Abstract
Key pathways like insulin signaling, AMP activated kinase (AMPK) activation and inflammatory signaling are involved in the complex pathological network of hepatic insulin resistance. Our aim is to investigate whether grape seed proanthocyanidins (GSP) and metformin (MET) target any of these pathways in insulin resistant rat liver. Albino Wistar rats were rendered insulin resistant by feeding a high fat-fructose diet (HFFD). Either GSP (100 mg/kg b.w), MET(50 mg/kg b.w) or both were administered to insulin resistant rats as therapeutic options. HFFD-feeding caused hyperglycemia, hyperinsulinemia, increased gluconeogenesis, decreased tyrosine phosphorylation of insulin receptor-β(IR-β) and insulin receptor substrate-1 (IRS-1) and increased serine phosphorylation of IRS-1. The association of p85α subunit of phosphotidyl inositol 3 kinase(PI3K) with IRS-1 and subsequent Akt phosphorylation were reduced while the expression of mitogen activated protein kinases (MAPK) were increased in HFFD rats. Both MET and GSP reduced hyperglycemia and hyperinsulinemia and improved glycolysis, tyrosine phosphorylation of IR-β and IRS-1, IRS-1-PI3K association and Akt activation. However, activation of tumor necrosis factor-α, interleukin-6, leptin and suppressor of cytokine signaling-3 and reduction in adiponectin caused by chronic HFFD feeding were reversed by GSP better than by MET. Activation of AMPK by GSP was much less compared to that by MET. These findings suggest that GSP might activate PI3K pathway and promote insulin action by reducing serine kinase activation and cytokine signaling and MET by targeting AMPK. The beneficial effects were enhanced during combination therapy. Thus, combination therapy with MET and GSP may be considered for the management of metabolic syndrome.
Keywords: High fat-fructose diet, Grape seed proanthocyanidins, Metformin, Insulin signaling, Inflammation
Introduction
Reduced receptivity of the cells to circulatory insulin, a condition termed insulin resistance progresses to type 2 diabetes mellitus (T2D). The metabolic actions of insulin in liver occurs, in response to the activation of the phosphotidyl inositol 3 kinase (PI3K) pathway in which insulin receptor (IR) gets activated by autophosphorylation following binding to insulin. Activated IR then phosphorylates selective tyrosine residues on insulin receptor substrates (IRS) and induces the binding of IRS-1 and 2 to PI3K which then serine phosphorylates and activates Akt or protein kinase B (PKB). Activation of Akt is essential for glucose transport in liver, muscle and adipose tissue, glycogen synthesis in liver and muscle and lipogenesis in adipose tissue.
IRS proteins also undergo serine phosphorylation by IRS kinases like c-Jun amino terminal kinase (JNK), extracellular regulatory kinase (ERK), p38 MAP kinase (p38 MAPK), inhibitor κB kinase (IKK), protein kinase C (PKC), and S6 kinase 1 (S6K1). Phosphorylation of IRS-1 by serine kinases disrupts the interaction between IR and IRS, thus preventing tyrosine phosphorylation of IRS (Werner et al. 2004).
Hepatic insulin resistance is also considered an inflammatory state associated with an upregulation of proinflammatory cytokines. Adipose tissue is crucial for the inflammatory status associated with insulin resistance, primarily due to macrophage infiltration, and secretion of a large number of pro- and anti-inflammatory cytokines like leptin, adiponectin, tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) (Kershaw and Flier 2004). These adipocytokines are important due to their wide range of biological effects on insulin signaling and other metabolic activities (Rabe et al. 2008).
Studies show that a member of the suppressor of cytokine signaling (SOCS) protein family, SOCS-3, binds to the receptors of leptin and insulin rendering them resistant to circulating hormones (Bjorbak et al. 2000; Ueki et al. 2004). SOCS-3 serves as one of the links between insulin resistance and cytokine signaling.
Metformin hydrochloride (MET) is one of the most recommended medicines for the treatment of T2D. It improves insulin sensitivity, reduces gluconeogenesis, promotes glucose uptake and reduces glucose production in the liver. MET is now known to influence glucose uptake, at least in part by increasing 5′-AMP-activated protein kinase (AMPK) activity (Zhou et al. 2001). AMPK is the master regulator of energy homeostasis and is suggested to be a target for the development of drugs aimed at T2D (Winder and Hardie 1999).
Proanthocyanidins are the major polyphenols in red wine as well as in grape seeds and are widely used as nutritional supplements. Proanthocyanidins exhibit antioxidant (Yousef et al. 2009), anti-inflammatory (Terra et al. 2009) and anti-cancer (Kaur et al. 2006) activities. It has been shown that grape seed proanthocyanidins (GSP) reduces oxidative stress, hyperglycemia and hyperinsulinemia in high fructose diet-induced insulin resistant rats (Suwannaphet et al. 2010). A natural mixture of grape polyphenols at nutritional doses to obese first degree relatives of T2D patients effectively prevented fructose-induced oxidative stress, insulin resistance and secured an unwavering metabolic state in them (Hokayem et al. 2013). Oligomeric proanthocyanidins activate the insulin receptor in vitro by inducing the tyrosine phosphorylation of insulin receptor to promote glucose translocation and uptake (Montagut et al. 2010). However, the effects of GSP on phosphotyrosine or phosphoserine status of IRS and on PI3K pathway have not been studied.
Chronic consumption of diet high in fat and fructose induces a proinflammatory state and causes abnormalities in the insulin signaling pathway (Coate et al. 2010). The present investigation was conducted to compare the modulatory effects of GSP and MET on some of the critical nodes associated with insulin signaling and their ability to affect adipocytokines and proinflammatory markers in high calorie diet-induced model of insulin resistance. The combined effect of the phytochemical GSP and MET was also investigated.
