Abstract
Activation of fibroblasts and their differentiation into myofibroblasts, excessive collagen production and fibrosis occurs in a number of bladder diseases. Similarly, conversion of epithelial cells into mesenchymal cells (EMT) has been shown to increase fibroblasts like cells. TGF-β1 can induce the EMT and the role of TGF-β1-induced EMT during bladder injury leading to fibrosis and possible organ failure is gaining increasing interest. Here we show that EMT and fibrosis in porcine bladder urothelial (UC) cells are Smad dependent. Fresh normal porcine bladder urothelial cells were grown in culture with or without TGF-β1 and EMT markers were assessed. TGF-β1 treatment induced changes in cellular morphology as depicted by a significant decrease in the expression of E-cadherin and corresponding increase in N-cadherin and α-SMA. We knocked down Smad2 and Smad3 by Smad specific siRNA. Downregulation of E-cadherin expression by TGF-β1 was Smad3-dependent, whereas N-cadherin and α-SMA were dependent on both Smad2 and Smad3. Connective tissue growth factor (CTGF/CCN2), matrix metalloproteinase-2 and -9 (MMP-2, MMP-9) has been shown to play important roles in the pathogenesis of fibrosis. Induction of these genes by TGF-β1 was found to be time dependent. Upregulation of CTGF/CCN2 by TGF-β1 was Smad3 dependent; whereas MMP-2 was Smad2 dependent. Smad2 and Smad3 both participated in MMP-9 expression. TGF-β1 reprogrammed mesenchymal fibroblast like cells robustly expressed collagen I and III and these was inhibited by SB-431542, a TGF-β receptor inhibitor. Our results indicate that EMT of porcine bladder UC cells is TGF-β1 dependent and is mediated through Smad2 and Smad3. TGF-β1 may be an important factor in the development of bladder fibrosis via an EMT mechanism. This identifies a potential amenable therapeutic target.
Keywords: TGF-β1; EMT; Smad2, and Smad3; Bladder fibrosis
Introduction
The bladder is a complex organ and large reservoir for storage of urine. Storage and emptying of urine requires coordinated interaction between bladder and central nervous system control loci. Physiologic dysfunctions of bladder causes bladder wall stress from stored urine, which results in the changes of apparent gene expression and causes changes in the bladder architecture. Severe partial bladder outlet obstruction (pBOO) is a common bladder dysfunction in adults with benign prostatic hyperplasia. In addition, pBOO often occurs in children with congenial abnormality of lower urinary tract and leads to voiding dysfunction. pBOO shows initial inflammatory response to stress, smooth muscle hypertrophy and eventual transition to fibrosis (Metcalfe et al. 2010). Fibrosis is characterized by excessive deposition of extracellular matrix (ECM) and is associated with overgrowth, hardening and scarring of tissues and is a frequent complication in chronic diseases of the lung, liver, kidney, heart and bladder (Iwano et al. 2002; Guarino et al. 2009). In the human adult bladder increased transforming growth factor – β1 (TGF-β1) is known to play a critical role in bladder fibrosis, altered collagen expression, smooth muscle atrophy and diminished compliance (Deveaud et al. 1998).
The TGF-β superfamily constitutes a large group of secreted polypeptides that play diverse roles during embryonic development (Siegel and Massague 2003). Apart from its role in fibrosis TGF-β1 was shown to significantly affect the normal growth and development of many different organs, including the bladder (Liu et al. 2010) via activation of diverse signaling pathways (Attisano and Wrana 2002). Members of the TGF-β superfamily, including TGF-βs, activins and bone morphogenetic protein (BMPs), mediate their pleiotropic effects by signaling through transmembrane serine/threonine kinase type I receptors and II. Binding of TGF-β1 to heterodimeric complexes of type I and type II TGF-β receptors leads to the phosphorylation of the type I receptors by constitutively active type II receptor. The activated type I receptor kinase phosphorylates Smad2 and Smad3, which then form a complex with Smad4 that translocate to the nucleus and regulates gene expression negatively or positively (Shi and Massague 2003). Studies have shown that Smad2 and Smad3 might mediate different actions of TGF-β1 signaling (Moustacas et al 2001). For instance, Smad3 has been established as a mediator of EMT in normal mammary epithelial cells (Piek et al. 1999). Furthermore, our group has recently shown requirements for Smad2 and Smad3 in bladder α-smooth muscle cell formation during embryonic bladder development (Islam et al. 2013).
Conversion of epithelial cells into mesenchymal cells occurs through a cellular program called epithelial-to-mesenchymal transition (EMT), which is a critical factor during development and tissue repair (Peinado et al. 2007). During embryogenesis, EMT plays an initial role in germ layer reorganization and later in organogenesis. The reorganization of the actin cytoskeleton in epithelial cells allows cells to migrate through basement membranes into other anatomical areas and tissues. When epithelial cells undergo EMT, changes occur in gene expression. Cells degrade the basement membrane to become migratory, with several epithelial markers such as, E-cadherin, cytokeratins being lost and mesenchymal markers, N-cadherin, fibronectin and α-SMA, induced (Zavadil and Bottinger 2005).
The molecular mechanisms underlying EMT in the bladder in response to TGF-β1 remains mostly unclear. TGF-β relays signals through Smad-dependent and alternative mitogen activated protein kinase (MAPK) pathways and both pathways have been shown to be involved in EMT (Derynck and Zhang 2003). TGF-β1 has been reported to induce EMT in many cells and tissues and is able to convert epithelial cell to mesenchymal cells and to differentiate fibroblasts to myofibroblasts (Kasai et al. 2005). In addition to TGF-β1, several other growth factors have been identified to function similarly as TGF-β1. One such example is connective tissue growth factor (CTGF/CCN2), which is produced by fibroblasts and is known to regulate extracellular matrix deposition via Smad signaling (reviewed by Verrecchia and Mauviel 2002). Numerous reports have indicated a role for EMT during tissue injury leading to organ fibrosis that is associated with progressive kidney diseases (Iwano et al. 2002). Recent studies have suggested that similar to TGF-β1, CTGF/CCN2 is widely regarded as mediator of fibrogenesis. CTGF/CCN2 is a member of the CCN family of proteins that regulate many biological functions including extracellular matrix synthesis, cellular proliferation and migration (Chen and Lau 2009). CTGF/CCN2 functions as a downstream mediator of TGF-β activity and plays important role in development and normal wound healing (Leask and Abraham 2003). CTGF/CCN2 is normally expressed at a low level, but in fibrotic conditions its expressions dramatically increases (Shi-Wen et al. 2008) with upregulation of extracellular matrix MMP-2 and MMP-9 production that further contributes to EMT by disrupting the basement membrane. However, the molecular mechanism that underlies the coordinated effects of TGF-β1, CCN2 and ECM production remains unclear in the process of bladder EMT and fibrosis.
Previous reports have shown that the pig is a suitable model to study bladder functions (Kumar et al. 2004). In this paper, we demonstrate that Smad2 and Smad3 are functionally linked to TGF-β1-induced EMT in porcine bladder UC cells. We show that Smad2 and Smad3 play specific roles in the TGF-β1-dependent expression of E-cadherin, N-cadherin and α-SMA in porcine bladder UC cells. In addition CTGF/CCN2, matrix metalloproteinase-2 and -9 (MMP-2, MMP-9) show at a time dependent induction by TGF-β1. We further show that upregulation of CTGF/CCN2 by TGF-β1 is Smad3 dependent, whereas MMP-2 is Smad2 dependent. Smad2 and Smad3 together produce significant effects on MMP-9 expression and collagen deposition. Our findings suggest that TGF-β1 plays a critical role in the induction of EMT and fibrosis that appears to be functionally dependent on Smad2 and Smad3.
Materials and methods
Reagents and antibodies
TGF-β1 was purchased from R&D System (Minneapolis, MN, USA). SB-431542 was purchased from SellTek Chemicals USA. RPMI-1640 was obtained from GIBCO (GIBCO, Canada). siRNA (small interfering RNA) targeting Smad2 and Smad3 and non-targeting control siRNA were obtained from Applied Biosystems (Austin, TX, USA). The transfection reagent Lipofectamine RNAiMAX was purchased from Life Technologies (Invitrogen, Carlsland, CA, USA). Dispase II was purchased from Roche (Laval, Quebec, Canada). Rabbit anti-E-cadherin, goat anti-N-cadherin, mouse anti-cytokeratin5, goat anti-cytokeratin14, rabbit anti-phospho-Smad2 and Smad3, goat anti-GAPDH, and rabbit anti-goat HRP conjugated secondary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), rabbit anti-α-SMA from Abcam (Cambridge, MA, USA), rabbit anti-CTGF/CCN2 from Cell Application Inc and mouse anti-MMP-2 and mouse anti-MMP-9 from New Markers (Freemont, CA). Mouse anti-collagen I and anti-collagen III were a kind gift from Dr Darius Bagli, The Hospital For Sick Children. VectaShield mounting medium with DAPI was obtained from Vector laboratories (Burlington, ON, Canada).
Pig bladder urothelial cell isolation and culture
Ten bladders from female pigs (25–30 kg) were used for this study and procedures were performed according to the CCAC and animal care guidelines of the Hospital for Sick Children. After euthanization, fresh bladders were resected and placed in sterile PBS. The urothelial cell layer was mechanically separated, minced with a sterile scalpel and washed in sterile PBS. Tissue fragments were then enzymatically digested in Dispase II solution (2.4U/ml) at 37°C and stirred slowly until the tissue dissociated. The dispersed cells were separated from the residual tissues by centrifugation and resuspended in RPMI 1640 (GIBCO, Canada) supplemented with 10 % fetal bovine serum (FBS). Cells were separated by centrifugation at 1500 rpm for 5 min and resuspended in RPMI 1640 supplemented with 10 % FBS, 1 % penicillin/streptomycin and epithelial cell growth factor (25 ng/ml). Cells were plated in 6-well plates at approximately 1 × 106 cells/well and incubated at 37°C in 5 % CO2 until confluent. Unless otherwise stated, passage 1 cultures at 90 % confluence were used for all experiments. In some experiments, cells were serum-starved overnight before the addition of TGF-β1. Cells were harvested by washing with PBS, trypsinized and recovered by centrifugation at 1500 rpm for 5 min.
AlamarBlue cell viability assay
AlamarBlue, which measures mitochondrial activity, was used to quantitatively measure cell survival. Preliminary experiments determined that the relative fluorescence of AlamarBlue correlated with cell number. UC cells were seeded at a density of 5 × 103 cells/well (48-well plate) in RPMI 1640 supplemented with 10 % FBS. Cells were allowed to adhere for 1 day and changed into media varying concentrations (0, 20, 40, 60, and 100 pM and 2, 4, and 6nM) of TGF-β1. UC cells were allowed to differentiate for 12 h, 24 h and 48 h. A total of 10 % AlmarBlue reagent was added to the culture and incubated at 37°C for 4 h Fluorescence intensity was determined in a microplate reader with excitation at 540 nm, emission at 590 nm and cutoff at 550 nm (Protocol provided by BioSource). Percent survival versus control (untreated) was reported as the mean +/− standard deviation.
