Abstract
Within the same population, individuals often differ in how they respond to changes in their environment. A recent series of models predicts that competition in a heterogeneous environment might promote between-individual variation in behavioural plasticity. We tested groups of sticklebacks in patchy foraging environments that differed in the level of competition. We also tested the same individuals across two different social groups and while alone to determine the social environment’s influence on behavioural plasticity. In support of model predictions, individuals consistently differed in behavioural plasticity when the presence of conspecifics influenced the potential payoffs of a foraging opportunity. Whether individuals maintained their level of behavioural plasticity when placed in a new social group depended on the environmental heterogeneity. By explicitly testing predictions of recent theoretical models, we provide evidence for the types of ecological conditions under which we would expect, and not expect, variation in behavioural plasticity to be favoured.
Keywords: behavioural types, consistent individual differences, foraging, Gasterosteaus aculeatus, personality, plasticity, repeatability, social context
INTRODUCTION
Consistent individual differences in behaviour are difficult to explain because we expect natural selection to favour a single optimal behavioural strategy. These average differences in behaviour among individuals (i.e. personality) are now well documented across many taxa and appear to be driven by a number of ecological factors with important consequences (Reale et al. 2007; Smith & Blumstein 2008). In addition to variation in personality, there is now growing evidence that individuals vary in the extent to which they adjust their behaviour according to the environment, including the social environment (i.e. behavioural plasticity, Dingemanse et al. 2010; Mathot et al. 2012; Dingemanse & Wolf in press). For example, some individuals within a population might be more likely to exploit new food patches, while other individuals consistently forage in old patches, regardless of the behaviour of others. Interest in between-individual variation in behavioural plasticity has triggered two key questions: First, are there adaptive reasons why individuals differ in plasticity? And second, what is the influence of changes in the environment on behavioural plasticity?
A growing number of models have provided ‘proof of principle’ support for the first question, showing that under certain conditions, variation in plasticity can be adaptive (reviewed in Dingemanse & Wolf in press). Adaptive mechanisms that might generate and/or maintain between-individual differences are state variable differences (Houston & McNamara 1999; Clark & Mangel 2000), temporal and spatial variation in environmental conditions (Wolf et al. 2008; Dubois et al. 2010) and repeated social interactions (Wolf et al. 2011). In these models, the presence of conspecifics can alter the payoff of the behavioural choices available to an individual (i.e. game theoretic dynamics). These competitive interactions can generate negative frequency-dependent payoffs to plastic individuals, thereby promoting between-individual variation in behaviour (e.g. Wolf et al. 2008, 2011), a prediction which has empirical support in anti-predator behaviour (Mathot et al. 2011). This mechanism could also be at work in other contexts; for example, in a patchy foraging context, individuals arrange themselves in a way that maximises their own food intake, which generally is proportional to the food input at each patch (‘ideal free distribution’, Fretwell & Lucas 1970). If a new food patch suddenly becomes available, a plastic individual may be able to take advantage of this opportunity more quickly than a less plastic individual. This should increase the payoff to the plastic individual, but also the non-plastic individual as the level of competition within a patch is reduced, increasing the likelihood of their using the same tactic again (i.e. positive feedback, Wolf et al. 2008). However, behavioural plasticity is assumed to carry a cost (DeWitt et al. 1998) and the advantage to a plastic individual is highest when it is rare in the population (Wolf et al. 2008). If all individuals in the population are plastic and constantly respond to changes in their environment, a non-plastic individual might do well if it can behave appropriately on average and not have to pay the costs associated with plasticity (Wolf et al. 2008). In this way, between-individual variation in behaviour might be maintained when individuals have a mutual interest in avoiding competition in a heterogeneous environment.
As suggested by these models, social dynamics can play a key role in promoting variation in plasticity; however, it is still unknown how changes in the social environment might influence behavioural plasticity (Reale et al. 2007; Stamps & Groothuis 2010). Individuals may be strongly influenced by the social composition and therefore exhibit context-specific behaviours that change as the context or social environment changes (sensu Coleman & Wilson 1998). For example, individual birds maintained a consistent producer or scrounger strategy in one social group, but switched strategies when they were placed in a new social group (David et al. 2011; Morand-Ferron et al. 2011). Alternatively, individuals might exhibit very domain-general (sensu Coleman & Wilson 1998) behaviours that do not change when placed in a new context. Hyper-aggressive water striders maintained high levels of aggression regardless of the composition of the social group, even though their hyper-aggressiveness decreased mating success (Sih & Watters 2005). If behaviours are domain-general, then the behaviour of an individual in a non-social situation is predictive of an individual’s behaviour in a social situation, and individuals maintain their behaviour across different social groups (e.g. Beauchamp 2000; Magnhagen & Bunnefeld 2009). However, given the dynamism and unpredictability of social interactions, we might also expect individuals to exhibit context-specific behaviours for each social environment.