Methods
Materials
Proanthocyanidin-rich extract from grape seed (GSP, Gravinol-SuperTM) was kindly provided by Kikkoman Co. (Chiba, Japan). The grape seed extract was composed of 89 % proanthocyanidin, 6 % monomers, and 5 % other materials. All reagents were purchased from Hi media Laboratories Pvt. Ltd., Mumbai, India. Human recombinant insulin (Humulin R) was purchased from Eli Lilly (Indianapolis, IN). Enzyme linked immunosorbent assay (ELISA) kits were purchased for analysing the levels of rat leptin, adiponectin (Invitrogen, Carlsbad, CA, USA), TNF-α, IL-6 (KOMA Biotech, Seoul, South Korea), glucose (Recon diagnostics Pvt Ltd., Baroda, India) and insulin (Accubind microwells, Monobind Inc., CA, USA). Antibodies such as anti-ERK-1, anti-JNK-1, anti-SOCS-3, anti-Akt-1, anti-IR beta, and anti-PI3K were purchased from Santacruz technologies (Santacruz, CA, USA). Anti-p-tyrosine, anti-p-serine, anti-pser473-Akt, anti-p(T183/Y185)-JNK-1, anti-p(T202/Y204)-ERK, anti-p(T180/Y182)-p38 MAPK and anti-pThr172-AMPK were obtained from Cell Signaling Technologies (Danvers, MA, USA). Enhanced chemiluminescence (ECL, Super Signal West Pico chemiluminescent substrate) assay kit was purchased from Thermo Scientific (Rockford, USA).
Animals diet and experimental design
Albino male Wistar rats of body weight 120–150 g were obtained from and maintained at the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai Nagar. The animals were housed under a controlled temperature (22 ± 2 °C) without limitation of access to water and chow. The study was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA). All procedures were approved by the Institutional Animal Ethical Committee (IAEC). The animals were fed with one of the following diets: normal rat chow (control) or high fat-fructose diet (HFFD). The commercial normal chow consisted of 60 % starch, 22.08 % protein and 4.38 % fat, the caloric content of which was 382.61 cal/100 g. HFFD was prepared fresh in our laboratory every week which provided 471.25 cal/100 g. HFFD contained 45 % fructose, 20 % fat (10 % beef tallow, 10 % groundnut oil) and 22.5 % casein.
A total of six groups were treated as follows: Group 1 were fed normal rat chow (CON), group 2 were fed HFFD, group 3 were fed HFFD and supplemented with GSP by gastric intubation (100 mg/kg b.w/day, HFFD + GSP), group 4 rats were fed HFFD and administered with MET through gastric intubation (50 mg/kg b.w/day, HFFD + MET), group 5 rats were fed HFFD and administered GSP and MET (HFFD + GSP + MET) through gastric intubation, and group 6 rats were fed normal rat chow and administered GSP (CON + GSP). Food and water were provided ad libitum to the animals. The total experimental duration was 45 days. GSP and MET were given for the last 15 days of the experimental period. In group 5, the rats were administered GSP first and MET was given 3–4 h later, once daily. At the end of experimental period, the animals in each group (n = 6) were fasted overnight and sacrificed to obtain blood and liver for further analyses.
Preparation of plasma and tissue extracts
Blood was collected by decapitation in heparinised tubes and centrifuged at 1,500 rpm for 10 min. Plasma was separated and used for assays. Total extract of liver was obtained by homogenising liver slices at 10 % (w/v) in extraction buffer (0.1 M Tris HCl buffer, pH 7.4) followed by centrifugation at 1,000 g for 5 min at 4 °C. Supernatants were used for assessing the glycogen content, glycolytic and gluconeogenic enzymes. Protein content was measured by the method of Lowry et al. 1951.
Glucose, insulin and insulin sensitivity indices
Plasma glucose and insulin levels were measured using ELISA kit according to manufacturer’s protocol. Insulin sensitivity indices like homeostasis model assessment (HOMA) and quantitative insulin check index (QUICKI) were computed using appropriate formulae
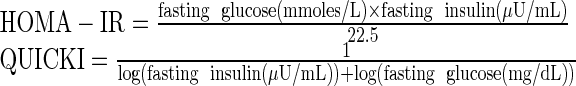 |
Carbohydrate metabolising enzymes and glycogen content in liver
The activities of key enzymes of carbohydrate-metabolism such as hexokinase and pyruvate kinase, glucose 6-phosphatase and fructose 1,6-bis phosphatase and total glycogen content in liver were assayed by methods outlined elsewhere (Anithanandhini et al. 2002).
Pro- and anti-inflammatory adipocytokines
Levels of TNF-α, IL-6, leptin and adiponectin were measured in plasma using ELISA method.
Insulin signaling
Following an overnight fast (∼15 h), animals (n = 4) were anesthetized (ketamine hydrochloride, 30 mg/kg b.w, i.p) and injected intraperitoneally with 15 unit/kg human recombinant insulin. After 30 min, animals were sacrificed. The liver tissue was removed, weighed and homogenised in ice-cold buffer (20 mM Tris–HCl at pH 7.4, 0.25 % SDS, 150 mM NaCl, 1 % NP-40, 0.5 % Triton X-100, 1 mM PMSF, 1 mM EDTA and protease inhibitor cocktail) and centrifuged at 12,000 × g, for 15 min at 4 °C. The supernatant was collected and protein content was measured (Lowry et al. 1951).
Immunoprecipitation
Tyr phosphorylation of IR-β, ser/tyr phosphorylation of IRS-1 and the association of IRS-1 with PI3K were assessed by immunoprecipitation and immunoblotting. Samples containing equal amount of protein from each group were subjected to immunoprecipitation at 4 °C using anti-IR beta or anti-IRS-1. The immune complexes thus formed were precipitated by the addition of 50 μl protein-A-CL agarose slurry to each sample and incubated for 2 h at 4 °C. Immune complex precipitates were pelleted at 4,000 × g for 10 min at 4 °C and washed thrice with homogenization buffer. The pellets were suspended in Laemmli sample buffer and boiled for 5 min. Proteins were resolved by 8–10 % SDS PAGE and electrotransferred onto polyvinylidene fluoride membranes. The membranes were then blocked in Tris buffered saline-Tween 20 (TBST), pH 7.4 containing 3 % bovine serum albumin for 2 h at room temperature. Proteins were immunoblotted with either anti-phospho tyr antibody (for insulin receptor-β and IRS-1) or anti-ser antibody (for IRS-1) or PI3K antibody (for IRS-1). The membranes were washed with TBST, incubated with respective secondary antibodies for 2 h at room temperature. Blots were subsequently stripped and reprobed with the same antibody used for immunoprecipitation to find the total (phosphor and dephospho forms) protein content. Protein bands were detected using ECL kit.