In-situ TUNEL apoptosis assay
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) was performed as described by manufacturer (Millipore, CA, USA). Briefly, porcine primary UC cells were cultured on glass cover slips in a 48-well plate. Cells were washed with PBS, fixed with 4 % paraformaldehyde, and permeabilized with 0.1 % Triton X-100 in 0.2 % BSA. After washes, cells were incubated with TdT end-labeling cocktail for 60 min at room temperature followed by a PBS wash for 2 min. Cells were then stained with avidin-FITC, mounted and visualized by fluorescence microscopy Q-Imaging Retiga 2000R, Olympus U-TV1 X (Japan). The apoptotic index (AI) was determined at 60X magnification as the proportion of TUNEL positive cells relative to the total number of cells per cover slip. Three different fields were counted to calculate the mean AI values.
Immunofluorescence
Selective primary antibodies were used to characterize the cells types present in the culture using immunofluorescence. Positive and negative controls were used to confirm the presence and absence of epithelial markers and mesenchymal markers in the cultures. Porcine primary UC cells were grown on sterile glass cover slips overnight at 37o C. Unless otherwise specified, all labeling procedures were conducted at room temperature. Cells were washed twice with PBS and fixed in pre-cooled Methanol at −20°C for 20 min. The cells were incubated in 4 % BSA for 30 min to block non-specific binding to IgG and then briefly washed with PBS. Cells were incubated with primary antibody diluted in 4 % BSA as follows: Rabbit anti-E-cadherin (1:100), goat anti-N-cadherin (1:100), goat anti-cytokeratin5 (1:100) mouse anti-cytokeratin14 (1:100), and rabbit anti-α-SMA (1:250), mouse anti-collagen I (1:200) and rabbit anti-collagen III (1:250). After primary antibody incubation, cells were washed three times with PBS for 5 min each and then incubated with secondary antibodies diluted in 4 % BSA in a dark chamber as follows: Alexa Fluor 594 chicken anti-mouse IgG (H + L), Alexa Fluor 594 goat anti- rabbit IgG (H + L), and donkey anti-goat (FITC). Cells were washed three times with PBS, mounted with aqueous mounting medium with DAPI and examined in a fluorescence microscope using Q-Imaging Retiga 2000R, Olympus U-TV1 X (Japan) to capture images.
SMADs siRNA design and transfection
Smad2 and Smad3 siRNAs were designed using the Ambion siRNA design tools. The sequences for Smad2 and Smad3 siRNAs are: Smad2 siRNA: sense 5′ GAGUUCACUCCACAUUCUCtt 3′, anti-sense 5′GAGAAUGUGGAGUGAACUCtt 3′; Smad3 siRNA: sense: 5′AUACGAUAGAUCAGUGGGAtt 3′ and anti-sense UCCCACUGAUCUAUCGUAUtt 3′. The transfection mixture was prepared separately by incubating (i) 10 μl of Lipofectamine-RNAiMAX and 190 μl of Opti-MEM, (ii) 10 μl of siRNA and 190 μl of Opti-MEM at room temperature. The combination of diluted (i) Lipofectamine-RNAiMAX and (ii) siRNA duplex was preincubated for 30 min at room temperature and then added to cell culture dishes containing RPMI-1640. Transfection was performed for 24 h at 37°C in a CO2 incubator. Medium was then changed to fresh medium for a further 12 h and TGF-β1 (6 nM) was added to the cultures and incubated in 5 % CO2 for 24 h before harvesting for analysis.
Western blotting
Overnight serum-starved cultures of UC cells were treated with 0, 0.05, 0.1, 0.5, 1, 2, 4 and 6nM of TGF-β1. Cultured urothelial cells were washed twice with ice cold PBS and lysed in ice-cold RIPA (Sigma, Canada) cell lysis buffer (150 mM sodium chloride, 1.0 % NP-40, 0.5 % sodium deoxycholate, 0,1 % SDS, 50 mM Tris, pH 8.0 and protease inhibitor cocktail). Lysates were cleared by centrifugation at 14,500 rpm for 15 min and 100 μg of each sample was separated by SDS-PAGE gel under reducing condition. Proteins were transferred to Nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) and blots were blocked with PBST with 0.1 % Tween 20 and 5 % fat free milk. The membranes were probed with primary antibody at 4°C overnight and dilutions as follows: rabbit anti-E-cadherin (1:300), goat anti-N-cadherin (1: 300), goat anti-cytokeratin5 (1:300), mouse anti-cytokeratin14 (1:200), rabbit anti-p-smad2 and Smad3 (1:300), rabbit anti-α-SMA (1:100), rabbit anti-CTGF (1:300), mouse anti-MMP-2 (1:200), mouse anti- MMP-9 (1:200), mouse anti-collagen I (1:500), rabbit anti-collagen III (1:200) and goat anti-GAPDH (1:100). Blots were incubated with horseradish peroxidase-labeled secondary antibody for 1 h at room temperature, developed with Super Signal West Pico Chemiluminescent substrate (Thermo Scientific, Rockford, IL, USA) and visualized using hyper film ECL photographic film. Bands were quantified using densitometry AlphaEaseFe, 6.0.0 (Alpha Innotech, USA). Results are expressed as the percentage changes in the band density as compared to control GAPDH values.
Wound healing assay
UC cells were seeded in a 48-well plate on glass cover slips and allowed to adhere overnight at a density of 1 × 105 cells/well in 500 μl culture medium in triplicate. Wells were marked with a straight black line on the bottom for orientation. At the time of 90 % confluence, cells were scratched using a marker guide across the monolayer in each well with a 200 μl pipette tip and non-adherent cells were washed off with medium. Fresh medium was added to the culture and treated with TGF-β1 (6nM) for 48 h. At the end of experiment cells were washed with PBS and fixed in 4 % paraformaldehyde. After 3 times washing in PBS, the cells were stained with 1 % Crystal violet (20 % methanol). Phase contrast light microscopic images (4X magnification) were taken at time point 0 h, 12 h, 24 h and 48 h. Migrated cells were counted manually to quantify the numbers of cells migrated to wound area using NIH Image J program. Each experiment was conducted three times in triplicate and one representative assay is shown.
Statistical analysis
Graphing and statistical analysis were performed with Prism GraphPad statistical software. Unpaired (Student’s) T-test and ANOVA were used to test significance and in all analyses the significance levels were specified at p ≤ 0.05. All the in-vitro experiments were done in triplicates.
Results
Characterization of cultured urothelial cells and TGF-β1 induced cell growth and apoptosis
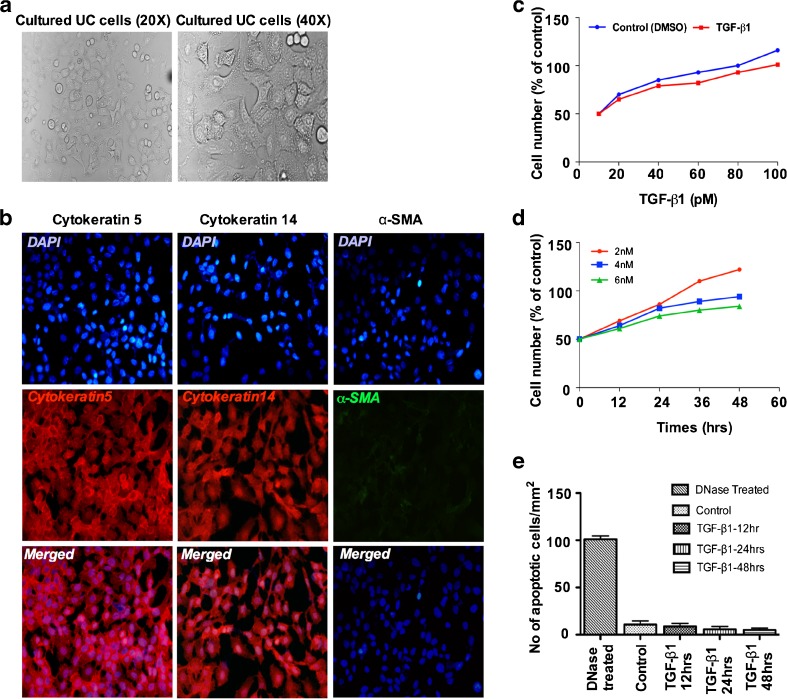
We employed porcine bladder primary urothelial cells (UC cells) to study the mechanisms of TGF-β1 mediated EMT. We first isolated and cultured the UC cells from bladder urothelium, for 5 days and further immunostained the cultures to confirm the UC cell phenotype for the expression of epithelial markers cytokeratin5 and cytokeratin14 versus mesenchymal marker α-SMA. Cultured UC cells appeared epithelial like with a smooth cell surface as confirmed by phase contrast microscopy (Fig. 1a). Epithelial markers cytokeratin5 and cytokeratin14 were abundantly expressed but no α-SMA positive cells could be detected in the cultures (Fig. 1b). We next evaluated the dose response of different concentrations (0, 20, 40, 60, 100 pM) of TGF-β1 on UC cells growth. We found that lower concentrations of TGF-β1 did not significantly inhibited UC cells growth (Fig. 1c). Next examined the effects of higher concentration (2, 4 and 6 nM) of TGF-β1 on cell growth and proliferation of primary UC cells at different time points (0, 12, 24, 36 and 48 h). The AlamarBlue assay showed that treatment with TGF-β1 for 48 h resulted in an increase in cell number but at a slower kinetics (p < 0.001) (Fig. 1d). Given the previous reports of TGF-β1-mediated apoptosis, we then investigated whether TGF-β1 treatment show any effects on apoptosis of UC cells. TUNEL assay did not reveal any apparent induction of apoptosis by TGF-β1. The apoptotic index (AI) score was found to be similar in untreated and TGF-β1 treated UC cells (∼5 % for control, 12 h and 24 h and ∼ 4 % for 48 h (p < 0.7836) (Fig. 1e).
Fig. 1.