Therefore, in this study, we (1) tested the prediction that there is more between-individual variation in plasticity in a social environment when there is the opportunity to avoid competition and (2) tested the influence of changes in the social environment on between-individual variation in plasticity in threespined sticklebacks (Gasterosteaus aculeatus). Sticklebacks are a small fish known for their variation in behaviour (Huntingford 1976; Bell 2005; Dingemanse et al. 2007). In addition, individual sticklebacks differ in their resource use (Bolnick et al. 2003) and how they behave in a patchy foraging environment (Milinski 1984, 1994), suggesting that some individuals may be more sensitive to changes in food availability than others. Therefore as a measure of behavioural plasticity, we measured how quickly individuals within social groups responded to a newly available food patch in a two-patch foraging environment. This method quantifies plasticity as a single variable: the speed with which an individual moves into a newly available food patch, allowing us to gather repeated measures of behavioural plasticity on the same individuals relatively quickly.
To alter the level of competition, we created two competitive regimes that differed in the number of food patches available at any one time: the ‘simultaneous patch’ regime had two patches available simultaneously, which gave plastic individuals the opportunity to reduce within-patch competition by moving into a newly available patch. The ‘sequential patch’ regime only had one patch available at a time therefore only one foraging opportunity was ever available. Given that most environments do not have perfectly reliable food patches, both regimes present ecologically relevant challenges with obvious fitness consequences (food payoff). To determine the role of the social environment on individual behaviour, we measured individuals’ behaviour in two different social groups and we also measured a subset of the individuals while alone (not in a social group).
MATERIALS AND METHODS
All fish were wild-caught females from Putah Creek, CA, a freshwater stream. Fish were housed in large groups (~ 30 individuals) in the laboratory for six months prior to experiments. Fish were fed an ad libitum diet of bloodworms, mysis and brine shrimp daily. Fish were permanently marked with subcutaneous UV elastomer (North-west Marine Technologies, Inc., Shaw Island, WA) at least 1 week prior to testing. Three days prior to testing, fish were also marked with a small plastic tag on their dorsal spine to allow visual identification (Webster & Laland 2009). All experimental procedures were approved by the University of Illinois’s Institutional Animal Care and Use Committee protocol #09204.
Measuring behavioural plasticity under two different competitive regimes
We created a feeding arena (113 × 30 × 35.5 cm) with two food patches to which food could be added independently (Fig. S1). Food was dropped into the patches by means of a conveyer belt with small cups; as the belt advanced, a cup upended into the patch. If the patch was receiving food, the cup contained a single small (~ 1 cm) bloodworm in a small amount of distilled water (~ 2 mL); if not, the cup only contained distilled water. Each patch was located on either end of the long axis of the aquarium and the aquarium was divided into three zones: two patches (30 cm long each) with a neutral zone (53 cm long) in between.
We created six groups of six non-reproductive, size-matched (42–45 mm) sticklebacks. We never detected an influence of body size on any behavioural measure (data not shown). Each group was tested in two trials per day on five consecutive days to assess the repeatability of behaviour. Groups were tested in one of two competitive regimes that differed in the level of within-patch competition (Fig. 1). In the ‘simultaneous patch’ regime, food was first added to one patch (12 bloodworms min−1) for 5 min and then at an equal rate to both patches for 5 min (6 bloodworms min−1). In this regime, individuals could reduce within-patch competition by switching to the new patch. In the ‘sequential patch’ regime food was first added to one patch for 5 min (12 bloodworms min−1) and then only to the other patch for 5 min (12 bloodworms min−1). In this regime, only one patch was available at a time, which forced foragers to switch patches and maintain a similar level of competition. In both regimes, the side that received food first was randomly assigned on the first trial of the day; however, the same side could not receive food first for more than two consecutive days. To control for potential side biases, on the second trial of the day, the opposite patch received food first.
Figure 1.
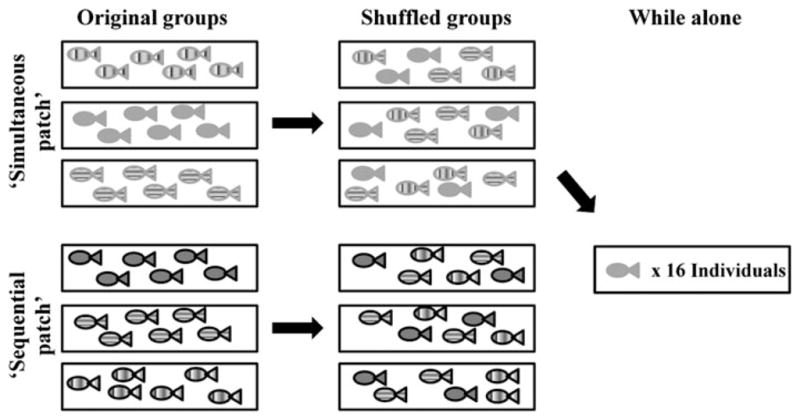
Experimental design. 18 individuals were tested in the ‘simultaneous patch’ regime and 18 individuals were tested in the ‘sequential patch’ regime. The three original groups within each regime are represented with different patterns. All individuals were first tested in an original group and then randomly reassigned and tested in a shuffled group. Individuals from the ‘simultaneous patch’ regime were then also tested while alone (see Methods). Each group was tested in 10 trials over 5 days.