Immunoblotting
Liver homogenates containing equal amount of protein were resolved by 10 % SDS PAGE and processed for western blot analysis as described above. To assess the activation status of Akt, AMPK, SOCS3, JNK, ERK and p38 MAPK, the membranes were incubated overnight at 4 °C with antibodies against SOCS3, phosphoSer473Akt, phosphoThr172 AMPKα, phospho(T183/Y185) JNK, phospho(T202/Y204) ERK, phospho(T180/Y182) p38 MAPK or their respective unphosphorylated forms (total-Akt, AMPK, JNK, ERK, p38 MAPK). The membranes were washed with TBST and incubated with the respective secondary antibody for 2 h at room temperature. Protein band detection and were performed as mentioned earlier.
Densitometry analysis
Quantitative comparison of the protein expression between groups were analysed after obtaining band densities using the inverted images of the protein bands in Image J software. The band density of each group was normalized with the band density of total protein in the respective group or β-actin for SOCS3 expression.
Data analysis
The values were expressed as means ± SD of six rats for all biochemical estimations and means ± SD of four rats for immunoblotting analysis from each group and statistically evaluated by one-way analysis of variance (ANOVA) followed by Bonferroni’s test for multiple comparisons. Value of P < 0.05 was considered statistically significant.
Results
General observations
Consumption of HFFD caused increase in body weight liver weight and liver index as compared to control whereas treatment groups (GSP, MET and combination) showed significantly reduced body and liver weights compared to rats fed HFFD alone (Table 1). Insulin and glucose levels (Table 2) in the HFFD-fed animals were higher than those seen in CON group. Both MET and GSP reduced glucose and insulin levels. However MET treated group showed mild improvement in the levels than GSP administered rats. The group fed HFFD and subsequently treated with both MET and GSP (HFFD + GSP + MET) had glucose and insulin levels closer to those of CON. Between the treatment groups, the combination of MET and GSP reduced glucose and insulin values better than individual treatment.
Table 1.
Body weight, liver weight and liver index of the experimental rats
| Parameters | CON | HFFD | HFFD + GSP | HFFD + MET | HFFD + GSP + MET | CON + GSP |
|---|---|---|---|---|---|---|
| Body wt | ||||||
| Initial (g) | 152.8 ± 11.2 | 154.2 ± 11.3 | 155.4 ± 10.5 | 156.8 ± 11.6 | 154.4 ± 11.9 | 153.6 ± 10.8 |
| Final (g) | 169.3 ± 14.3 | 220.5 ± 12.2a | 183.2 ± 10.3b | 192.3 ± 14.9b | 180.5 ± 13.6b | 170.2 ± 14.6b |
| Liver wt (g) | 7.19 ± 0.3 | 8.70 ± 0.2a | 7.67 ± 0.2b | 8.03 ± 0.6a,f | 7.37 ± 0.4b | 7.27 ± 0.3b,d |
| Liver index (%) | 4.24 ± 0.1 | 3.95 ± 0.2a | 4.19 ± 0.2b | 4.17 ± 0.3b | 4.08 ± 0.1b | 4.27 ± 0.2b |
CON control, HFFD high fat, fructose diet, HFFD + GSP high fat, fructose diet + grape seed proanthocyanidins, HFFD + MET high fat, fructose diet + metformin, HFFD + GSP + MET high fat, fructose diet + grape seed proanthocyanidins + metformin, CON + GSP control + grape seed proanthocyanidins
Liver index (%) = liver weight (g)/body weight (g) × 100
Values are means ± SD of six rats from each group. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Table 2.
Plasma glucose and insulin and insulin sensitivity indices at the end of experimental study
| Parameters | CON | HFFD | HFFD + GSP | HFFD + MET | HFFD + GSP + MET | CON + GSP |
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 82.4 ± 5.75 | 222.1 ± 5.31a | 162.4 ± 4.09a,b | 134.5 ± 6.93a,b,c | 101.4 ± 5.39a,b,c,d | 81.8 ± 4.51b,c,d,e |
| Insulin (μIU/mL) | 16.2 ± 0.77 | 38.9 ± 1.29a | 24.9 ± 0.98a,b | 20.5 ± 1.43a,b,c | 18.3 ± 0.69a,b,c,d | 17.1 ± 0.9b,c,d |
| HOMA | 3.30 ± 0.12 | 18.4 ± 0.35a | 8.12 ± 0.36a,b | 5.70 ± 0.27a,b,c | 4.45 ± 0.22a,b,c,d | 3.46 ± 0.19b,c,d,e |
| QUICKI | 0.43 ± 0.0 | 0.28 ± 0.01a | 0.35 ± 0.01a,b | 0.38 ± 0.01a,b,c | 0.40 ± 0.02a,b,c | 0.42 ± 0.01b,c,d |
HOMA homeostasis model assessment, QUICKI quantitative insulin check index, CON control, HFFD high fat, fructose diet, HFFD + GSP high fat, fructose diet + grape seed proanthocyanidins, HFFD + MET high fat, fructose diet + metformin, HFFD + GSP + MET high fat, fructose diet + grape seed proanthocyanidins + metformin, CON + GSP control + grape seed proanthocyanidins
Values are means ± SD of six rats from each group. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
HFFD-fed animals were less sensitive to circulating insulin than the control rats, as expressed by the insulin sensitivity indices HOMA and QUICKI. The combined use of MET and GSP increased the insulin sensitivity similar to that of individual treatment of MET and GSP and the values are statistically different from those of the HFFD group (Table 2).