Characterization of porcine bladder primary UC cells and TGF-β1 induced growth and apoptosis: Porcine bladder primary UC cells were grown to confluence in RPMI 1640 supplemented with 10 % FBS and Penicillin and streptomycin. a Morphology of cultured UC cells shown under phase contrast microscopy and pebble-like shape and cell-cell adhesion is clearly observed (Magnification X20 and X40). b Characterization of UC cells by immunofluorescence staining. Cytokeratin5 and cytokeratin14 were abundantly expressed in the culture however no α-SMA expression was observed blue-DAPI, red-cytokeratin5 and cytokeratin14, green- α-SMA (Scale bar 2 μM) c Confluent UC cells were incubated with various concentrations (0, 20, 40, 60, 80, and 100 pM) of TGF-β1 for 48 h and Alamar Blue reagent was added to the culture and incubated for 4 h at 37 ° C and the absorbance (OD) was recorded at 540 nm by Gemini Spectrophotometer. No significance differences were detected at all concentrations of TGF-β1 treatment compared to untreated controls, p < 0.05. d TGF-β1 (2nM, 4nM and 6nM) were added to the cultures and incubated for 0, 12, 24, 36 and 48 h. AlamarBlue was added to the culture and incubated for 4 h at 37°C and the absorbance (OD) was recorded at 540 nm by Gemini Spectrophotometer. e TUNEL apoptosis assay of TGF-β1 (6nM) treated porcine primary UC cells (p < 0. 001). The experiments were repeated at least three times. Statistically significant p value was p < 0.0001
TGF-β1 treatment induces EMT related genes in porcine bladder primary UC cells
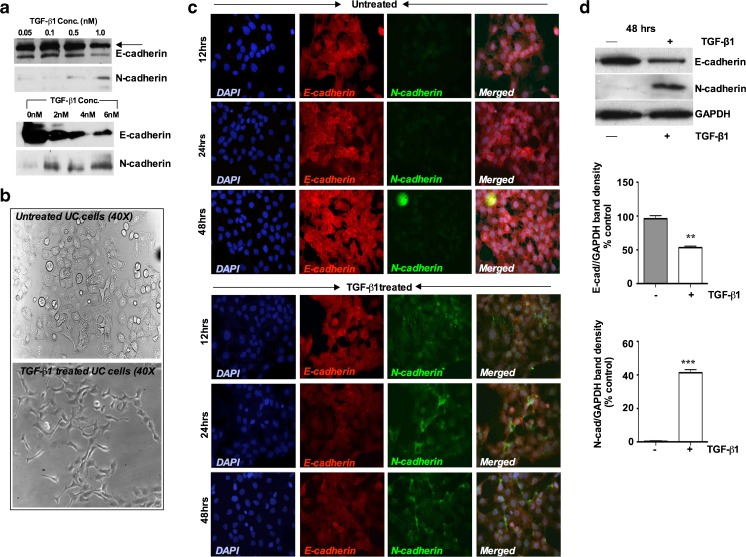
EMT is defined by changes in gene expression in which epithelial marker E-cadherin decreases while mesenchymal markers N-cadherin and α-SMA increase. In order to address the question of whether EMT can occur in this primary UC cells, we first determined the optimal concentration of TGF-β1 sufficient to induce EMT in UC cells. E-cadherin and N-cadherin expression were assessed after treating the cells with TGF-β1 in a dose range from 0.05, 0.1, 0.5 and 1 nM and results show that EMT markers did not show significant expression below 1nM of TGF-β1, however N-cadherin became detectable at 1 nM TGF-β1 (Fig. 2a). We then evaluated higher concentrations of TGF-β1 in the ranges from 0nM, 2nM, 4nM and 6nM. We observed that, 2nM concentration was further induced N-cadherin, a phenotypic marker of EMT while coordinately reducing E-cadherin. A maximal effect on both genes was obtained at 6nM of TGF-β1 (Fig. 2a). We therefore used 6nM TGF-β1 in our subsequent experiments. We then determined the morphological and biochemical changes after treatment with TGF-β1 (6nM) for 48 h. Figure 2b shows the morphological changes of UC cell after treating the cells with TGF-β1 (6nM). Untreated porcine bladder primary UC cells retained an epithelial cobble stone pattern morphology. In the TGF-β1 treated cultures, the cellular morphology changed to a more elongated spindle fibroblast like shape as seen under phase contrast microscopy (Fig. 2b). Since these morphological changes resembled that of EMT, we examined whether the expression and localization of E-cadherin and N-cadherin were concomitantly altered. Immunofluorescence staining for E-cadherin and N-cadherin was performed on these cells in a time dependant manner. Untreated confluent cells uniformly expressed E-cadherin, however no N-cadherin expression could be detected over the 48 h period in the untreated cultures (Fig. 2c). Stimulation with TGF-β1 for 12 h showed sufficient expression of N-cadherin, whereas, after 24 h, marked levels of N-cadherin expression was observed, however the expression was restricted to a fraction of the cells within the monolayer (Fig. 2c). Coordinately expression of E-cadherin in TGF-β1 treated cells disappeared after 24 and 48 h. To confirm the EMT changes at the protein level we assessed the effects of TGF-β1 on E-cadherin and N-cadherin protein expression by Western blot. Immunoblotting of cell lysates demonstrated that E-cadherin protein levels were downregulated by 65 % within 48 h of incubation with TGF-β1 (Fig. 2d). On the other hand, N-cadherin normally did not show any expression in untreated UC cell; however, N-cadherin protein became detectable after 48 h of TGF-β1 treatment (Fig. 2d). The results indicate that E-cadherin expression only diminished to the level undetectable by immunofluorescence while N-cadherin was induced to a high level
Fig. 2.
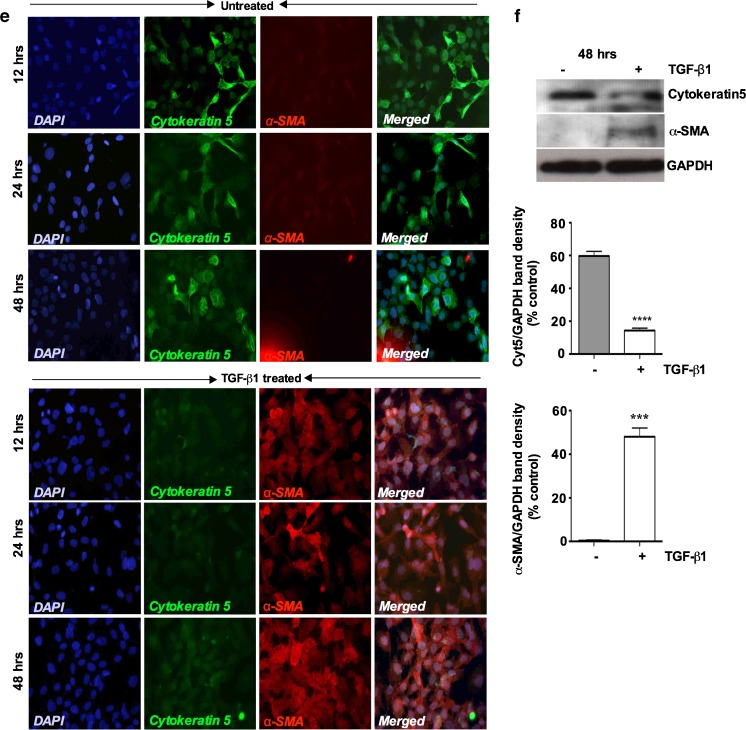
TGF-β1 treatment induces EMT-related genes in porcine bladder UC cells. a Western blot analysis UC cell lysates after different concentration of TGF-β1 treatment. Cultured UC cells were incubated with 0.05, 0.1, 0.5 and 1nM of TGF-β1 in the serum free medium and induction of EMT markers were assessed (Upper panel). Similar experiment was repeated in a higher concentration of TGF-β1 to assess the EMT induction. Cells were treated with 0nM, 2nM, 4nM and 6nM of TGF-β1 for up to 48 h. Western blot analysis showed no significance changes in E-cadherin and N-cadherin expression in lower TGF-β1 concentration. However, at 1nM and 2nM TGF-β1 concentration showed a decrease in E-cadherin expression whereas N-cadherin expression started to increase. b Morphology of the UC cells by phase-contrast microscopy after TGF-β1 stimulation. TGF-β1 treated cells showed a decrease in cell-cell contacts and adopted a more elongated and spindle fibroblasts like shape (Magnification X40). c Immunofluorescence staining showing switching of epithelial marker E-cadherin to mesenchymal marker N-cadherin in a time dependent manner Magnification × 40 (Scale Bar 2 μM). d Representative Western blots showing decreased expression of E-cadherin and increased expression of N-cadherin, in UC cells treated with TGF-β1 (6nM) for 48 h with quantitative densitometric analysis (p < 0.05). e Immunofluorescence staining showing loss of epithelial marker cytokeratin5 and gain of mesenchymal marker α-SMA after TGF-β1 treatment for 48 h (Scale bar 2 μM). f Representative Western blots showing decreased expression of cytokeratin5 and increased expression of α-SMA in UC cells after TGF-β1 treatment for 48 h, with quantitative densitometric analysis (p < 0.05)
We further investigated the expressions of cytokeratin5 and α-SMA in the cultures. Similar to E-cadherin (Fig. 2c), untreated confluent cells uniformly expressed cytokeratin5, whereas α-SMA expression was barely detectable (Fig. 2e). Cytokeratin5 expression was undetectable after 12 h of TGF-β1 stimulation while α-SMA expression first appeared at this same time period (Fig. 2e). After 24 and 48 h, α-SMA expression was significantly elevated, which was also confirmed by Western blot and densitomeric analysis (Fig. 2f) These results indicate that TGF-β1 induces porcine UC cells to undergo EMT changes, which involved in the downregulation of epithelial markers and the upregulation of mesenchymal markers. Again, Western blot revealed a strong but not complete downregulation of the epithelial markers cytokeratin5 and an absolute induction of the mesenchymal α-SMA marker.
TGF-β1 induces phosphorylation of Smad2 and Smad3 and Smad specific siRNA treatment attenuates TGF-β1-induced EMT in cultured porcine bladder primary UC cells
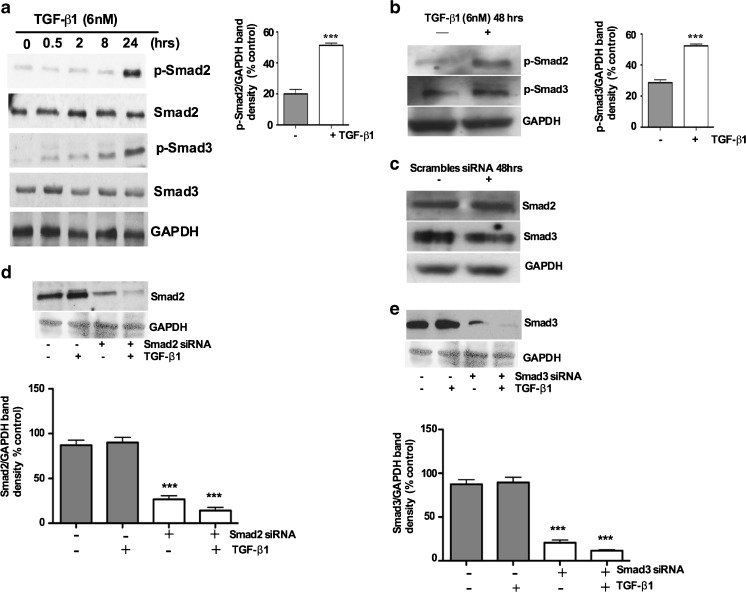
Phosphorylation of C-terminal serine residues in R-Smads by type I receptor kinase is a crucial step in TGF-β family signaling (Abdollah et al. 1997). R-Smads, Smad2 and Smad3 respond to the TGF-β superfamily and become phosphorylated, while Smad1, Smad5 and Smad8 are phosphorylated primarily through BMP signaling. There is now good evidence that Smad2 and Smad3 have distinct functional and non-overlapping roles in TGF-β signaling (Brown et al. 2007). Intracellular factors, which control the relative activation state of Smad2 and Smad3 signaling, have a central role in determining the final outcome of the TGF-β response. To examine the effects of TGF-β1 on the levels of phosphorylated Smad2 and Smad3 in the TGF-β1 treated UC cells we analyzed the expression levels of p-Smad2 and p-Smad3 by Western blot. First we evaluated the activation of p-Smad2 and p-Smad3 expression levels by TGF-β1 in a time ranging from 0, 0.5, 2, 8 and, 24 h. As shown in Fig. 3a both phosphorylated Smad2 and Smad3 were activated in UC cells as early as 24 h after TGF-β1 induction. Smad3 showed a clear expression 8 h after TGF-β1 induction. The basal expression of Smads remained similar for both Smads. In comparison, TGF-β1 significantly increased the levels of p-Smad expression at 24 and 48 h (Fig. 3b). This led us to investigate the roles of Smad2 and Smad3 in TGF-β1-induced EMT in these primary UC cells.