Influence of the social environment on behavioural plasticity
We assessed the influence of changes in the social environment by measuring individual behaviour in two different social groups: their ‘original’ social group (described above) and a ‘shuffled’ social group. We randomly shuffled the fish from their original groups into three new groups with the restriction that only two individuals from each original group went into any one shuffled group. Fish were tested in the same regime in both their original and shuffled groups (Fig. 1). Each shuffled group was tested in 10 trials as before.
We also tested whether individual behaviour while in a group was related to individual behaviour while alone. To do this, we measured the behaviour of individuals from the ‘simultaneous patch’ regime while they were alone (Fig. 1). Preliminary observations of lone sticklebacks suggested that individuals were unwilling to move into a new patch if the old patch was still receiving food, and if an individual switched to the new patch, it usually did so within 2 min. Pilot experiments showed that single fish became satiated after consuming ~ 25 bloodworms. Therefore in the ‘alone’ trials, individuals were tested in a modified regime where one patch received food for 2 min (5 bloodworms min−1) and then the other patch received food for 2 min (5 bloodworms min−1).
To test individuals while alone, a group was placed in the feeding arena where it remained for the week of testing. For this assay, two opaque dividers were lowered on either end of the feeding arena during testing (Fig. S1), but remained up at other times so that fish could swim freely around the aquarium when a trial was not in session. When a trial began, all individuals except one were gently herded behind one divider. We then measured the behaviour of the remaining individual in the feeding arena. Then, this fish was gently herded under the opposite divider. A new individual was gently herded from under the other divider into the arena and tested. This continued until all fish had been tested in one trial. We waited 15 min and repeated the process until all fish had been tested a second time. This method allowed us to minimise stress to the fish from excessive netting and isolation from their group mates. Fish were tested for five consecutive days and were performed two months after tests in the original and shuffled groups.
Data collection
Each trial was video-recorded with a high-definition JVC Everio camcorder and the videos were coded using JWatcher (Blumstein, Daniel & Evans; UCLA & Macquarie University). In all trials, we never observed overt aggressive interactions among group mates suggesting that behavioural differences were not simply the result of differences in dominance. We measured 18 individuals in each regime in the original groups (n = 36); however, one individual in the ‘sequential patch’ regime and two in the ‘simultaneous patch’ regime died before they could be tested in their shuffled group (n = 33). They were replaced with other fish to maintain the same group size but we did not include the extra fish’s behaviour in the dataset. The two fish that died in the ‘simultaneous patch’ regime also meant that our sample size was n = 16 for the ‘alone’ trials.
In each trial, we recorded three variables for each fish. First, we recorded switch delay: the latency of an individual to move into the newly available food patch. If an individual never switched to the newly available food patch they were given a maximum switch delay of 5 min; if an individual was already within the new food patch before food was added, we could not assess whether they would have switched quickly or not at all so they were not given a switch delay for that trial. Second, we measured the number of food items an individual consumed in a single trial. Finally, we recorded an individual’s sampling behaviour as the number of times an individual moved from one patch to another. We restrict our results and discussion to variation in switch delay as we interpret this as a measure of behavioural plasticity in response to a change in the environment; sampling behaviour occurred throughout the trial before and after the change in food availability and therefore we could not determine whether this was in direct response to the change in food. Sampling behaviour results are in the Supporting Information (Appendix S1).
Data analysis
We used Bayesian statistics with Markov Chain Monte Carlo simulations using the MCMCglmm package (Hadfield 2010) in R 2.15 (http://www.r-project.org/). We first tested for differences in average behaviour between competitive regimes by including Regime as a fixed effect. To account for the non-independence of observations, we included Group and Individual (nested within Group) as random effects. For all analyses, we used non-informative proper priors (Hadfield 2010) with 500 000 iterations, thinning of 10 iterations and a burn-in of 1000 iterations (Appendix S2).
To address our first research question of whether the opportunity to avoid competition promotes greater between-individual variation in behavioural plasticity, we estimated the repeatability of switch delay (our measure of behavioural plasticity) over the entire trial week within each of our regimes. Then, to determine whether between-individual variation in plasticity increased with time spent in the social group, we estimated repeatability of switch delay using only the first two, and the last two days of the trial week in each regime. Throughout all the following analyses, we mean-centred and scaled the variance to one for all our variables within each regime, although we present raw values in the figures for ease of interpretation. Repeatability (r) is the proportion of total variation that can be attributed to between-individual differences and we estimated ‘r’ using MCMC simulations which reports 95% credibility intervals which we use to interpret significance (Nakagawa & Schielzeth 2010; Dingemanse & Dochtermann 2013). We did not include any fixed effects in our models but rather only Group and Individual (nested within Group) as random effects. As all individuals were exposed to the same levels of any potential fixed effect (e.g. trial day), variation attributable to these factors would remain in the residual variance, thereby providing a conservative repeatability estimate (Nakagawa & Schielzeth 2010). Preliminary analysis showed that inclusion of the fixed effects had little effect on our estimates of repeatability (< 0.02 change in r estimate and no change in CI interpretation, data not shown); therefore, we provide the non-’adjusted’ repeatability estimates to allow for broader generalisation of our results (Nakagawa & Schielzeth 2010; Dingemanse & Dochtermann 2013).