Glycolysis, gluconeogenesis, and glycogen content in liver
The activities of hexokinase and pyruvate kinase, were decreased in HFFD fed animals compared to control rats but increased in all the three treatment groups compared to that of HFFD rats (Table 3). Individual administration of MET and GSP produced similar effects in restoring the activities of these enzymes but showed significant difference between themselves. MET was more effective than GSP in reducing the activities glycolytic enzymes. Administration of both GSP and MET to HFFD fed rats increased the activation of glycolytic enzymes better than individual treatment.
Table 3.
Activities of insulin-sensitive enzymes and glycogen content in liver at the end of experimental study
| Parameters | CON | HFFD | HFFD + GSP | HFFD + MET | HFFD + GSP + MET | CON + GSP |
|---|---|---|---|---|---|---|
| Hexokinase (A) | 8.06 ± 0.36 | 3.46 ± 0.23 a | 5.74 ± 0.23a,b | 6.80 ± 0.26a,b,c | 7.41 ± 0.35a,b,c,d | 8.13 ± 0.48b,c,d,e |
| Pyruvate kinase (B) | 12.96 ± 0.17 | 5.12 ± 0.38a | 8.95 ± 0.31a,b | 9.96 ± 0.39a,b,c | 11.12 ± 0.58a,b,c,d | 12.95 ± 0.39 b,c,d,e |
| Glucose-6-phosphatase (C) | 4.15 ± 0.21 | 17.23 ± 1.43a | 8.22 ± 0.55a,b | 6.84 ± 0.49a,b,c | 4.88 ± 0.26a,,b,c,d | 3.93 ± 0.19b,c,d,e |
| Fructose 1,6 bis phosphatase (C) | 4.13 ± 0.17 | 10.72 ± 0.67a | 6.04 ± 0.29a,b | 5.73 ± 0.37a,b,c | 5.17 ± 0.34a,b,c,d | 4.13 ± 0.16b,c,d,e |
| Glycogen (D) | 52.14 ± 1.47 | 33.56 ± 2.57a | 42.33 ± 2.35a,b | 48.78 ± 3.07a,b,c | 50.99 ± 3.19b,c | 51.93 ± 3.28b,c,d,e |
A μmol of glucose phosporylated.h−1. mg ptn−1, B μmol of pyruvate formed min−1.mg ptn−1, C μmol of Pi liberated.min−1.mg ptn−1; D-mg of glucose/g tissue, CON control, HFFD high fat, fructose diet, HFFD + GSP high fat, fructose diet + grape seed proanthocyanidins, HFFD + MET high fat, fructose diet + metformin, HFFD + GSP + MET high fat, fructose diet + grape seed proanthocyanidins + metformin, CON + GSP control + grape seed proanthocyanidins
Values are means ± SD of six rats from each group. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Activities of glucose-6-phosphatase and fructose 1,6-bisphosphatase and glycogen content are also presented in Table 3. The activities of these enzymes were higher while glycogen content was lower in HFFD-fed rats compared to control. MET and GSP individually and in combination reduced the activities of gluconeogenic enzymes and restored the glycogen content. Again, MET was more effective than GSP but the combination treatment with both MET and GSP showed best reduction among the three treatments.
Insulin signaling in the liver
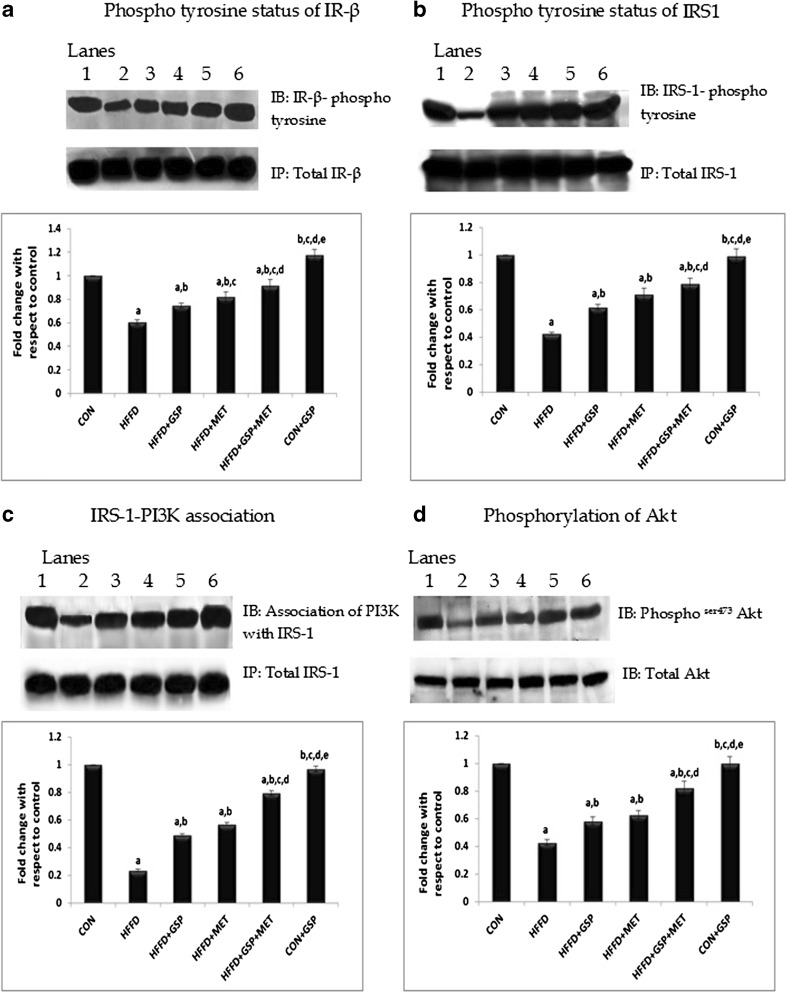
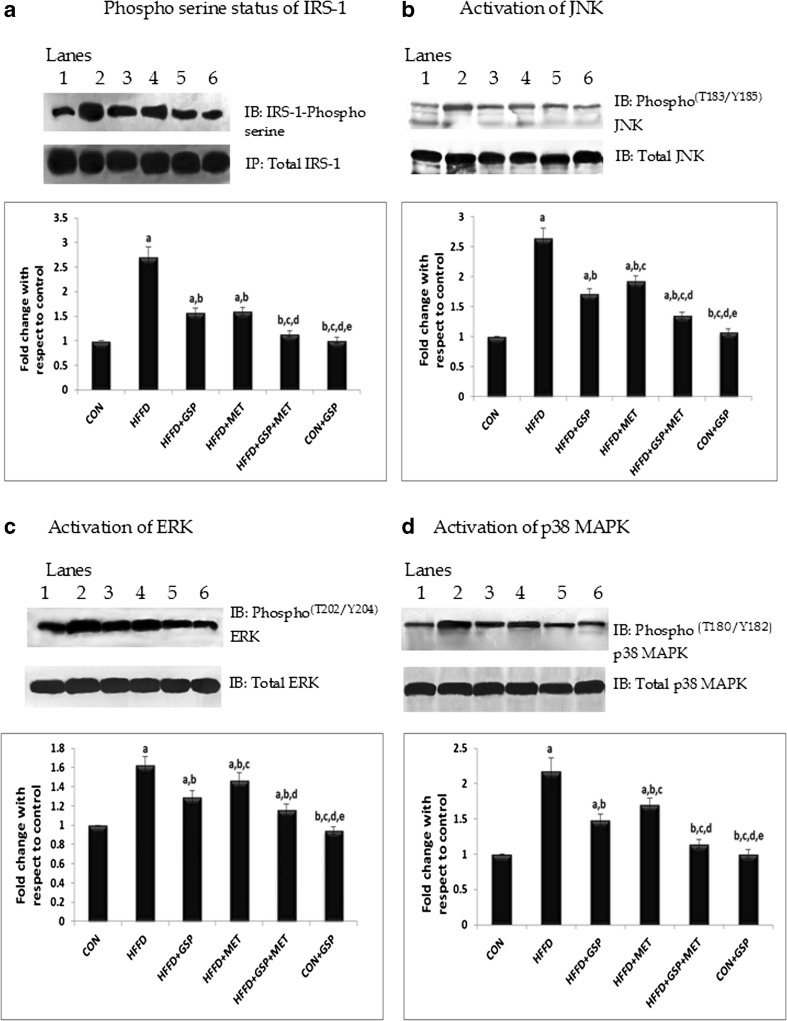