Fig. 3.
TGF-β1 stimulation induces phosphorylation of Smad2 and Smad3 in cultured porcine bladder primary UC cells: Porcine bladder UC cells were cultured as described in Materials and methods. TGF-β1 was added to the cultures and incubated for indicated times (0.5, 2, 8 and 24 h). a Western blots showing time (0, 0.5, 2, 8, 24 h) dependent activation of p-Smad2 and p-Smad3 after TGF-β1 treatment. b Activation of p-Smad2 and p-Smad3 48 h after TGF-β1 treatment compared to untreated (control) cells, with quantitative densitometric analysis (p < 0.01). Porcine bladder UC cells were transfected with Smad2 and Smad3 siRNA and incubated for 24 h at 37°C. TGF-β1 was added to the culture and further incubated for 24 h (total incubation time 48 h). Cells were lysed and expression of Smads was assessed by Western blot. c Smad2 and Smad3 showing unchanged expression of respective Smads in scrambled non-specific siRNA treatment. d and e siRNA treatment resulted in about 65 % reduction in the band density in respective proteins assessed 24 h after the start of transfection, with quantitative densitometric analysis (p < 0.01)
To delineate the role of Smad2 and Smad3 in TGF-β1-induced EMT, we silenced Smad2 and Smad3 expression by Smad specific siRNA knockdown. The results show that there were no apparent effects on Smad2 and Smad3 protein levels by control scrambled non-targeting siRNA (Fig. 3c). In contrast, Smad2 and Smad3 siRNA treatment resulted in an approximately 65 % decrease in the expression levels of respective Smad proteins 48 h after siRNA transfection (Fig. 3d and e). The silencing of Smad2 and Smad3 expression by siRNA knockdown was highly specific for both genes (Fig. 3d and e).
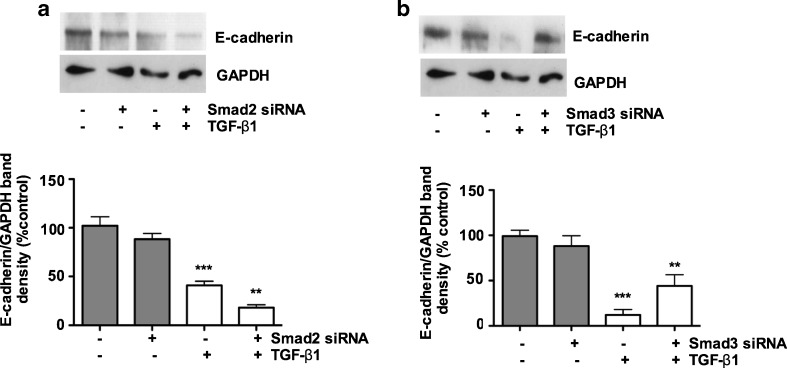
Downregulation of E-cadherin expression in porcine bladder UC cells in response to TGF-β1 is Smad3-dependent
Epithelial cell junctions are critical in maintaining the cell polarization, and expression of EMT related proteins. Downregulation of the epithelial marker E-cadherin represents an early event in the EMT. We therefore investigated whether primary UC cells undergo EMT in response TGF-β1. As shown in Fig. 2a, downregulation of E-cadherin and upregulation of N-cadherin was observed at 1nM of TGF-β1. Here, we found that, TGF-β1 (6nM) stimulation resulted in a 60 % and 80 % decrease in E-cadherin expression in porcine bladder UC cells at the protein level (Fig. 4a and b). To determine whether Smad2 and/or Smad3 mediated TGF-β1–induced downregulation of E-cadherin in these cells, we examined the effects of Smad2 and Smad3 siRNA treatment on E-cadherin expression. The TGF-β1-mediated downregulation of E-cadherin expression was prevented by Smad3 knockdown. In contrast, Smad2 knockdown showed no significant effects on E-cadherin expression (Fig. 4a and b). Neither Smad2 nor Smad3 showed any significant effects on the basal E-cadherin expression. These results illustrate that downregulation of E-cadherin expression by TGF-β1-mediated EMT in porcine bladder urothelial cells may be mediated by Smad3.
Fig. 4.
Downregulation of E-cadherin expression in porcine bladder primary UC cells in response to TGF-β1 is Smad3 dependant: Porcine bladder UC cells transfected with Smad2 and Smad3 siRNA and incubated for 24 h at 37 ° C followed by TGF-β1 treatment for further 24 h. Cells were lysed and an expression level of E-cadherin was assessed by Western blotting. a Silencing of Smad2 did not show significant inhibitory effects in E-cadherin (p < 0.01). b Silencing of Smad3 significantly prevented the inhibitory effects on E-cadherin (p < 0.01)
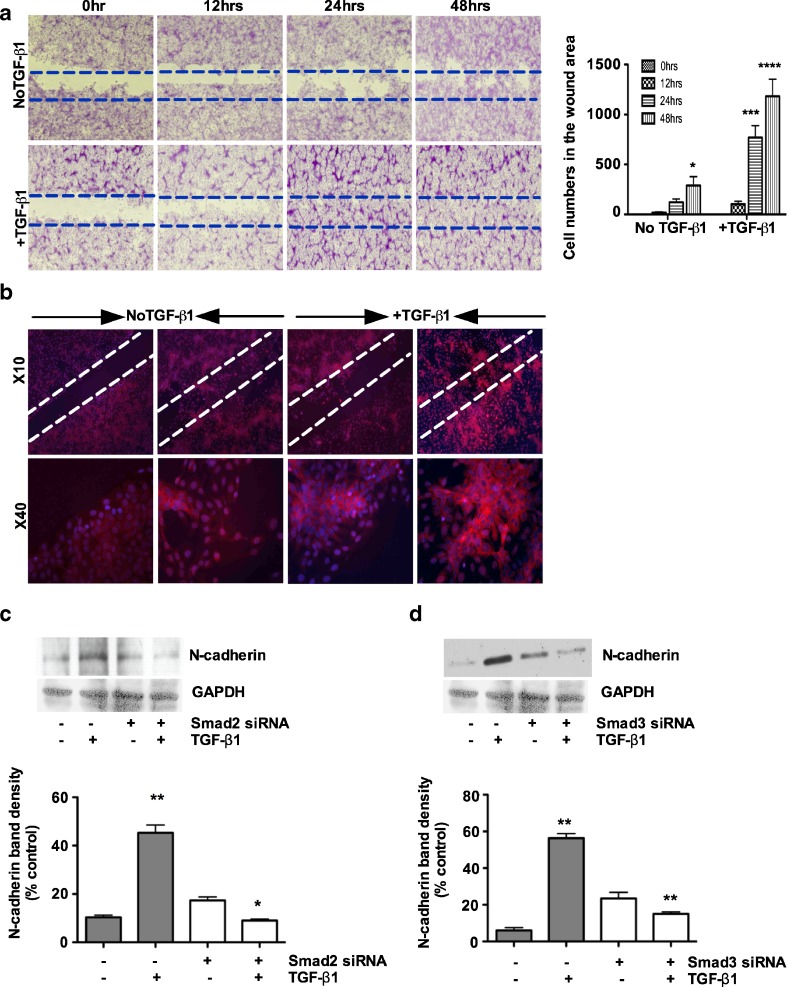
Smad2 and Smad3 regulate TGF-β1 mediated N-cadherin expression in porcine bladder primary UC cells
A previous study had shown that TGF-β induced invasiveness by upregulating N-cadherin (De Wever et al. 2004). Based on this observation, we first investigated the migratory behavior of these cells using the wound scratch assay. We quantified the cells that had migrated into the cell-free wound area during a 48 h post-injury period. We found that UC cells migration was faster by more than 90 % in the cultures treated with TGF-β1 compared to untreated control cultures (Fig. 5a). We next examined the phenotypic expression of N-cadherin in the wound area by immunofluorescence staining. We observed that N-cadherin was expressed by the migrated cells in the wound area treated with TGF-β1 (Fig. 5b). These results suggests a stimulatory effects of TGF-β signaling on UC cell migration and also suggest a role for TGF-β1 in EMT during wound healing. We next silenced Smad2 and Smad3 to investigate their inhibitory effects on TGF-β1-mediated N-cadherin expression at the protein level. As shown in Fig. 5c and d, TGF-β1 stimulation for 48 h resulted in a significant increase in N-cadherin protein expression. Knockdown of Smad2 and/or Smad3 individually showed significant effects on the downregulation of N-cadherin by TGF-β1 (Fig. 5c and d). However, treatment with TGF-β1 did not reverse the expression of N-cadherin in this cell. These observations demonstrate that both Smad2 and Smad3 are required for the expression of the mesenchymal marker N-cadherin during TGF-β1-induced EMT in porcine bladder primary UC cells.
Fig. 5.
