper urinary tract stone development were evaluated in a univariate fashion using Fisher's exact test reporting p values and the 95% CI. Multivariable logistic regression was used to identify factors significantly associated with upper tract stone disease. Model variables were all dichotomous, including the dependent outcome variable. A p value <0.05 was determined significant for all tests.
Results
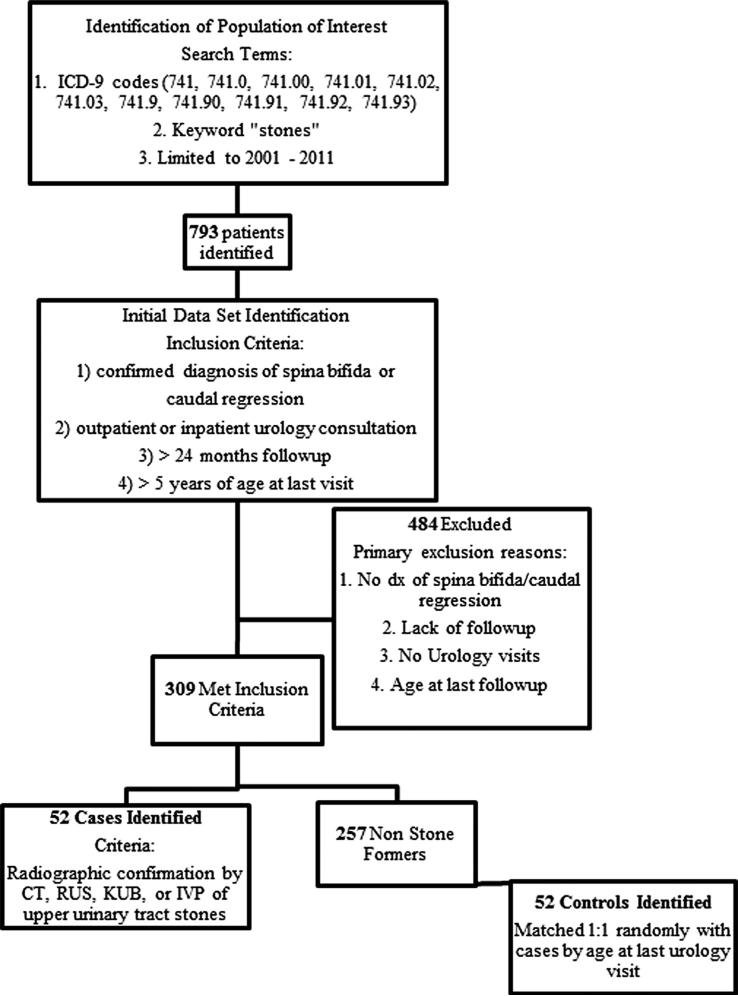
Fifty-two patients with upper urinary tract stones met inclusion criteria and served as the cases while 52 controls were identified. Fig. 1 depicts the process of identifying cases and controls. Table 1 shows general characteristics of the study population. No significant differences were noted in age at last urology follow-up or months of urologic follow-up between cases and controls. Cases were more likely to be male, although the difference was not statistically significant (p = 0.11). Cases were also significantly more likely to have dysraphism involving the thoracic spine, to be non-ambulatory, to have bowel interposed in the urinary tract, and to have a history of bladder stones. All 24 cases with a history of bladder stones prior to or concomitant with upper tract stone disease had a confirmed bladder stone during the study period at Vanderbilt. Seven of the nine controls with bladder stones had confirmed lower tract stones at Vanderbilt and two had a verbal history of previous bladder stones. Seventeen of the 24 cases with bladder stones had previously undergone bladder augmentation compared with six of the nine controls (p = 1.00, 95% CI 0.23–6.27).
Fig. 1.
Process of identifying cases and controls.
Table 1.