HFFD feeding to rats significantly (P < 0.05) reduced insulin-stimulated tyrosine phosphorylation of IR-β (C: 100 % vs HFFD: 60 %, Fig. 1a) and IRS-1 compared with control (C: 100 % vs HFFD: 42 %, Fig. 1b) whereas the treatment groups showed improved insulin stimulated IRS-1 tyrosine phosphorylation (HFFD + GSP: 62 %; HFFD + MET: 71 %; HFFD + GSP + MET: 79 %) and IR-β tyrosine phosphorylation (HFFD + GSP: 77 %; HFFD + MET: 82 %, HFFD + GSP + MET: 91 %) as compared to rats fed HFFD alone. In addition, HFFD significantly increased the basal serine phosphorylation of IRS-1 (CON: 100 %, HFFD: 270 %, P = 0.02, Fig. 4a) compared with control. GSP and MET when given alone or in combination (HFFD + GSP: 157 %; HFFD + MET: 160 %; HFFD + GSP + MET: 114 %) reduced this HFFD-induced IRS-1 serine phosphorylation. There were negligible variations in the protein levels of insulin receptor and IRS-1 between the groups.
Fig. 1.
Effect of GSP, MET and both on phosphotyrosine status of IR-β and IRS1, association of IRS-1 with PI3K and Akt activation. Lane 1: CON, Lane 2: HFFD, Lane 3: HFFD + GSP, Lane 4: HFFD + MET, Lane 5: HFFD + GSP + MET, Lane 6: CON + GSP. Immunoblots and representative graphs of phosphotyrosine status of IR-β (a) and IRS-1 (b), association status of IRS-1 with PI3K (c) and activation of Akt (d) show reduced induction of insulin signaling in HFFD fed rats compared to control. GSP and MET improved these changes and the combination treatment was found to be more effective than others. For quantitative assessment, the band intensity was measured in a densitometer and normalized with respective total proteins and expressed as fold change with respect to control. The changes relative to control are represented in the bar diagram. Data shown are means ± S.D of four experiments. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Fig. 4.
Effect of GSP, MET and both on phospho serine status of IRS-1, phospho JNK, phospho ERK and phospho p38 MAPK. Lane 1: CON, Lane 2: HFFD, Lane 3: HFFD + GSP, Lane 4: HFFD + MET, Lane 5: HFFD + GSP + MET, Lane 6: CON + GSP. Immunoblots and representative graphs of phosphoserine status of IRS-1 (a), JNK (b), ERK (c) and p38 MAPK (d) show increased serine phosphorylation of IRS1 in HFFD fed rats compared to control. GSP attenuated serine phosphorylation of IRS1 by reducing JNK, ERK and p38 MAPK activation better than MET. The combination of GSP and MET brought down the expression to near normal. For quantitative assessment, the band intensity was measured in a densitometer and normalized with respective total proteins and expressed as fold change with respect to control. The changes relative to control are represented in the bar diagram. Data shown are means ± S.D of four experiments. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
A similar behaviour was seen for the association of IRS-1 and p85 subunit of PI3K (C: 100 %, HFFD: 23.4 %, HFFD + GSP: 49 %; HFFD + MET: 56.9 %; HFFD + GSP + MET: 79.3 %, Fig. 1c).
However, animals that consumed HFFD showed a decrease in insulin-induced Akt serine473 phosphorylation, which was reversed after treatment (C: 100 %, HFFD: 42 %, HFFD + GSP: 58 %, HFFD + MET: 62 %, HFFD + GSP + MET: 82 %; Fig. 1d.)