To determine how food intake and behaviour were related, we used bivariate mixed models to estimate the covariance between food items and switch delay (Dingemanse & Dochtermann 2013). We ran a separate model for each regime and included Group and Individual (nested within Group) as random effects. This method allowed us to partition the covariance between the two behaviours at the between- (i.e. Individual covariance; e.g. individuals that switched quickly on average, ate more food, on average) and within-Individual level (i.e. residual covariance; e.g. when an individual switched more quickly during a trial, it ate more food compared to other trials).
Our second research question was whether individuals maintain their behaviour when placed in a new social environment. We used a bivariate mixed model where we considered each individual’s switch delay in the original and shuffled groups as separate response variables and estimated the covariance between these variables (Dingemanse & Dochtermann 2013).
RESULTS
The opportunity to avoid competition promotes between-individual variation in behavioural plasticity
Across both competitive regimes, individuals switched to the new patch in, on average, 120 ± 6 (SE) seconds, but there was individual variation in switch delay: some individuals switched within 2.4 s, whereas others never switched (Fig. 2a,b). We did not detect a difference in switch delay between regimes [‘sequential patch’: 114 ± 8 s; ‘simultaneous patch’: 127 ± 9 s; posterior Regime estimate = 9.75 (−13.7, 34.6)].
Figure 2.
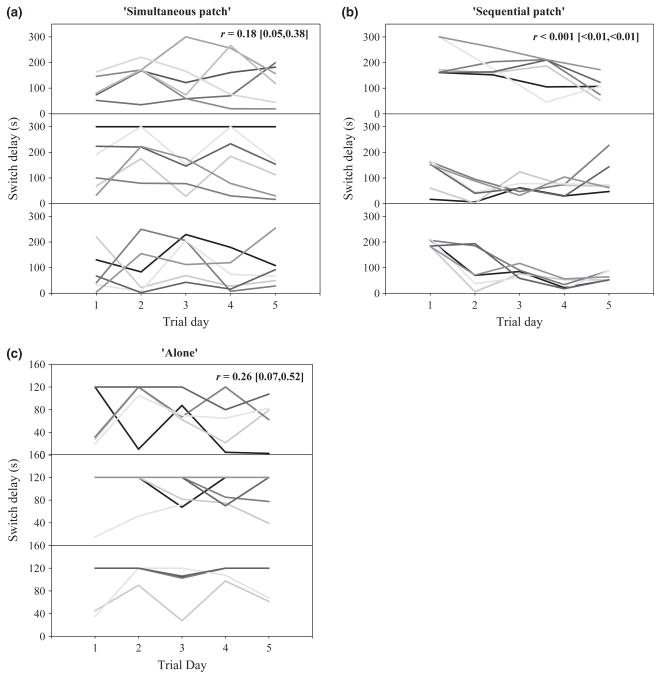
Individuals consistently differed in switch delay in the ‘simultaneous patch’ regime (a) and while alone (c), but not the ‘sequential patch’ regime (b). Each line represents a different individual and the panels show different original groups within each regime. The value (95% CI) in the upper right of each panel represents the repeatability estimate for that regime over the entire testing week. When the lines do not cross, there is perfect rank-order consistency over time, for example, between days 4 and 5 in the second panel of (a). One cause of a low estimate of repeatability is when there is little between-individual variation, for example, in (b).
In support of our hypothesis, individuals in the ‘simultaneous patch’ regime exhibited consistent individual differences in switch delay [repeatability ‘r’ = 0.18, 95% CI: (0.05, 0.38), Fig. 2a]. Individuals in the ‘sequential patch’ regime exhibited very low between-individual variation (Appendix S1), resulting in a repeatability estimate of 0 [95% CI: (3.0 × 10−10, 6.6 × 10−9), Fig. 2b). Importantly, the CI’s of the Individual variance (Appendix S1) and repeatability estimates of switch delay in each regime do not overlap demonstrating that there is greater between-individual variation in switch delay in the ‘simultaneous patch’ regime than in the ‘sequential patch’ regime. Moreover, the repeatability of switch delay in the ‘simultaneous patch’ regime significantly increased later in the testing week [first 2 days: r = 0.006 (0.0009, 0.015); last 2 days: r = 0.20 (0.05, 0.42)], driven by a significant increase in the Individual variance component (Appendix S1), which is consistent with positive feedback increasing between-individual differences. This pattern was not apparent in the ‘sequential patch’ regime; the Individual variance component (Appendix S1) and repeatability of switch delay was always nearly zero [first 2 days: r = 0.00 (1.9 × 10−10, 4.5 × 10−9); last 2 days: r = 0.00 (1.2 × 10−12, 3.7 × 10−11)].
We suspected individuals in the ‘sequential patch’ regime might be more influenced by their group mates than individuals in the ‘simultaneous patch’ regime. To assess this, we compared the amount of variation explained by the Group variance component. Over the entire week, in the ‘sequential patch’ regime, ~ 21% of the variance could be attributable to variation among Groups [0.23 (0.04, 0.56)], whereas only 3% of the variance was attributable to variation among Groups [0.05 (0.009, 0.12)] in the ‘simultaneous patch’ regime, a suggestive, but not significant difference. However, by the end of the week, the Group variance component explained significantly more variation in the ‘sequential patch’ regime [0.38 (0.007, 0.94)] than in the ‘simultaneous patch’ regime [0.00 (< 0.001, < 0.001), Appendix S1].