Characteristics of cases and controls.
| Characteristics | Cases (n = 52) | Controls (n = 52) | p Value | 95% CI |
|---|---|---|---|---|
| Mean age at last visit (years) | 28.5 (8.62–61.3) | 26.1 (8.61–55.44) | 0.25 | –1.77 to 6.77 |
| Mean follow-up (months) | 111.5 (27–210) | 113.6 (24–184) | 0.81 | –19.6 to 15.32 |
| Male | 26 (50%) | 17 (32%) | 0.11 | 0.21–1.07 |
| Caucasian | 50 (94%) | 49 (96%) | 1.00 | 0.10–4.1 |
| Dysraphism involving thoracic spine | 22 (40%) | 10 (17%) | 0.01 | 1.31–8.02 |
| Ventriculoperitoneal shunt | 43 (82%) | 46 (88%) | 0.58 | 0.56–4.88 |
| Ambulatory with or without assist device | 8 (15%) | 20 (38%) | 0.01 | 1.35–8.78 |
| Lower urinary tract reconstruction | 38 (73%) | 24 (46%) | 0.01 | 1.32–6.63 |
| History of bladder stones | 24 (46%) | 9 (17%) | 0.002 | 0.069–0.47 |
Table 2 depicts the bladder management strategy at the time of stone development in cases and bladder management at the time of last follow-up in controls. Cases were less likely to have native, unreconstructed bladders than controls and, conversely, were more likely to have bowel–urinary tract interposition either by a non-continent bowel diversion or by enterocystoplasty. Specific surgical dates were identifiable in 26 of the 38 cases and 19 of the 24 controls having undergone LUTR or urinary diversion. Mean age at the time of these procedures was similar in the two groups (13.5 years in cases vs. 11.1 years in controls, p = 0.54; 95% CI –3.72–6.76). Table 3 shows univariate analysis of five selected clinical predictor variables in cases and controls. Odds ratios showed male sex, non-ambulatory status, thoracic level dysraphism, bowel–urinary tract interposition, and a history of prior or concomitant bladder stones were all significantly associated with upper tract stones.
Table 2.
Bladder management.
| Characteristics | Cases (n = 52) | Controls (n = 52) | p Value | 95% CI |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Unreconstructed bladder | 12 (23) | 24 (46) | 0.02 | 0.15–0.81 |
| Volitional voiding | 0 | 4 | ||
| Native bladder – incontinent | 0 | 7 | ||
| Native bladder – CIC | 12 | 13 | ||
| Bowel–urinary tract interposition | 38 (73) | 24 (46) | 0.01 | 1.32–6.63 |
| Bladder augmentation | 24 | 17 | ||
| Ileal conduit diversion | 13 | 5 | ||
| Mitrofanoff only | 1 | 2 | ||
| Non-bowel urinary diversion | 2 (4) | 4 (8) | 0.67 | 0.083–2.74 |
| Vesicostomy | 2 | 2 | ||
| Catheterizable bladder tube | 0 | 2 | ||
CIC: Clean intermittent catheterization.
Table 3.
Univariate analysis of clinical predictor variables in cases and controls.