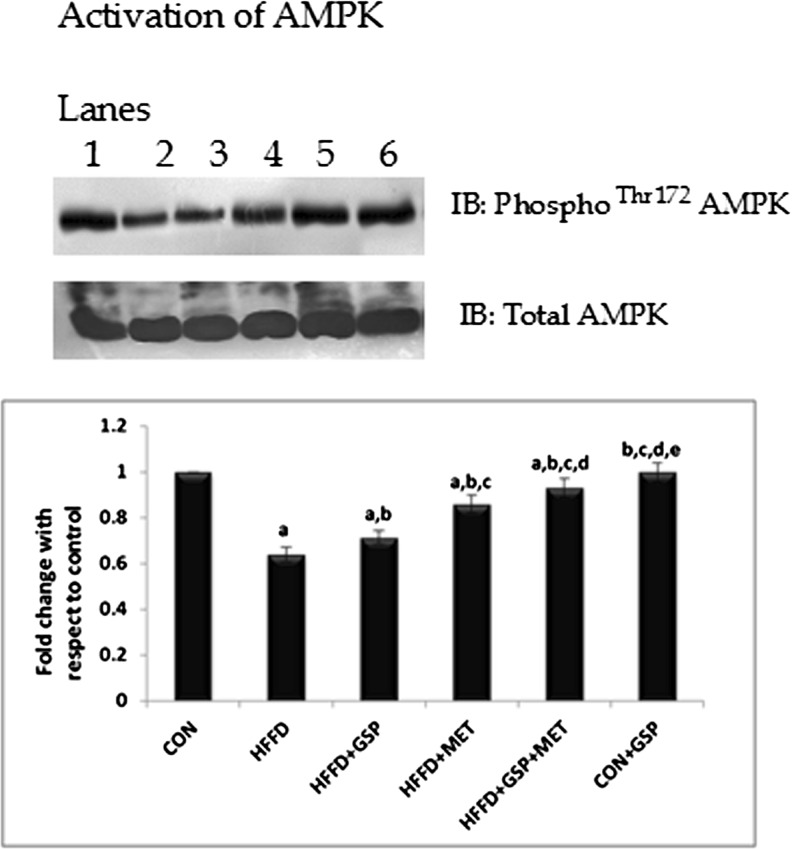
The expression of phospho AMPK in all the six groups was analysed. The study found that, unlike MET, GSP did not improve the expression of p-AMPK drastically in HFFD-fed rats. And hence it will be more prudent to conclude that the increased p-AMPK expression in the group HFFD + GSP + MET could be due to the presence of MET rather than GSP (C: 100 %, HFFD: 63.9 %, HFFD + GSP: 71.4 %, HFFD + MET: 85.9 %, HFFD + GSP + MET: 93 %, Fig. 2.).
Fig. 2.
Effect of GSP, MET and both on the activation of AMPK. Lane 1: CON, Lane 2: HFFD, Lane 3: HFFD + GSP, Lane 4: HFFD + MET, Lane 5: HFFD + GSP + MET, Lane 6: CON + GSP. Immunoblot and representative graph of AMPK phosphorylation show that GSP weakly induces AMPK activation compared to MET, suggesting that GSP may not be a direct activator. The marked increase in AMPK phosphorylation in the combination treatment may be due to the presence of MET. For quantitative assessment, the band intensity was measured in a densitometer and normalized with total protein and expressed as fold change with respect to control. The changes relative to control are represented in the bar diagram. Data shown are means ± S.D of four experiments. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Activation of inflammatory cytokine signals and its supressor
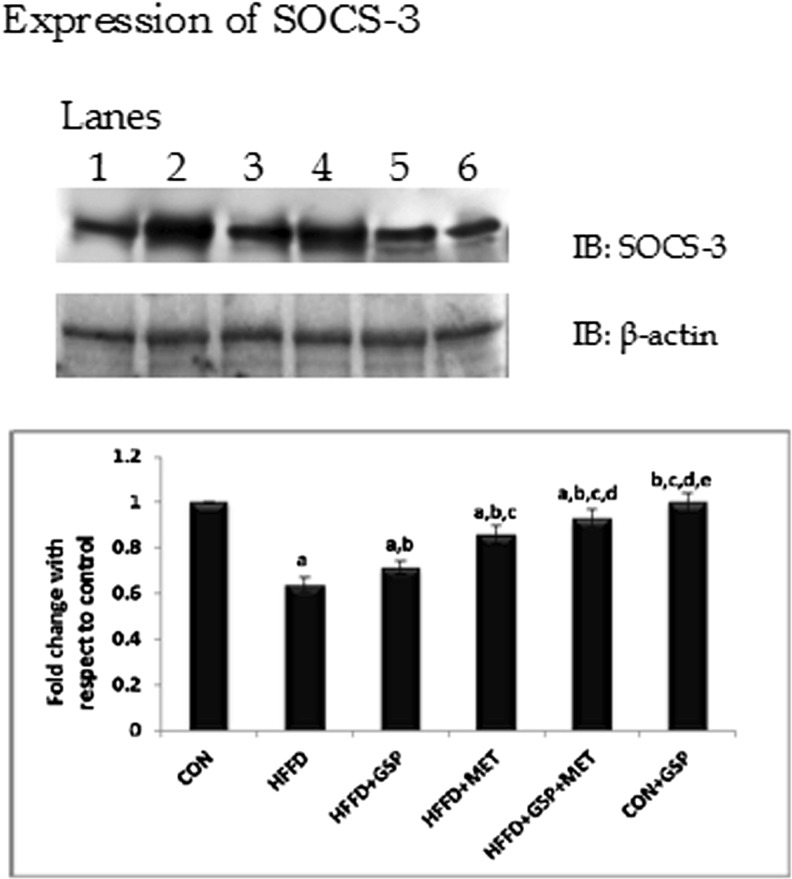
The levels of TNFα, IL-6 and leptin were increased in circulation accompanied by an increase in the expression of the suppressor protein, SOCS-3 in liver of HFFD fed rats indicating inflammation and possibly leptin resistance. Adiponectin levels were decreased in HFFD fed rats. Individual and combination administration of GSP and MET to HFFD fed rats increased adiponectin levels and decreased the levels of TNF, IL-6 and leptin (Table 4). The expression of SOCS-3 increased with HFFD feeding and decreased after treatment (C: 100 %, HFFD: 255 %, HFFD + GSP: 168 %, HFFD + MET: 192 %; HFFD + GSP + MET: 126 %, Fig. 3.).