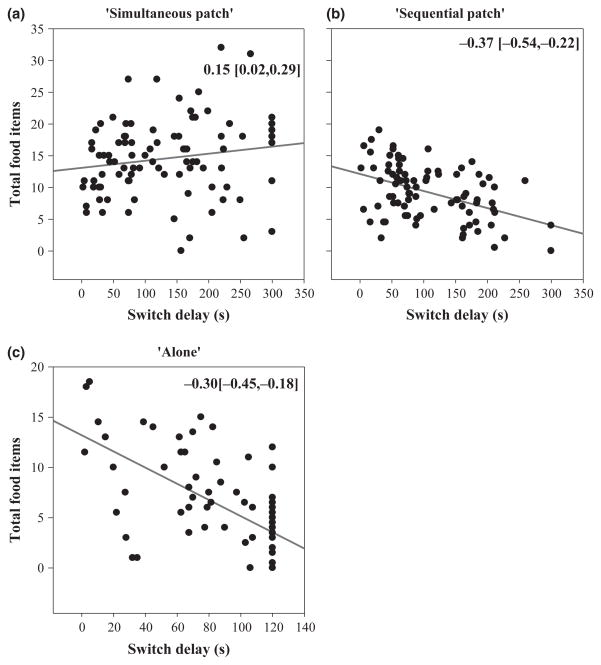
We found evidence for within-individual correlations across trials in how many food items an individual consumed and their switch delay, though in opposite directions in the two regimes. In the ‘simultaneous patch’ regime, when individuals took longer to switch (larger switch delays), they consumed more food items in that trial [residual covariance = 0.15 (0.02, 0.29), Fig. 3a). Not surprisingly, the opposite pattern was apparent in the ‘sequential patch’ regime [residual covariance = −0.37 (−0.54, −0.22), Fig. 3b] as only individuals that switched would receive food in the second period of the trial. While we found no evidence for a significant covariance between an individual’s average switch delay and average food items in either regime, this may have been influenced by the relatively small sample size of our study [Individual covariance: ‘simultaneous patch’ = 0.03 (−0.14, 0.21); ‘sequential patch’ = −8.9 × 10−5 (−2.2 × 10−5, 2.3 × 10−5)].
Figure 3.
When individuals switched more quickly in the ‘simultaneous patch’ regime they received less food during that trial compared to other trials (a), but when individuals switched more quickly in the ‘sequential patch’ regime (b) and while alone (c) they received more food during that trial compared to other trials. Each dot represents a trial so a single individual is represented by ten dots. The value in the upper right represents the residual covariance between switch delay and food items (95% CI). The regression line is included for illustrative purposes only.
Individuals maintain behavioural plasticity across two social environments in the ‘simultaneous patch’ regime
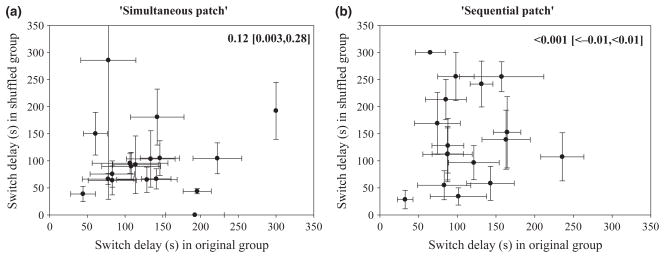
We evaluated whether individuals were influenced by changes in their social environment by measuring individual switch delay in their original group and in a new shuffled group. We observed between-individual variation in switch delay within the original social groups in the ‘simultaneous patch’ regime and there was significant covariance between an individual’s switch delay in their original group and their switch delay in the shuffled group [Individual covariance = 0.12 (0.003, 0.28), Fig. 4a]. In contrast, in the ‘sequential patch’ regime, we could not accurately estimate covariance between individual switch delay across the two social groups, as there was essentially zero variation in individual behaviour within the original groups (Fig. 2b, Appendix S1), which further supports that there is little carryover in behaviour from one social context to the other.
Figure 4.
There was significant covariance between individuals’ switch delay across two social groups in the ‘simultaneous patch’ regime, but not in the ‘sequential patch’ regime. Each dot represents one individual and their average behaviour in their original and shuffled group ± 1 SE. The value in the upper right shows the covariance estimate between individual switch delay (95% CI).
As only individuals in the ‘simultaneous patch’ regime exhibited significant between-individual variation in switch delay, we also measured these individuals while alone. We found that these individuals also exhibited significantly repeatable variation in switch delay while alone [r = 0.26 (0.07, 0.52)]. While not strongly supported, we did find evidence for cross-context repeatability in individual behaviour while alone and in a social group as there were positive covariances between individual switch delay while alone and in their original groups [Individual covariance = 0.10 (−0.10, 0.31)] and while alone and in their shuffled groups [Individual covariance = 0.10 (−0.02, 0.24)]. The fact that the CI’s overlapped zero should be interpreted cautiously but this still suggests that individuals with shorter switch delays while alone tended to have shorter switch delays in the social groups.