| Characteristics | Cases (no.) | Controls (no.) | Or | p value | 95% CI |
|---|---|---|---|---|---|
| Male sex | 26 | 17 | 2.05 | 0.11 | 0.93–4.59 |
| Bowel in urinary tract | 38 | 24 | 3.16 | 0.009 | 1.39–7.19 |
| Non-ambulatory | 8 | 20 | 2.95 | 0.01 | 1.31–6.64 |
| Bladder stones | 24 | 7 | 5.09 | 0.01 | 1.94–13.4 |
| Thoracic level dysraphism | 22 | 10 | 3.08 | 0.01 | 1.27–7.44 |
A multivariable logistic regression analysis was conducted to predict the formation of upper urinary tract stone disease using the predictors listed in Table 3. In the model, independent predictors of stone formation were male sex (odds ratio, 2.82; p = 0.02; 95% CI 1.12–7.09), dysraphism involving the thoracic spine (odds ratio, 3.37; p = 0.014; 95% CI 1.27–8.93) bowel–urinary tract interposition (odds ratio, 2.611; p = 0.38; 95% CI 1.054–6.46) and a history of bladder stones (odds ratio, 3.57; p = 0.015; 95% CI 1.28–9.95) (Table 4). The model did not change when the interaction between a history of bladder stones and bowel–urinary tract interposition was included nor did it change when interaction between non-ambulatory status and thoracic level dysraphism was included. Table 5 demonstrates the degree of stone burden seen in the 52 cases. The mean age at the time of the first stone at our institution was 22.83 years and most had more than one stone event. Sixty percent required surgical intervention for stone disease with 38% requiring at least one percutaneous procedure. Three patients underwent nephrectomy as a result of stone disease and 14 patients lost renal function over time due to upper tract stones. Loss of renal function was considered present if serial renography demonstrated decreases in function to values less than 40% of differential renal function or if renal sonography revealed renal atrophy in a kidney with multiple stone episodes.
Table 4.
Multivariate analysis of clinical predictor variables in cases and controls.
| Characteristics | Cases (no.) | Controls (no.) | Or | p Value | 95% CI |
|---|---|---|---|---|---|
| Male sex | 26 | 17 | 2.82 | 0.02 | 1.12–7.09 |
| Thoracic level dysraphism | 22 | 10 | 3.37 | 0.014 | 1.27–8.93 |
| Bowel in urinary tract | 38 | 24 | 2.61 | 0.038 | 1.05–6.46 |
| Bladder stones | 24 | 7 | 3.57 | 0.015 | 1.28–9.95 |
Table 5.
Degree of stone burden.
| Characteristics | Value | 95% CI of mean |
|---|---|---|
| Total no. stone events | 79 | – |
| Mean age at first stone event at institution (years) | 22.83 (3.9–60.5) | 19.95–25.72 |
| Mean number of events | 1.48 (1–4) | 1.3–1.65 |
| Mean size of stone burden per event (cm) | 2.17 (0.1–9.3) | 1.65–2.69 |
| No. bilateral stone events (%) | 30 (38%) | – |
| No. events with surgery required (%) | 48 (60%) | – |
| No. patients requiring at least one PCNL (%) | 30 (38%) | – |
| No. patients requiring multiple PCNL (%) | 18 (22%) | – |
PCNL, percutaneous nephrolithotomy.
Discussion
Urologic management of the neurogenic bladder is an evolving process throughout the life of these patients. Yet critical decisions regarding optimal management of the neurogenic bladder are increasingly made during childhood and early adolescence. Such decisions can have life-long impact upon future health. One readily acknowledged, yet understudied, aspect of neurogenic bladder management is the development of upper urinary tract stone disease. In patients with congenital neurogenic bladder, renal stones often present a challenging problem because of complicating factors like altered body habitus, severe scoliosis and kyphosis, significant medical comorbidity, altered renal anatomy, and sheer stone burden. It was our clinical impression that patients who develop bladder stones are at increased risk of developing upper urinary tract stones. In this retrospective, case–control analysis we were able to utilize a unique institutional database to select an appropriate group of cases and respective controls. Using these populations, the current analysis identified a history of bladder stones prior to or at the time of upper urinary tract stone diagnosis as an independent predictor of forming an upper tract stone. Several additional independent predictors of upper urinary tract stone disease identified were male sex, thoracic spinal dysraphism, and bowel–urinary tract interposition either by enterocystoplasty or non-continent ileal conduit. Moreover, upper tract stone burden in the 52 cases was not trivial, as evidenced by an average stone burden of at least 2.17 cm per stone episode, 38% with bilateral stone burden, 60% ultimately requiring surgery, and 38% requiring at least one percutaneous nephrolithotomy (PCNL).