Smad2 and Smad3 regulates TGF-β1 mediated N-cadherin expression in porcine bladder primary UC cells: a Confluent monolayers of serum-starved UC cells were wounded with a 200 μL pipette tip. Migration of UC cells was assessed for 48 h after treating the cells with TGF-β1 and viewed under phase contrast microscopy (Magnification × 4) and quantification of wound area was measured by counting the migrated cell numbers. b N-cadherin expression was evaluated in the wound area using immunofluorescence. c and d Porcine bladder primary urothelial cells were transfected with Smad2 and Smad3 siRNA and incubated for 24 h at 37 ° C. TGF-β1 was added to the culture and further incubated for 24 h. Cells were lysed and N-cadherin expression level was determined by Western blotting. TGF-β1 treatment for 24 h resulted in a 3-fold increase in N-cadherin expression. Smad2 knockdown resulted in about 85 % decrease in TGF-β1 mediated N-cadherin expression (p < 0.01). Knock down of Smad3 resulted in about 75 % decrease in TGF-β1-mediated N-cadherin expression (p < 0.01). The results were expressed as mean ± SD (n = 3). (*P < 0.01 and ***p < 0.01 compared with untreated group)
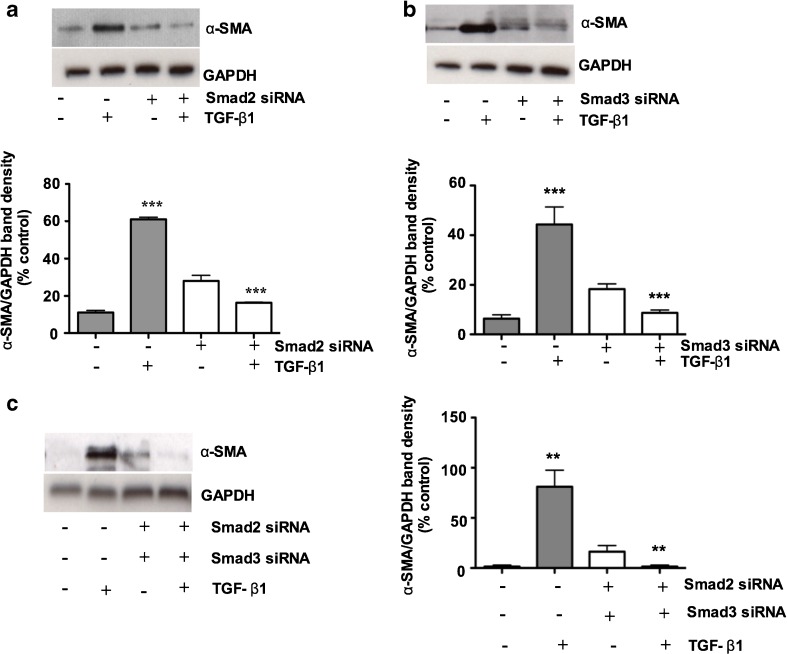
Smad2 and Smad3 regulate TGF-β1-induced α-SMA expression in porcine bladder primary UC cells
α-SMA is one of the signature markers of EMT and is characteristically expressed by myofibroblasts. As we found TGF-β1 stimulation for 48 h resulted in the expression of α-SMA by immunofluorescence and Western blotting (Fig. 2e and f). We therefore sought if Smad2 and/or Smad3 regulate α-SMA expression. We silenced the expression of Smad2 and Smad3 by Smad specific siRNA in the in vitro culture system. We found a 5-fold increase in α-SMA expression after TGF-β1 treatment and α-SMA expression was reduced by Smad2 and Smad3 knockdown (Fig. 6a and b). In individual Smad2 and Smad3 knockdown cell cultures, a small induction (~2 fold increase) by TGF-β1 could still be observed. Consistent with the notion that both Smad2 and Smad3 play overlapping roles in transducing the TGF-β1 signal, we therefore simultaneously knocked down Smad2 and Smad3 and found complete disappearance of α-SMA expression in porcine bladder primary urothelial cells (Fig. 6c), suggesting that the double knockdown brought Smad2 and Smad3 expressions below an effective functional threshold.
Fig. 6.
Smad2 and Smad3 regulate TGF-β1-induced α-SMA expression in porcine bladder primary UC cells: Porcine bladder primary urothelial cells were transfected with Smad2 and Smad3 siRNA and incubated for 24 h at 37°C. TGF-β1 was added to the culture and further incubated for 24 h. Cells were lysed and α-SMA expression level was determined by Western blotting. a TGF-β1 treatment for 24 h resulted in a 3-fold increase in α-SMA expression. Smad2 knockdown resulted in a 50–55 % decrease in TGF-β1 mediated α-SMA expression (p < 0.001). b TGF-β1 treatment for 24 h resulted in a 3-fold increase in α-SMA expression and knock down of Smad3 resulted in a 45–50 % decrease in TGF-β1 -mediated α-SMA expression (p < 0.001) c Simultaneous knockdown of both Smad2 and Smad3 resulted in complete disappearance of TGF-β1 mediated α-SMA expression (p < 0.001)
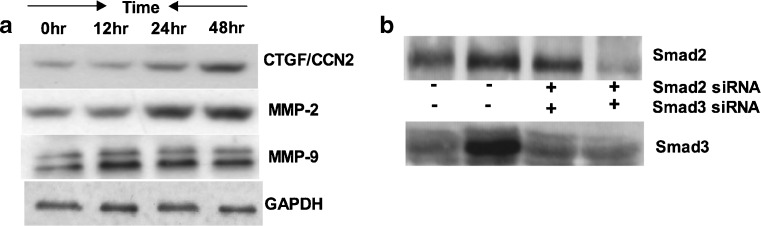
Smad3 regulates TGF- β1 induced upregulation of CTGF/CCN2, whereas upregulation of MMP-2 is Smad2 dependent, and Smad2 and Smad3 both participate in MMP-9 reduction
Studies have shown that TGF-β1 plays a critical role in wound healing, deposition of matrix molecules and induces fibrotic response in vivo (Leask and Abraham 2004). Furthermore, several lines of evidence support the pro-fibrotic actions of CTGF/CCN2 and MMPs in many tissues including kidneys and bladder (Phanish et al. 2010; Metcalfe et al. 2010). To investigate the effects of TGF-β1 on CTGF/CCN2, MMP-2 and MMP-9 expression in porcine bladder primary UC cells, cells were incubated in 6-well plates in the presence or absence of TGF-β1 (6nM) for 0, 12, 24 and 48 h and cellular protein expression were analyzed for CTGF/CCN2, MMP-2 and MMP-9 by Western blot. Our results demonstrated that there was a time-dependent upregulation of CTGF/CCN2, MMP-2 and MMP-9 above baseline expression when cells were stimulated with TGF-β1 (Fig. 7a). CTGF and MMP-2 showed significant increase after 12 h and 24 h and reached the maximum levels after 48 h. In contrast, MMP-9 also showed marked increase after 12 h and remained at the same level after 24 h and 48 h. To establish the role of Smad2 and Smad3 in CTGF/CCN2, MMP-2, MMP-9 expression, we knocked down Smad2 and Smad3 by Smad specific siRNA. The results shown in Fig. 7b resemble the knockdown effects of respective Smads similar to that presented in Fig. 3f and g.
Fig. 7.
TGF-β1 induces CCN2, MMP-2 and MMP-9 in porcine bladder UC cells in a time dependent manner: Porcine bladder UC cells were cultured in 6-well plates. a UC cells were treated with TGF-β1 (6nM) for 0, 12, 24 and 48 h. Whole cell extracted protein was analyzed for CTGF/CCN2, MMP-2 and MMP-9 by Western blot. Results showing increased expression of CTGF/CCN2, MMP-2 and MMP-9 after TGF-β1 treatment. b UC cells were transfected with Smad2 and Smad3 siRNA and incubated for 24 h. TGF-β1 was added to the culture and further incubated for 24 h. Expression levels of Smads was analyzed by Western blot
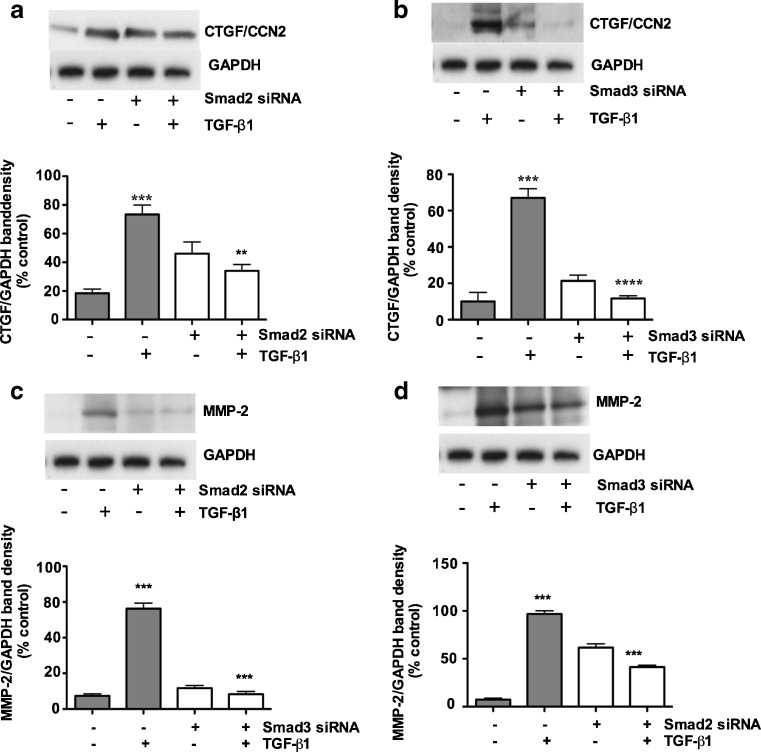
To investigate whether CTGF/CCN2, MMP-2 and MMP-9 expression were Smad2 and/or Smad3 dependent, we silenced Smad2 and Smad3 with Smad specific siRNA. Figure 8a and b show that CTGF/CCN2 expression was significantly downregulated in these siRNA treated cells. Our results also demonstrate that CTGF/CCN2 had minimal effects on the expression of CTGF/CCN2 when Smad2 was knocked down by Smad2 specific siRNA (Fig. 8a), By comparison, TGF-β1 treatment for 48 h significantly upregulated CTGF/CCN2 expression and this induction was markedly reduced when the cultures were treated with Smad3 specific siRNA (Fig. 8b), suggesting that induction of CTGF/CCN2 by TGF-β1 in porcine bladder primary UC cells is Smad3 dependent. In all cases non-targeting scrambled siRNA was used as controls and showed no expression of these genes.
Fig. 8.