Not surprisingly, when tested while alone, individuals that switched more quickly during a trial also received more food during that trial [residual covariance = −0.30 (−0.45, −0.18)]. Individuals that switched more quickly on average, tended to get more food overall, although this was not not strictly significant [Individual covariance = −0.16 (−0.43, 0.05)].
DISCUSSION
While there is evidence that limited behavioural plasticity can constrain optimal behaviour (Sih et al. 2004; Johnson & Sih 2005; Sih & Watters 2005), recent models have shown that individual variation in plasticity can be adaptive under some circumstances (e.g. Wolf et al. 2008, 2011). Some of the most intriguing models have shown that competitive interactions among individuals might promote between-individual variation in behaviour, including plasticity, when there is environmental heterogeneity (e.g. Wolf et al. 2008, 2011; McNamara et al. 2009; Dubois et al. 2010). These models predict that individual differences in plasticity are most likely to emerge when access to foraging opportunities is temporally and/or spatially variable and these opportunities are limited by the presence of competitors. In this article, we provide strong support for this prediction by showing that consistent individual differences in switch delay were only apparent in the ‘simultaneous patch’ regime, that is, under conditions where individuals that quickly switched to the new food patch could exploit a different foraging opportunity than non-plastic individuals. In addition, under these conditions (‘simultaneous patch’ regime), between-individual variation in switch delay increased the longer a group had been together, consistent with positive feedback, which is another prediction of these models (Wolf et al. 2008, 2011). We also showed that the relative success of this behaviour depended on the competitive regime, suggesting that ecological factors such as food availability and predictability might influence variation in plasticity. Finally, we showed that the influence of the social environment on individual behaviour depended on the competitive regime: individuals in the ‘simultaneous patch’ regime exhibited similar behaviour across two social groups and while alone, whereas individuals in the ‘sequential patch’ regime did not.
The Ideal Free Distribution theory predicts that groups of foraging animals arrange themselves among patches to avoid competition and increase individual payoffs (Fretwell & Lucas 1970). We showed that this pattern might be driven by only a few individuals in the group that consistently respond more quickly than others to new foraging opportunities. Recent models argue that the presence of plastic individuals reduces within-patch competition: as plastic individuals utilise a new patch and the non-plastic individuals stay at the old patch, the payoffs to all individuals increase (Wolf et al. 2008). Because of this increase in payoff, all individuals, including non-plastic individuals, should be more likely to use the same strategy again (Wolf et al. 2008, 2011). The repeatability of switch delay increased later in the week, demonstrating that between-individual variation in this behaviour increased the longer the group had been together, suggestive of positive feedback. However, this positive feedback could be caused by several potential mechanisms. One possibility is that individuals became familiar with the patchiness of the competitive regime; as food at one patch became unavailable or reduced, they learned to quickly search for another. Alternatively, increasing familiarity among group mates may have caused positive feedback. As individuals learned their group mates’ reputation for patch use, they were able to avoid competition because individuals could be relied upon to behave in a certain way (e.g. Dall et al. 2004; McNamara et al. 2009). A promising future research direction is to determine if familiarity with the competitive regime or familiarity among group mates is the cause of the positive feedback.
Comparing the two competitive regimes revealed that how quickly an individual exploited a new patch influenced individual payoffs, but the direction of the relationship depended on the variation in food availability. In the ‘sequential patch’ regime, when an individual quickly responded to the change in food availability (faster switch delay), it received more food during that trial compared to other trials; however, in the ‘simultaneous patch’ regime the opposite pattern was the case. In this regime, when an individual quickly switched to the new food patch, it did worse during that trial (Fig. 4). The fact that individuals that quickly responded did worse in the ‘simultaneous patch’ regime suggests that there is a cost associated with behavioural plasticity, which is a crucial assumption of these models (Wolf et al. 2008, 2011). The most obvious cost under these conditions is travel time between patches, however, other costs have been suggested (e.g. maintenance and production costs, DeWitt et al. 1998; Wolf et al. 2008). Determining how costs maintain variation in behavioural plasticity is a promising topic for further work. The Wolf et al. (2011) model suggests that plastic individuals might be favoured whenever individuals vary in their average behaviour and our results suggest that the benefits of plasticity can also depend on variation in abiotic environmental factors, such as resource availability. Similar results have been found elsewhere that demonstrated that the success of different behavioural types depended on the distribution and abundance of resources (Dingemanse et al. 2004; Morand-Ferron et al. 2011). Environmental heterogeneity is pervasive in natural populations and along with maintaining variation in personality, might play a role in maintaining variation in behavioural plasticity within and across populations as well. Whether this extends to the maintenance of genetic variation in plasticity in sticklebacks is an obvious question for future work.
While adaptive models explain the origin and maintenance of variation in behaviour over evolutionary time, another outstanding question is the extent to which current social conditions influence behaviour within the individual’s lifetime (e.g. Stamps & Groothuis 2010; Dingemanse & Wolf in press). Multiple studies have demonstrated the impact of changes in the social environment on individual behaviour (e.g. Sih & Watters 2005; Magnhagen & Bunnefeld 2009), and here we offer insight into how changes in social group composition influence an individual’s reaction to foraging opportunities. One extreme view might be that behaviour is context-specific and therefore the most important influence on an individual’s behaviour is their current environment. Therefore, we would expect to see little carry-over in behaviour from one situation to the next. In contrast, another view suggests that behaviour is most heavily determined by innate factors such as genetics and therefore we would expect individuals to exhibit very domain-general behaviours across multiple environments. In our study, we found evidence for both perspectives: the relative importance of the social environment varied between regimes.