Prior to discussing the current findings, several limitations of our study must be addressed. Despite our initial hopes for the analysis, the retrospective design and the use of the SD hampered our ability to evaluate several potentially critical pieces of information in cases and controls. Namely, the presence or absence of urea-splitting organisms in the urinary tract, urine culture data in general, 24-h urine and serum metabolic studies, and, finally, chemical stone analysis. Much of these data are either not currently included in the SD or were not routinely collected in patients with congenital neurogenic bladder and upper urinary tract stones. Stone composition may be the most important missing factor as it is believed that most stones in patients with neurogenic bladder are infection stones. Two separate reports have highlighted the changing composition of renal stones in patients with neurologic bladder. A study from Indiana University in 2006 reported the stone composition in 32 patients with spinal cord injury or myelomeningocele undergoing PCNL. The authors found only 37% to be struvite while 63% were metabolically derived [9]. A follow-up study from the same authors in 2011 reported stone analysis following PCNL was most commonly a metabolic stone, with calcium phosphate predominating [10]. Examining stone composition in our cases would have improved our analysis and likely furthered the point that many upper tract stones in this population could be prevented. Additionally, the ability to characterize the use of bladder irrigation protocols or patient compliance with such protocols was not possible. The importance of bladder irrigation to eliminate mucus formation following enterocystoplasty has been previously reported [11].
Reporting an increased risk of upper urinary tract stones in patients with neurogenic bladder is not new. A report from Duke University in 1999 examined 327 patients with neurogenic bladder and suggested that stone formation occurs at a greater rate in this population than in healthy controls [12]. The authors identified 20 patients with upper urinary tract stones reporting a 6.1% incidence. Factors associated with renal stones included vesicoureteral reflux, pelvocaliectasis, renal scarring on ultrasound, LUTR, and thoracic level dysraphism. In contrast to the current study, the Duke report compared stone formers and non-stone formers as a whole. This resulted in vast age differences between the groups with stone formers on average being 10 years older than non-stone formers. In designing the current study, similar observations were made regarding our initial data set of 309 patients. In comparing stone formers and non-stone formers in this large population we found stone formers were significantly older at last follow-up. This prompted us to redesign our study as a case–control analysis.
The current report does not aim to suggest a causal relationship between bladder calculi and upper tract stones; rather, an association between the two entities is likely. It is plausible that development of bladder calculi may signal a propensity for stone formation in the urinary tract and might serve as an impetus for increased upper tract surveillance by the treating clinician. To deepen the analysis of the relationship between bladder calculi and upper tract stones, more sophisticated data points will certainly be necessary including chemical analysis of bladder stones and 24-h urine studies.
Aside from the Duke report, few data exist regarding risk factors for stone disease in the congenital neurogenic bladder population. Evidence from studies of spinal cord injury patients suggests an increased risk of upper tract stone formation that nears 20% by 20 years after injury [13]. However, the generalizability of those is limited, primarily due to the contrastingly acute setting of spinal cord injury compared with the chronic nature of congenital neurogenic bladder disease. In several reports, risk for upper tract stone formation appeared to be greatest in the first 3–6 months following spinal injury with a period of acute immobilization and over time might be more closely related to the use of an indwelling Foley catheter [14,15].
We propose that the information gleaned from this report and that from Duke University should serve as the basis for a collaborative multi-institutional study to prospectively examine more sophisticated risk factors in these high-risk groups. We submit that important information would include periodic urinary tract cultures, serial metabolic studies (both serum and 24-h urine studies), chemical analysis of renal and bladder stones if previous history, and assessment of compliance with irrigation protocols.
Conclusions
From the current data, it is clear that a distinct group of patients with congenital neurogenic bladder are at increased risk for upper tract stones. The stone burden experienced by these patients is clinically significant. Treating physicians should be mindful of the increased risk of upper urinary tract stones in patients with a known history of bladder calculi. More detailed analysis of patients with congenital neurogenic bladder will provide insight into the modifiable risk factors of upper tract stone disease.