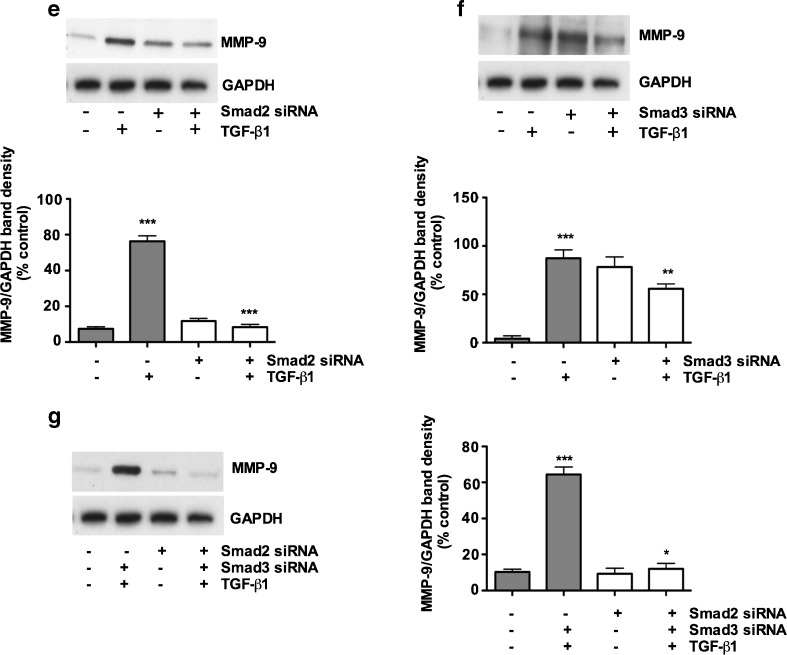
Smad3 regulates TGF- β1 induced upregulation of CCN2, whereas upregulation of MMP-2 is Smad2 dependent, Smad2 and Smad3 both participate in MMP-9 reduction. Porcine bladder UC cells transfected with Smad2 and Smad3 siRNA and incubated for 24 h at 37 ° C. TGF-β1 was added to the culture and further incubated for 24 h. Cells were lysed and silencing of Smads were assessed by Western blotting. a TGF-β1 treatment for 24 h resulted in a significant increase in CTGF/CCN2 expression. Smad2 knockdown resulted in no significant decrease in TGF-β1 mediated CTGF/CCN2 expression (p < 0.01). b TGF-β1 treatment resulted in a significant increase in CTGF/CCN2 expression. Knock down of Smad3 resulted in about 90 % decrease in TGF-β1 mediated CCN2 expression (p < 0.01). c Silencing of Smad2 did cause significant downregulation of MMP-2 and treatment by TGF-β1 prevented the expression of MMP-2 (p < 0.01). d Silencing of Smad3 did not cause significant downregulation in MMP-2 expression. e and f Silencing of Smad2 and Smad3 caused partial abrogation of MMP-9, g Simultaneous knockdown of both Smad2 and Smad3 significantly abrogated MMP-9 expression. Non-targeting scrambled siRNA did not show any effects on CTGF/CCN2, MMP-2 and MMP-9 expression
MMP-2 and MMP-9 play significant roles in proteolysis of the basement membrane. MMP-2 is produced by fibroblasts and endothelial cells, while MMP-9 is produced by inflammatory cells (Hoshino et al. 1998). In this study we investigated TGF-β1 induced MMP-2 and MMP-9 expression in the porcine bladder primary UC cells. We found that TGF-β1 stimulation for 48 h significantly induced MMP-2 and MMP-9 compared to non-targeting scrambled siRNA (Fig. 8c and d). We then investigated which Smad was responsible for TGF-β1 induced MMP-2 and MMP-9 induction. We knocked out Smad2 and Smad3 by Smad specific siRNA. Our results clearly demonstrate that, MMP-2 induction was only inhibited by Smad2 siRNA (Fig. 8c), suggesting a role for Smad2 in the induction of MMP-2. Compared to CCN2 and MMP-2, it appears that MMP-9 induction is associated with both Smad2 and Smad3. We observed that knock down of Smad2 and Smad3 did not significantly abolish MMP-9 expression and a fair amount of MMP-9 expression was still visible (Fig. 8e and f). Consistent with the notion of overlapping roles of Smad2 and Smad3, we performed a simultaneous knockdown for Smad2 and Smad3. As expected simultaneous knockdown of Smad2 and Smad3 significantly reduced the expression of MMP-9 (Fig. 8g), suggesting both Smads may play a role in the induction of MMP-9 expression.
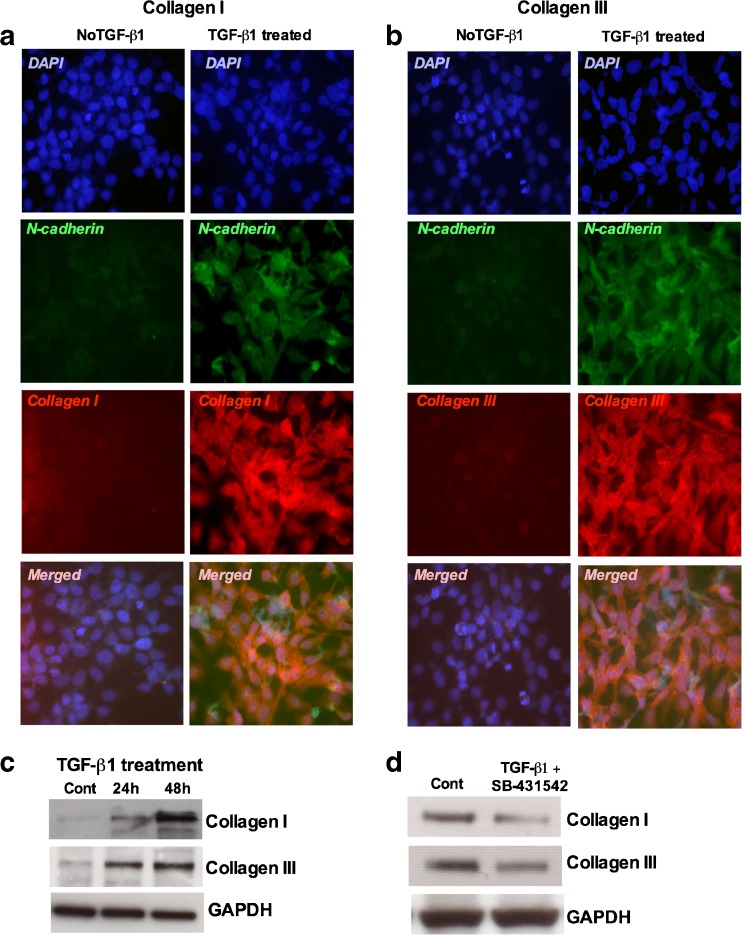
Numerous studies have proposed that several growth factors and cytokines released from fibroblasts have a role in the induction of collagen I and III (Inagaki and Okazaki 2007). TGF-β1 is considered one of the potent regulators of collagen synthesis. We observed that there were significant differences in staining intensity in the TGF-β1 treated cultures with those in controls (Fig. 9a and b). Increased collagen I and collagen III expression was seen 48 h after TGF-β1 treatment concomitant with the expression of N-cadherin (Fig. 9b). The expression levels for collagen I and collagen III were also confirmed by Western blot analysis. A significant increase in collagen I and collagen III expression was seen at the protein level 48 h after TGF-β1 treatment (Fig. 9c). To investigate the role of TGF-β responsive Smads we treated the cultures with a potent TβRI kinase inhibitor SB-431542 and analyzed the expression of collagen I and collagen III at the protein levels. The results revealed that collagen I and collagen III expression decreased significantly in the TβRI kinase inhibitor SB-431542 treated cultures compared to controls (Fig. 9d). Addition of TGF-β1 did not reverse the upregulated expression of either collagen, suggesting potential roles of Smad2 and/or Smad3 in the collagen synthesis.
Fig. 9.
TGF-β inhibitor SB-431542 inhibited the expression of Collagen and Collagen III expression in porcine bladder primary UC cells: Porcine bladder UC cells were cultured as described in Materials and Methods. TGF-β1 (6nM) was added to the culture and incubated for 48 h. a and b Showing the immunofluorescence staining for collagen I and collagen III expression 48 h after TGF-β1 treatment (Scale bar 2 μM). c Western blot showing the expression of collagen I and collagen III at 24 and 48 h. d Cells were treated with SB-431542 (24 h) and TGF-β1 (24 h) and analyzed for collagen I and collagen III by Western blot. GAPDH was used as loading control
Discussion
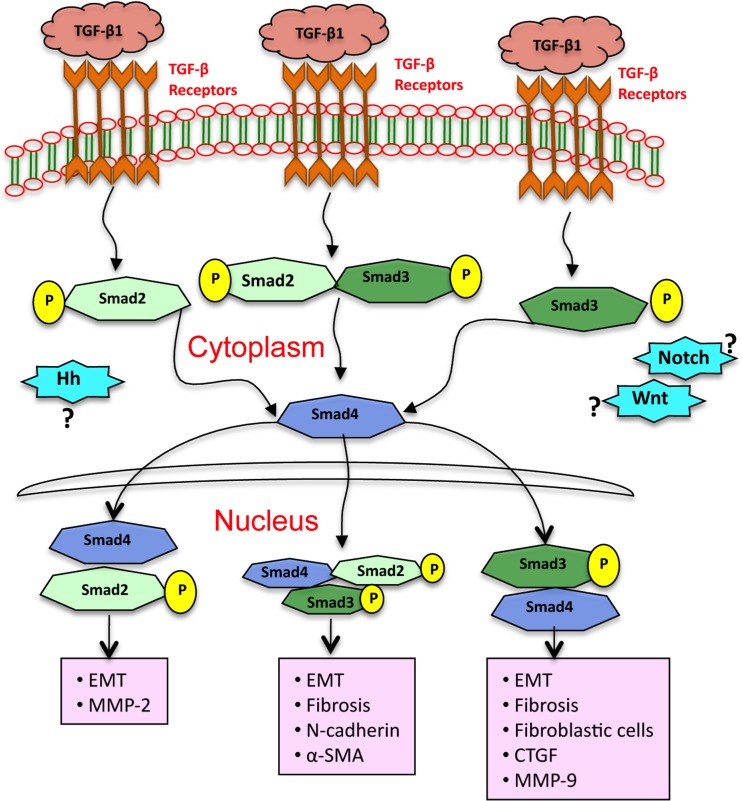
TGF-β1 is a multifunctional cytokine and functions as a master switch in tissue repair and wound healing. There is strong evidence that aberrant expression of TGF-β1 may lead to fibrosis. Furthermore, data suggests that EMT contributes to fibrotic remodeling in many organs including, lung, and kidney. Thus far there is a little evidence that EMT occurs in bladder urothelial cells (UC cells) and if EMT leads to bladder fibrosis and the possible signaling mechanisms in this process. In the present study, we demonstrate that in vitro bladder UC cells are capable to undergo EMT through Smad2 and Smad3 pathways. Additionally, we have shown that TGF-β1 decreases expression of epithelial markers E-cadherin, cytokeratin5 and cytokeratin14 and increases expression of mesenchymal markers N-cadherin, α-SMA and pro-fibrotic markers CTGF/CCN2, MMP-2 and MMP-9. The results further suggest that Smad3 is essential for the downregulation of E-cadherin and induction of CTGF/CCN2. In contrast, Smad2 is essential for the induction of MMP-2 while both Smad2 and Smad3 participate in the upregulation of N-cadherin, α-SMA and MMP-9 in response to TGF-β1 in porcine bladder primary UC cells. In addition, TβRI kinase inhibitor SB-431542 treatment downregulated both collagen I and collagen III expression in the UC cell cultures. These results indicate that primary bladder UC cells activate TGF-β1-induced EMT and fibrosis through Smad pathways. These signals further contribute to expression of ECM deposition in these cells. Figure 10 depicts how TGF-β1operates through cognate receptors to activate intermediate Smads and differentially activate the Smads 2/3/4 transcriptional complexes referenced for EMT and fibrotic responses. The fine-tuning of the process remains to be delineated; however the end points are evident.