Individuals in the ‘simultaneous patch’ regime maintained a similar level of behavioural plasticity across two different social contexts (original and shuffled groups), supporting the domain-generality of this behaviour. If individuals do not change their behaviour according to their social group, this might favour social selection, that is, for individuals to choose the ‘best’ group to join (e.g. Saltz 2011). There is support for this in sticklebacks: sticklebacks prefer to associate with familiar individuals (Barber & Ruxton 2000; Ward et al. 2002), groups of familiar sticklebacks find more food overall (Ward & Hart 2005) and share food more equitably with each other than non-familiar sticklebacks (Utne-Palm & Hart 2000). We also found tentative support that individual switch delay while alone was related to their switch delay while in a social group; however, this result was not strictly significant, potentially due to our low sample size. This suggests that individuals may inherently differ in their response to a new food source, but that the presence of conspecifics may alter the benefits of this type of plasticity.
In contrast, results from the ‘sequential patch’ regime supported the hypothesis that behaviour is strongly context-specific. Individuals in this regime did not display consistent individual differences in switch delay between the two social groups, demonstrating that the current social situation heavily influenced behaviour. The failure to detect variation in plasticity in the ‘sequential patch’ regime was caused by low levels of between-individual variation in switch delay: individuals may have been displaying consistent levels of plasticity over time (low within-individual variation), but they did not consistently differ from one another (Fig. 2). All individuals appeared to switch quickly, as would be expected given experience with the competitive regime as only individuals that switched would receive food in the second period of the trial. Interestingly, differences among the three original groups accounted for a significant portion of variation, suggesting that individuals may also have been using social cues from one another about when to switch. Similar among-group behavioural differences have been found in other studies (Mathot et al. 2011) and might be expected as sticklebacks are a schooling fish and behavioural synchrony within social groups is often vital for species that rely on social defenses against predation (Magurran & Pitcher 1987; Webster & Hart 2006).
Although there is accumulating evidence that between-individual variation in behavioural plasticity is common, we still know little about the ecological factors contributing to its evolution. Our study provides strong evidence that competition can play a key role in promoting variation in behavioural plasticity. The field of animal personality will continue to progress as more studies test the predictions of models that articulate when and why we expect to observe consistent individual differences in behaviour.
Supplementary Material
Acknowledgments
We thank Max Wolf, Niels Dingemanse, members of the Bell laboratory, the editor and two anonymous referees for helpful comments on this manuscript. We are indebted to Dan Sewell and Justin McGrath for help with the R coding. We thank Kelly Hogan and Nick Yohanna for help with the hours of behavioural observations and video coding.
Footnotes
AUTHORSHIP
KLL & AMB designed the experiment, KLL conducted the experiment and analysed the data, KLL & AMB wrote the manuscript
Additional Supporting Information may be downloaded via the online version of this article at WileyOnline Library (www.ecologyletters.com).
References
- Barber I, Ruxton GD. The importance of stable schooling: do familiar sticklebacks stick together? Proc R Soc B. 2000;267:151–155. doi: 10.1098/rspb.2000.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp G. Learning rules for social foragers: implications for the producer-scrounger game and ideal free distribution theory. J Theo Biol. 2000;207:21–35. doi: 10.1006/jtbi.2000.2153. [DOI] [PubMed] [Google Scholar]
- Bell AM. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus) J Evol Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CAD, et al. The ecology of individuals: incidence and implications of individual specialization. Am Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Clark CJ, Mangel M. Dynamic State-Dependent Models in Ecology. Oxford University Press; New York, NY: 2000. [Google Scholar]
- Coleman K, Wilson DS. Shyness and boldness in pumpkinseed sunfish: individual differences are context-specific. Anim Behav. 1998;56:927–936. doi: 10.1006/anbe.1998.0852. [DOI] [PubMed] [Google Scholar]
- Dall SRX, Houston AI, McNamara JM. The behavioural ecology of personality: consistent individual differences from an adaptive perspective. Ecol Lett. 2004;7:734–739. [Google Scholar]
- David M, Cezilly F, Giraldeau LA. Personality affects zebra finch feeding success in a producer-scrounger game. Anim Behav. 2011;82:61–67. [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol Evol. 1998;13:77–81. doi: 10.1016/s0169-5347(97)01274-3. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Dochtermann N. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J Anim Ecol. 2013;82:39–54. doi: 10.1111/1365-2656.12013. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Wolf M. Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim Behav. 2013 doi: 10.1016/j.anbehav.2012.12.032. [DOI] [Google Scholar]