Table 4.
Levels of adipocytokines in plasma at the end of experimental study
| Parameters | CON | HFFD | HFFD + GSP | HFFD + MET | HFFD + GSP + MET | CON + GSP |
|---|---|---|---|---|---|---|
| Leptin (ng/mL) | 0.31 ± 0.01 | 1.97 ± 0.07a | 0.61 ± 0.03a,b | 1.04 ± 0.04a,b,c | 0.57 ± 0.02a,b,d | 0.32 ± 0.01b,c,d,e |
| Adiponectin (μg/mL) | 4.65 ± 0.23 | 1.87 ± 0.17a | 3.09 ± 0.12a,b | 2.55 ± 0.16a,b,c | 4.07 ± 0.25a,b,c,d | 4.62 ± 0.21b,c,d,e |
| TNF-α (pg/mL) | 18.8 ± 0.81 | 52.9 ± 3.16a | 34.6 ± 2.87a,b | 46.9 ± 1.91a,b,c | 28.2 ± 0.52a,b,c,d | 16.7 ± 0.07b,c,d,e |
| IL-6 (pg/mL) | 75.6 ± 3.01 | 206.5 ± 12.1a | 144.7 ± 10.6a,b | 182.6 ± 13.7a,b,c | 94.2 ± 14.7a,b,c,d | 76.1 ± 3.56b,c,d,e |
CON control, HFFD high fat, fructose diet, HFFD + GSP high fat, fructose diet + grape seed proanthocyanidins, HFFD + MET high fat, fructose diet + metformin, HFFD + GSP + MET high fat, fructose diet + grape seed proanthocyanidins + metformin, CON + GSP control + grape seed proanthocyanidins
Values are means ± SD of six rats from each group. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Fig. 3.
Effect of GSP, MET and both on SOCS-3 expression. Lane 1: CON, Lane 2: HFFD, Lane 3: HFFD + GSP, Lane 4: HFFD + MET, Lane 5: HFFD + GSP + MET, Lane 6: CON + GSP. Immunoblots and representative graph of SOCS-3 indicates that the expression of SOCS3 is increased by HFFD treatment and that GSP reduces the expression of SOCS-3 better than MET. Combination treatment showed better reduction in SOCS-3 expression than individual treatments. For quantitative assessment, the band intensity was measured in a densitometer and normalized with β-actin and expressed as fold change with respect to control. The changes relative to control are represented in the bar diagram. Data shown are means ± S.D of four experiments. a = values significantly different from CON; b = values significantly different from HFFD; c = values significantly different from HFFD + GSP; d = values significantly different from HFFD + MET; e = values significantly different from HFFD + GSP + MET; f = values significantly different from CON + GSP. [One way ANOVA followed by Bonferroni test for multiple comparisons. Value that has p < 0.05 was considered statistically significant]
Mitogen activated protein kinases
Increased p T183/Y185 -JNK was observed in the HFFD fed rats compared with control. The rise in Thr183/Tyr185 phosphorlation due to HFFD was reversed in the treatment groups (C: 100 %, HFFD: 263 %, HFFD + GSP: 170 %, HFFD + MET: 192 %, HFFD + GSP + MET: 134 %, Fig. 4b). HFFD feeding increased the Thr202/Tyr204 phosphorlation of ERK in vivo whereas pT202/Y204-ERK forms were reduced during all the three treatments significantly (C:100 %, HFFD: 162 %, HFFD + GSP: 128 %, HFFD + MET: 146 %, HFFD + GSP + MET: 115 %, Fig. 4c). Similar results were obtained for pT180/Y182-p38 MAPK (C: 100 %, HFFD: 217 %, HFFD + GSP: 148 %, HFFD + MET: 170 %, HFFD + GSP + MET: 114 %, Fig. 4d)
Discussion
This study found for the first time that the administration of either GSP or MET improves hepatic insulin sensitivity by modulating the insulin signaling pathway in HFFD-induced insulin resistant rats. Combination treatment was more effective than individual treatments.
Although MET and GSP were individually effective in improved insulin signaling, the mechanistic actions of each of these seem to be different with respect to the expression pattern of AMPK, proinflammatory cytokines and MAPKs. GSP modulated insulin signaling, by reducing the activation of serine/threonine kinases, like JNK, ERK and p38 MAPK. GSP also activated AMPK significantly in HFFD group, but this increase (7.5 %) was lower than that of the increase induced by MET (22 %). Hence the observed modulation on insulin signaling by GSP might be attributed to reduction in cytokines like TNF-α, IL-6 rather than direct activation of AMPK. MET, on the other hand, improved insulin signalling by increasing the expression of AMPK (14.5 % increase as compared to GSP) and was found to be less efficient in controlling the adipocyte secretion compared to GSP.
Our study proves beyond doubt that the improved insulin sensitivity during all the three treatments is mediated via increased tyrosine phosphorylation of IR-β and IRS-1 followed by activation of Akt. Once Akt gets activated, it signals for the transport of glucose into the cell. Subsequent actions have resulted in the increased glycolysis and decreased gluconeogenesis.
Importance of adipokines in influencing insulin resistance is well documented. TNF-α interferes with early steps of insulin signaling, and causes ubiquitinylation and loss of Akt in the adipocytes (Medina et al. 2005). TNF-α is known to be involved in the disturbance of insulin/IR-initiated signals by activating JNK1 (Miller et al. 1996). This leads to progressive accumulation of IRS1 molecules phosphorylated at Ser307 which in turn hosts unknown signaling molecules that hinder its interaction with the IR (Aguirre et al. 2000; Taniguchi et al. 2006).
In our study, GSP administration to HFFD-fed insulin resistant rats showed reduced activation of JNK. This could have been achieved by controlling the activities of adipokines, primarily, TNF-α. Administration of both MET and GSP was more effective in reducing the expression of TNFα and JNK1 in HFFD fed rats than any of these two alone.