Fig. 10.
Schematic illustration depicting how signaling can lead to multiple EMT related changes after TGF-β1 treatment in porcine bladder primary UC cells. Smad2 and Smad3 are necessary for both bladder UC cells EMT and fibrosis. Our data suggest that EMT and fibrosis is initiated by TGF-β1 and mediated by Smad2 and Smad3 dependent expression of EMT markers E-cadherin, N-cadherin and α-SMA and fibrotic markers collagen I, collagen III, MMP-2 and MMP-9
The signal transduction by TGF-β receptors involves two parallel pathways, the Smad proteins and MAPK (Derynck and Zhang 2003). It is thought that Smad2 is critical during embryogenesis while Smad3 contributes to most TGF-β-dependent responses in the adult (ten Dijke and Hill 2004; Massague et al. 2005). TGF-β1 has long been shown to induce EMT in many normal and malignant cells. In addition, EMT has long been considered as a process leading to organ fibrosis (Lan 2003) with Smad3 playing a critical role in the EMT process in diverse cell types (Saika et al. 2004). TGF-β1 induced downregulation of E-cadherin, cytokeratin5 and cytokeratin14 at the protein level was the earliest event in EMT we observed and reached maximal effect within 48 h. Consistent with the downregulation of epithelial markers we observed a gradual upregulation of mesenchymal markers N-cadherin and α-SMA at the same time points, further establishing the capability of UC cells to undergo EMT. A series of in vitro and in vivo studies have strongly established the role of Smad signaling, specifically Smad2 and Smad3 in TGF-β1-induced EMT (Valcourt et al. 2005). We therefore investigated the functional roles of Smad2 and Smad3 in TGF-β1 mediated EMT in porcine bladder primary UC cells. To test our hypothesis, we silenced Smad2 and Smad3 in porcine bladder UC cells by using Smad specific siRNA. We found that silencing of Smad3, but not Smad2, attenuates TGF-β1 mediated EMT as confirmed by loss of epithelial marker E-cadherin and gain of mesenchymal markers N-cadherin and α-SMA. These results are consistent with previous data suggesting the involvement of Smad3 in EMT in other tissues (Phanish et al. 2006). It is now well established that, many fibrotic genes, such as collagens and EMT marker E-cadherin are Smad3 dependent (Piek et al. 2001). In our studies of porcine bladder primary UC cells, TGF-β1 treatment significantly downregulated epithelial marker E-cadherin expression, albeit with slower kinetics. We assume that being a junctional protein this might be due to the stability of E-cadherin protein at the junctions and that, for effective downregulation of E-cadherin, the cells need to achieve a certain level of consistent Smad signaling to function efficiently. Furthermore, it is likely that TGF-β1-dependent regulation of E-cadherin by Smad2 and/or Smad3 is tissue and cell-specific dependent. Given the differential roles of Smads, we suggest further investigations are needed to examine the mechanism of TGF-β1-mediated Smad3 dependent loss of E-cadherin as shown in many cell types, such as, tubular epithelial cells where Snail mediated loss of E-cadherin (Peinado et al. 2003) was observed.
Several in vitro studies have previously shown that TGF-β1 is capable of inducing EMT at lower concentrations (10pM to 500pM) of TGF-β1 (Miettinen et al. 1994; Piek et al. 1999). In comparison, in pig thyrocytes, TGF-β and FGF functioned synergistically while TGF-β alone could not induce EMT as high as 10 ng/ml of TGF-β1 concentration (Grande et al. 2002). Our dose response experiment shows that at the lower concentration of TGF-β1 (2nM) EMT was minimally induced, however overt EMT was documented at 6nM of TGF-β1 suggesting a cell type specific EMT. Thus we note that the UC cells are highly sensitive to TGF-β1. Therefore we postulate that at the low TGF-β1 concentration there could be a requirement for additional growth factors or the higher concentration of TGF-β1 is sufficient to stimulate the EMT mechanism. Concordantly a longer induction period is likely required to switch from the epithelial phenotype. These different results may reflect not only differences in cell types, but also differences in the way involved kinase activities function. The phosphorylation of Smad2 and Smad3 are apparently crucial events during EMT. Smad2/3/4 complex formation depends on a constant TGF-β1 stimulation and phosphorylation of these Smads. It is possible that in the UC cells the degree of Smad phosphorylation has to reach a critical threshold, this taking a longer time to effect subsequent downstream signaling events. An additional explanation may be that UC cells are intrinsically resistant to EMT, thus a longer stimulation time and higher TGF-β1 concentrations are required to activate the EMT mechanism. Additional studies are needed to dissect the complex phenomenon of EMT in UC cells.
We further investigated whether the regulation of mesenchymal phenotypic marker N-cadherin by TGF-β1 is Smad-dependent. N-cadherin is normally expressed in neuroectoderm- and mesoderm-derived tissues (Gumbiner 2005). Furthermore many studies have shown that N-cadherin promotes cell motility. In certain cancers, overexpression of N-cadherin is associated with increased tumor progression when expressed by epithelial cells (Hazan et al. 2000) and inhibition of N-cadherin expression has been shown to block motility and invasion (Diamond et al. 2008). Increased motility is also an important feature of EMT. These observations prompted us to investigate the TGF-β1 mediated N-cadherin expression in porcine bladder primary UC cells. To investigate the N-cadherin expression during wound healing processes, we performed a wound scratch assay. We found that the infiltration of UC cells into the wound area was faster compared to untreated cells suggesting key role for TGF-β1 in the EMT and wound healing processes. Our findings of N-cadherin in the wound area further supports our hypothesis that EMT and fibrosis function concomitantly in UC cells. In a recent study we have shown that TGF-β inhibitor SB-431542 effectively inhibits the phosphorylation of Smad2 and Smad3 and blocked α-SMA formation during embryonic mouse bladder development (Islam et al. 2013). Although increased expression of N-cadherin is TGF-β1 dependent, the mechanism by which TGF-β1 regulates N-cadherin expression in bladder primary UC cells is not known. In the present study, we have demonstrated that TGF-β1 significantly increased the expression of N-cadherin and blocking of Smad2 and Smad3 by siRNA decreased the expression of N-cadherin, suggesting that TGF-β1 mediated N-cadherin expression is regulated by both Smad2 and Smad3. Based on this observation we speculate that Smad2 and/or Smad3 may play combined and independent roles in this process. The molecular details of the induction of N-cadherin contributes to EMT have not been defined clearly therefore further investigation is warranted to fully understand the roles of N-cadherin in bladder EMT and fibrosis.
Acquisition of the TGF-β1 mediated myofibroblastic morphology is another key event in EMT. Myofibroblasts are large spindle-shaped cells, which exhibit long cytoplasmic extensions containing α-SMA positive stress fibers (Deveaud et al. 1998). A well-established modulator of myofibroblasts and smooth muscle cells is TGF-β1, which has also been shown to play multiple roles in vascular remodeling and wound healing (Bobik 2006; Leask and Abraham 2004). During wound healing, TGF-β1 activates fibroblasts to elicit contraction and production of extracellular matrix component. This is also associated with fibrosis, which leads to an abundance of myofibroblasts that cause scaring (Leask and Abraham 2004). TGF-β1 may have differential and opposite effects depending on the tissue or cell type (Robert and Sporn 1993). It has been established that TGF-β1 plays a vital role via activation of Smads although other factors are known to act concomitantly with Smad-dependent and Smad -independent pathways to induce gene transcription (Chen et al. 2006). On the other hand, the mechanism by which Smad signaling regulates α-SMA expression in bladder urothelial cells remains unclear. In this study we first tested for the expression of α-SMA in untreated UC cell cultures. No expression of α-SMA was found in the absence of TGF-β1, but stimulation of UC cells by TGF-β1 robustly increased the expression of α-SMA at the protein level. Furthermore, knock down of Smad2 and Smad3 by siRNA revealed that both Smad2 and Smad3 cooperated to regulate α-SMA expression in TGF-β1-induced EMT of bladder urothelial cells, suggesting key regulatory and cooperative roles of Smad2 and Smad3 in TGF-β1 mediated EMT.
Now the questions remains if both Smad2 and Smad3 form a better interaction with Smad4 or both Smad2 and Smad3 are needed to target the Smad2/Smad3/Smad4 complex for N-cadherin and α-SMA expression regulation. One theory is that, Smad2 and Smad3 may have a different subset of target genes and regulate distinct cellular process. Smads must cooperate with other transcription factors to activate or repress target genes. Smad was shown to activate p38 and subsequently Rho during TGF-β1-induced endothelial barrier dysfunction (Lu et al. 2006). On the other hand Rho was shown to modulate Smad2 and Smad3 phosphorylation and regulate N-cadherin (Diamond et al. 2008) and α-SMA (Chen et al. 2006) expression. We assume that the questions raised by the Smad complex regulation of N-cadherin and α-SMA expression during EMT are far from being answered; other molecules might be also involved in these regulation. Accumulating evidence obtained from different model systems of TGF-β-induced EMT suggest that several parallel signaling systems cooperate in order to execute the complex cellular program of EMT (Sebe et al. 2008). We observed both a-SMA and N-cadherin expression when cells were treated with siRNA but to a significantly lesser degree. It is obvious that disturbing baseline Smad signaling alone starts to switch phenotypic programming. Our observations suggest for effective EMT programming it is necessary to drive the process with TGF-β1, which then recruits the appropriate Smad signaling pathway. We cannot rule out the involvement of other signaling cascades in this system. However, further studies are needed to establish these possibilities.