Acknowledgments
Funding
The project described was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethical approval
The Institutional Review Board at Vanderbilt University has approved this study.
Abbreviations
- LUTR
Lower urinary tract reconstruction
- SD
Synthetic Derivative
- PCNL
Percutaneous nephrolithotomy
Footnotes
Conflict of interest
None.
References
- 1.Lopez PP, Moreno Valle JA, Espinosa L, Alonso Dorrego JM, Burgos LL, Martinez Urrutia MJ, et al. Enterocystoplasty in children with neuropathic bladders: long-term follow-up. J Pediatr Urol. 2008;4:27–31. doi: 10.1016/j.jpurol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Medel R, Ruarte AC, Herrera M, Castera R, Podesta ML. Urinary continence outcome after augmentation ileocystoplasty as a single surgical procedure in patients with myelodysplasia. J Urol. 2002;168:1849–52. doi: 10.1097/01.ju.0000029549.55124.6d. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe PD, Cain MD, Kaefer M, Gilley DA, Meldrum KK, Misseri R, et al. What is the need for additional bladder surgery after bladder augmentation in childhood? J Urol. 2006;176:1801–5. doi: 10.1016/j.juro.2006.03.126. discussion 1805. [DOI] [PubMed] [Google Scholar]
- 4.Palmer LS, Palmer JS, Firlit BM, Firlit CF. Recurrent urolithiasis after augmentation gastrocystoplasty. J Urol. 1998;159:1331–2. [PubMed] [Google Scholar]
- 5.DeFoor W, Minevich E, Reddy P, Sekhon D, Polsky E, Wacksman J, et al. Bladder calculi after augmentation cystoplasty: risk factors and prevention strategies. J Urol. 2004;172:1964–6. doi: 10.1097/01.ju.0000140911.43898.15. [DOI] [PubMed] [Google Scholar]
- 6.Mathoera RB, Kok DJ, Nijman RJ. Bladder calculi in augmentation cystoplasty in children. Urology. 2000;56:482–7. doi: 10.1016/s0090-4295(00)00663-4. [DOI] [PubMed] [Google Scholar]
- 7.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–9. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)da metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matlaga BR, Kim SC, Watkins SL, Kuo RL, Munch LC, Lingeman JE. Changing composition of renal calculi in patients with neurogenic bladder. J Urol. 2006;175:1716–9. doi: 10.1016/S0022-5347(05)01015-3. discussion 1719. [DOI] [PubMed] [Google Scholar]
- 10.Gnessin E, Mandeville JA, Handa SE, Lingeman JE. Changing composition of renal calculi in patients with musculoskeletal anomalies. J Endourol. 2011;25:1519–23. doi: 10.1089/end.2010.0698. [DOI] [PubMed] [Google Scholar]
- 11.Khoury A E, Salomon M, Doche R, Soboh F, Ackerley C, Jayanthi R, et al. Stone formation after augmentation cystoplasty: the role of intestinal mucus. J Urol. 1997;158:1133–7. [PubMed] [Google Scholar]
- 12.Raj GV, Bennett RT, Preminger GM, King LR, Wiener JS. The incidence of nephrolithiasis in patients with spinal neural tube defects. J Urol. 1999;162:1238–42. doi: 10.1016/S0022-5347(01)68146-1. [DOI] [PubMed] [Google Scholar]
- 13.Welk B, Fuller A, Razvi H, Denstedt J. Renal stone disease in spinal-cord-injured patients. J Endourol. 2012;26:954–9. doi: 10.1089/end.2012.0063. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord. 2000;38:346–53. doi: 10.1038/sj.sc.3101008. [DOI] [PubMed] [Google Scholar]
- 15.Hansen RB, Biering-Sorensen F, Kristensen JK. Urinary calculi following traumatic spinal cord injury. Scand J Urol Nephrol. 2007;41:115–9. doi: 10.1080/00365590600991383. [DOI] [PubMed] [Google Scholar]