SOCS-3 protein, also induced by cytokines like TNF-α and IL-6, attenuates insulin signaling by binding to the IR and reducing its ability to phosphorylate IRS proteins (Johnston et al. 2003; Ueki et al. 2004). Better reduction in the expression of SOCS-3 by GSP compared to MET in insulin resistant rats was observed.
Adiponectin, a 30 kDa protein, is an anti-inflammatory, anti-atherogenic and insulin-sensitizing hormone whose levels are found to be reduced in obesity (Whitehead et al. 2006; Phillips and Prins 2008). Adiponectin favours insulin action, inhibits stress-kinase activation and improves fatty acid oxidation by activating AMPK. Leptin also activates AMPK allowing increased fatty acid oxidation, however, obesity leads to leptin resistance (Greenberg and Obin 2006). High blood levels of leptin, plays a major role in insulin resistance by inducing production of pro-inflammatory cytokines (like IL-6, TNF-α) and vice versa (Sarraf et al. 1997; Otero et al. 2005).
With all the above observations, it can be suggested that, adipokines, in particular, TNF-α, IL-6, leptin and adiponectin and the serine kinases like (JNK1, p38 MAPK and ERK) may be the direct targets of GSP while AMPK could be a target for MET. GSP produces a combined positive effect on insulin signalling along with MET, which needs further evaluation in cell lines and other animal models of obesity.
Insulin resistance is a multifactorial disease and a combination of drugs is often used to treat the associated complications. However, the use of MET in combination with either rosiglitazone (Bailey and Day 2004), thiazolidinediones (Strowig and Raskin 2005) or fenofibrate (Li et al. 2011) has been shown to have limitations due to the accompanying complications in heart, liver and kidney. Thus, it is more prudent to include bioactive natural agents with less side effects with MET. In addition, GSP and MET appear to target different pathways. In view of this, the combination of GSP and MET could be a promising and effective treatment for insulin resistance and associated disorders. Future studies involving a preventive protocol with GSP and/or MET to HFFD rats would be useful since in a human study grape polyphenol treatment prevented the development of T2D in first degree relatives of T2D patients (Hokayem et al. 2013).
Conclusion
In conclusion, oral administration of GSP improves hepatic insulin signaling and glucose metabolism in a diet-induced model of obesity by reducing the proinflammatory cytokine production and inhibiting the inflammatory pathways independent of AMPK activation. The standard drug MET activated AMPK and promoted insulin signaling. The effects of GSP in liver are more pronounced in combination with MET. This study suggests a potential role for GSP as a combination drug along with MET in patients with insulin resistance and T2D.
Acknowledgments
We gratefully acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for providing financial assistance in the form of Senior Research Fellowship (SRF) to the first author, Yogalakshmi. B.
References
- Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- Anithanandhini AT, Balakrishnan SD, Anuradha CV. Taurine modulates antioxidant potential and controls lipid peroxidation in the aorta of high fructose-fed rats. J Biochem Mol Biol Biophys. 2002;6:129–133. doi: 10.1080/10258140290027261. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Day C. Avandamet: combined metformin-rosiglitazone treatment for insulin resistance in type 2 diabetes. Int J Clin Pract. 2004;58:867–876. doi: 10.1111/j.1742-1241.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Bjorbak C, Lavery HJ, Bates SH, Olson RK, Davis SM, Flier JS, Myers MG., Jr SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- Coate KC, Scott M, Farmer B, Moore MC, Smith M, Roop J, Neal DW, Williams P, Cherrington AD. Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. Am J Physiol Endocrinol Metab. 2010;299:887–898. doi: 10.1152/ajpendo.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Hokayem M, Blond E, Vidal H, Lambert K, Meugnier E, Feillet-Coudray C, Coudray C, Pesenti S, Luyton C, Lambert-Porcheron S, Sauvinet V, Fedou C, Brun JF, Rieusset J, Bisbal C, Sultan A, Mercier J, Goudable J, Dupuy AM, Cristol JP, Laville M, Avignon A. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2013;36:1454–1461. doi: 10.2337/dc12-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AM, Pirola L, Van Obberghen E. Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signaling. FEBS Lett. 2003;546:32–36. doi: 10.1016/S0014-5793(03)00438-1. [DOI] [PubMed] [Google Scholar]
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Li XM, Li Y, Zhang NN, Xie YH, Shi YQ. Combination therapy with metformin and fenofibrate for insulin resistance in obesity. J Int Med Res. 2011;39:1876–1882. doi: 10.1177/147323001103900531. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough N, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–725. [PubMed] [Google Scholar]
- Medina EA, Afsari RR, Ravid T, Castillo SS, Erickson KL, Goldkorn T. Tumor necrosis factor-{alpha} decreases Akt protein levels in 3T3-L1 adipocytes via the caspase-dependent ubiquitination of Akt. Endocrinology. 2005;146:2726–2735. doi: 10.1210/en.2004-1074. [DOI] [PubMed] [Google Scholar]
- Miller BS, Shankavaram UT, Horney MJ, Gore ACS, Kurtz DT, Rosenzweig SA. Activation of cJun NH2-terminal kinase/stress-activated protein kinase by insulin. Biochemistry. 1996;35:8769–8775. doi: 10.1021/bi952651r. [DOI] [PubMed] [Google Scholar]
- Montagut G, Onnockx S, Vaqué M, Bladé C, Blay M, Fernández-Larrea J, Pujadas G, Salvadó MJ, Arola L, Pirson I, Ardévol A, Pinent M. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J Nutr Biochem. 2010;21:476–481. doi: 10.1016/j.jnutbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gómez-Reino JJ, Gualillo O. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005;579:295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Phillips L, Prins J. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10:156–164. doi: 10.1007/s11906-008-0029-7. [DOI] [PubMed] [Google Scholar]
- Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig SM, Raskin P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes Metab. 2005;7:633–641. doi: 10.1111/j.1463-1326.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- Suwannaphet W, Meeprom A, Yibchok-Anun S, Adisakwattanna S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem Toxicol. 2010;48:1853–1857. doi: 10.1016/j.fct.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Brice E, Kahn CR. Critical nodes in signaling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, Blay M. Grape-seed procyanidins prevent low-GSPde inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin—a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. The AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]