Many recent reports demonstrate that the induction of CTGF/CCN2, MMP-2 and MMP-9 by TGF-β1 in tissue biopsies with induction in culture at detectable levels (Iwano et al. 2002; Shi-Wen et al. 2008). Consistent with these data, we have also demonstrated that TGF-β1 stimulates a time dependent increase in CTGF/CCN2, MMP-2 and MMP-9 in porcine bladder UC cells, suggesting that overproduction of CTGF/CCN2, MMP-2 and MMP-9 could be a potential pathway leading to fibrosis mediated by mechanism involving TGF-β responsive Smad signaling. The mechanisms that mediate the TGF-β1 induced CTGF/CCN2; MMP-2 and MMP-9 in porcine bladder UC cells is unknown. We used Smad specific siRNA to knock down Smad2 and Smad3, which enabled us to investigate the role of Smad2 and Smad3 in TGF-β1 induced differential cellular response of CTGF/CCN2, MMP-2 and MMP-9 in porcine bladder UC cells. We found that, Smad3, but not Smad2 is required for TGF-β1 mediated CTGF/CCN2 expression, whereas MMP-2-induced expression requires Smad2, but not Smad3. These results suggest Smad3 and Smad2 are essential for the induction of pro-fibrotic markers CTGF/CCN2 and MMP-2 proteins in response to TGF-β1. We further observed detectable changes in the expression of Smad dependent MMP-9 expression. Consistent with the notion that, both Smad2 and Smad3 may play overlapping roles, we therefore simultaneously knocked down Smad2 and Smad3 in the cultures. We found that, the simultaneous double knock down of Smad2 and smad3 significantly abrogated TGF-β1-induced MMP-9 expression, suggesting both Smads are involved in the upregulation of MMP-9. MMP-2 activity is thought to be an important contributor to EMT by facilitating basement membrane or ECM breakdown (Lenz et al. 2000). Smad3 and Snail have also been identified as key pro-fibrotic molecules, which contribute to induction of EMT in hepatic fibrosis (Saika et al. 2004; Flanders 2004). Taken together these findings propose that Smad2 and Smad3 pathways are involved in CTGF/CCN2, MMP-2 and MMP-9 production in porcine bladder UC cells. In addition, with the increase in CTGF/CCN2, MMP-2 and MMP-9 expression, it is reasonable to speculate that porcine UC cells undergoing EMT gain a migratory phenotype during fibrosis. Although Smad3 is widely studied in relation to the EMT concept, we found Smad2 was also associated with EMT and therefore cannot rule out an intrinsic role for Smad2 in EMT and fibrosis. Similar to TGF-β1, CTGF/CCN2 promotes fibroblast proliferation and ECM production. Furthermore, altered collagen composition has been shown in the hypertrophic bladder with increased collagen I and collagen III expression during fibrosis (Kim et al. 2000). In our studies, we found that UC cells showed increased levels of collagen I and collagen III expression in the TGF-β1 treated cultures corroborating the observations on CTGF/CCN2, MMP-2 and MMP-9. Notably, the expressions of both collagen I and collagen III were downregulated when the cultures were treated with SB-431542 suggesting potential roles for Smad2 and/or Smad3 in collagen synthesis. Previous studies have shown that collagen I and collagen II expression were dependent on Smad3 in vitro system (Verrecchia, et al. 2001). Furthermore, Smad3 deficient mice showed diminished type I pro-collagen mRNA expression in the lung compared with wild type mice after bleomycin injury (Zhao and Hoffman 2002).
In conclusion, our results provide additional insights into bladder EMT, potentially a critical mechanism in bladder fibrosis. We demonstrated that TGF-β1 is a potent inducer of EMT and fibrosis in porcine bladder primary UC cells in vitro via Smad2 and Smad3 mechanisms. While the mechanism appears complex, the results provided in this study could open the door for future investigation of EMT in human bladder epithelial cells, which in turn could contribute to a better understanding of bladder fibrosis and to development of new therapeutic approaches.
Acknowledgments
Competing interest
The authors declare that they have no competing interests.
Authors’ contribution
SSI designed the study, performed experiments, wrote the manuscript and analyzed the results, RBM, YEH performed experiments, MAA, MAU assisted in the manuscript preparation, HY, WAF designed the study, critically analyzed the results and assisted in the writing the manuscript.
Abbreviations
- CTGF
Connective tissue growth factor
- EMT
Epithelial-to-mesenchymal transition
- TGF-β1
Transforming growth factor β1
- MMP
Matrix metalloproteinase
- pBOO
Partial bladder obstruction
- UC
Urothelial cells
Contributor Information
Syed S. Islam, Email: syed.islam@sickkids.ca
Reza Bayat Mokhtari, Email: reza.mokhtari@sickkids.ca.
Yaser El Hout, Email: vicryl90@hotmail.com.
M. A. Azadi, Email: maazadi@yahoo.com
M. Alauddin, Email: alauddin_bmb@yahoo.com
Herman Yeger, Email: hermie@sickkids.ca.
Walid A. Farhat, Phone: +1-416-8136580, FAX: +1-416-8136461, Email: walid.farhat@sickkids.ca
References
- Abdollah S, Macia-Silva M, Tsukazaki T, Hayashi H, Attisano L, Wrana JL. TβRI phosphorylation of Smad2 on Ser465 and Ser467 is required for Smad2-Smad4 complex formation and signaling. J Biol Chem. 1997;272:27678–27685. doi: 10.1074/jbc.272.44.27678. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana J. Signal transduction by TGF-β superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- Bobik A. Transforming growth factor βs and vascular disorders. Arterioscler Thromb Vasc Biol. 2006;26:1712–1720. doi: 10.1161/01.ATV.0000225287.20034.2c. [DOI] [PubMed] [Google Scholar]
- Brown KA, Pietenpol JA, Moses HJ. A tale of two proteins: differential roles and regulation of Sma2 and Smad3 in TGF-beta signaling. J Cell Biochem. 2007;101:9–33. doi: 10.1002/jcb.21255. [DOI] [PubMed] [Google Scholar]
- Chen S, Crawford M, Day RM, et al. RhoA modulates Smad signaling during transforming growth factor β-induced smooth muscle differentiation. J Biol Chem. 2006;281:1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependant and Smad-independent pathways in TGF-β family signaling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- De Wever O, Westbroek W, Verlose A, Bloemen N, Bracke M, Gespach C, Bryuneel E, Mareel M. Critical role of N-cadherin in myofibroblast invasion and migration in vitro stimulated by colon cancer derived TGF-β or wounding. J Cell Sci. 2004;117(20):4691–4703. doi: 10.1242/jcs.01322. [DOI] [PubMed] [Google Scholar]
- Deveaud CM, Macarak EJ, Kucich U, Ewalt DH, Abrams WR, Howard PS. Molecular analysis of collagens in bladder fibrosis. J Urol. 1998;160:1518–1527. doi: 10.1016/S0022-5347(01)62606-5. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Sun L, Ottaviano AJ, et al. Differential growth factor regulation of N-cadherin expression and motility in normal and malignant oral epithelium. J Cell Sci. 2008;121:2197–2207. doi: 10.1242/jcs.021782. [DOI] [PubMed] [Google Scholar]
- Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelia-to-mesenchymal transition (EMT) through MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci. 2002;15:4227–4236. doi: 10.1242/jcs.00091. [DOI] [PubMed] [Google Scholar]
- Guarino M, Tosoni A, Nebuloni M. Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition. Hum Pathol. 2009;40:1365–1376. doi: 10.1016/j.humpath.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Natl Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton I, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immonol. 1998;102:783–788. doi: 10.1016/S0091-6749(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Inagaki Y, Okazaki I. Emerging insight into transforming growth factor beta/Smad signaling in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SS, Mokhtari RB, Kumar S, Maalouf J, Arab S, Yeger H, Farhat W. Spatio-temporal distribution of Smads and roles of Smad/TGF-β/BMP-4 in the regulation of mouse bladder development. PLoS ONE. 2013;8(4):e63140. doi: 10.1371/journal.pone.0061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta 1 induces human alveolar epithelial cells to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Yoon JY, Seo SI, Hwang TK, Park YH. Effects of partial bladder obstruction and its relief on type I and type III collagen and detrusor contractility in the rat. Neurourol Urodyn. 2000;19:29–42. doi: 10.1002/(SICI)1520-6777(2000)19:1<29::AID-NAU5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chapple CC, Chess-Williams R. Characteristics of adenosine triphosphate releaser from perception from porcine and human normal bladder. J Urol. 2004;172:744–747. doi: 10.1097/01.ju.0000131244.67160.f4ABSTRACT. [DOI] [PubMed] [Google Scholar]
- Lan HY. Tubular epithelial-myofibroblast transdifferentiations mechanisms in proximal tubule cells. Curr. Opin. Nephrol. Hyertens. 2003;12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein in fibroblast biology. Biochem Cell Biol. 2003;81:355–366. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Lenz O, Elliot SJ, Stevenson WG. Matrix metalloproteinase’s in renal development disease. J Am Soc Nephrol. 2000;11(3):574–581. doi: 10.1681/ASN.V113574. [DOI] [PubMed] [Google Scholar]
- Liu B, Feng D, Lin G, et al. Signaling molecules involved in mouse bladder smooth muscle cellular differentiation. Int J Dev Biol. 2010;54(1):175–180. doi: 10.1387/ijdb.082610bl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Harrington EO, Jackson H, Morin N, Shannon C, Rounds S. Transforming growth factor-beta1-induced endothelial barrier dysfunction involves Smad-dependent p38 activation and subsequent Rho activation. J Appl Physiol. 2006;101:375–384. doi: 10.1152/japplphysiol.01515.2005. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19(23):2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Metcalfe PD, Wang J, Jiao H, Huang Y, Hori K, Moore RB, Tredget EE. Bladder outlet obstruction: progression from inflammation to fibrosis. BJU Int. 2010;116(11):1686–1694. doi: 10.1111/j.1464-410X.2010.09445.x. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner P, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127(6):2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustacas A, Souchelnytsky S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Peinado H, Quintanilo M, Cano A. Transforming growth factor β1 induces Snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transition. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat Rev Cancer 415–428 [DOI] [PubMed]
- Phanish MK, Wahab NA, Colville-Nash P, et al. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGF-β1 responses in human proximal-tubule epithelial cells. Biochem J. 2006;393:601–607. doi: 10.1042/BJ20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phanish MK, Winn SK, Dockrell ME. Connective tissue growth factor (CTGF, CCN2)- a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp Nephrol. 2010;114:e83–e92. doi: 10.1159/000262316. [DOI] [PubMed] [Google Scholar]
- Piek E, Moustakas A, Kurisaki A, et al. TGF-β type I receptor/ALK-5 and Smad protein mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CC, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor-β signaling in Smad2 and Smad3 deficient fibroblast. J Biol Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- Robert AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor –β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anjano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe A, Leivonen SK, Fintha A, Masszi A, Rosival L, Kahari VM, Mucsi I. Transforming growth factor-β-induced alpha-smooth muscle cell actin expression in renal proximal tubular cells is regulated by p38β mitogen-activated protein kinase, extracellular signal-regulated protein kinase1,2 and the Smad-signaling during epithelial-myofibroblast transdifferentiation. Nephrol Dial Transplant. 2008;23:1537–1545. doi: 10.1093/ndt/gfm789. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Leask A, Abraham DJ. Regulation and function of connective tissue growth factor/CCN2 in tissue repairs, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanism of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–820. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Hill CS. New insight into TGF-beta-Smad signaling. Trends Biochem Sci. 2004;29(5):265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustacas A. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mo, Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. Transforming growth factor- β signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- Zhao BM, Hoffman FM (2002) Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol 282:L585–L593 [DOI] [PubMed]