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. Fitness consequences of avian personalities in a fluctuating environment. Proc R Soc B. 2004;271:847–852. doi: 10.1098/rspb.2004.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse NJ, Wright J, Kazem AJN, Thomas DK, Hickling R, Dawnay N. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol. 2007;76:1128–1138. doi: 10.1111/j.1365-2656.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Dingemanse NJ, Kazem AJN, Reale D, Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Dubois F, Morand-Ferron J, Giraldeau LA. Learning in a game context: strategy choice by some keeps learning from evolving in others. Proc R Soc B. 2010;277:3609–3616. doi: 10.1098/rspb.2010.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fretwell SD, Lucas HJ. On territorial behaviour and other factors influencing habitat distribution in birds. Acta Biotheor. 1970;19:16–36. [Google Scholar]
- Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Soft. 2010;33:1–22. [Google Scholar]
- Houston AI, McNamara JM. Models of Adaptive Behaviour. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Huntingford FA. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback, Gasterosteus aculeatus. Anim Behav. 1976;24:245–260. [Google Scholar]
- Johnson JC, Sih A. Precopulatory sexual cannibalism in fishing spiders (Dolomedes triton): a role for behavioral syndromes. Behav Ecol Sociobiol. 2005;58:390–396. [Google Scholar]
- Magnhagen C, Bunnefeld N. Express your personality or go along with the group: what determines the behaviour of shoaling perch? Proc R Soc B. 2009;276:3369. doi: 10.1098/rspb.2009.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A, Pitcher T. Provenance, shoal size and the sociobiology of predator-evasion behaviour in minnow shoals. Proc R Soc B. 1987;229:439–465. [Google Scholar]
- Mathot KJ, van den Hout PJ, Piersma T, Kempenaers B, Reale D, Dingemanse NJ. Disentangling the roles of frequency- vs. state-dependence in generating individual differences in behavioural plasticity. Ecol Lett. 2011;14:1254–1262. doi: 10.1111/j.1461-0248.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- Mathot KJ, Wright J, Kempenaers B, Dingemanse N. Adaptive strategies for managing uncertainty may explain personality-related differences in behavioural plasticity. Oikos. 2012;121:1009–1020. [Google Scholar]
- McNamara JM, Stephens PA, Dall SRX, Houston AI. Evolution of trust and trustworthiness: social awareness favours personality differences. Proc R Soc B. 2009;276:605–613. doi: 10.1098/rspb.2008.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski M. Competitive resource sharing: an experimental test of a learning rule for ESSs. Anim Behav. 1984;32:233–242. [Google Scholar]
- Milinski M. Long-term memory for food patches and implications for ideal free distributions in sticklebacks. Ecology. 1994;75:1150–1156. [Google Scholar]
- Morand-Ferron J, Gi-Mike W, Giraldeau LA. Persisent individual differences in tactic use in a producer-scrounger game are group dependent. Anim Behav. 2011;82:811–816. [Google Scholar]
- Nakagawa S, Schielzeth H. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev. 2010;85:935–956. doi: 10.1111/j.1469-185X.2010.00141.x. [DOI] [PubMed] [Google Scholar]
- Reale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. Integrating animal temperament within ecology and evolution. Biol Rev. 2007;82:291–318. doi: 10.1111/j.1469-185X.2007.00010.x. [DOI] [PubMed] [Google Scholar]
- Saltz JB. Natural genetic variation in social environment choice: context-dependent gene-environment correlation in Drosophila melanogaster. Evolution. 2011;65:2325–2334. doi: 10.1111/j.1558-5646.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Sih A, Watters JV. The mix matters: behavioural types and group dynamics in water striders. Behaviour. 2005;142:1417–1431. [Google Scholar]
- Sih A, Bell AM, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol. 2004;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Smith BR, Blumstein DT. Fitness consequences of personality: a meta-analysis. Behav Ecol. 2008;19:448–455. [Google Scholar]
- Stamps JA, Groothuis TGG. Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differencese. Philos Trans R Soc B-Biol Sci. 2010;365:4029–4041. doi: 10.1098/rstb.2010.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utne-Palm AC, Hart PJB. The effects of familiarity on competitive interactions between threespined sticklebacks. Oikos. 2000;91:225–232. [Google Scholar]
- Ward AJW, Hart PJB. Foraging benefits of shoaling with familiars may be exploited by outsiders. Anim Behav. 2005;69:329–335. [Google Scholar]
- Ward AJW, Botham MS, Hoare DJ, James R, Broom M, Godin JGJ, et al. Association patterns and shoal fidelity in the three-spined stickleback. Proc R Soc B. 2002;269:2451–2455. doi: 10.1098/rspb.2002.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MM, Hart PJB. Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav Ecol Socio. 2006;60:77–86. [Google Scholar]
- Webster MM, Laland KN. Evaluation of a non-invasive tagging system for laboratory studies using three-spined sticklebacks Gasterosteus aculeatus. J Fish Biol. 2009;75:1868–1873. doi: 10.1111/j.1095-8649.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- Wolf M, van Doorn GS, Weissing FJ. Evolutionary emergence of responsive and unresponsive personalities. PNAS. 2008;105:15825–15830. doi: 10.1073/pnas.0805473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Van Doorn GS, Weissing FJ. On the coevolution of social responsiveness and behavioural consistency. Proc R Soc B. 2011;278:440–448. doi: 10.1098/rspb.2010.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.