Abstract
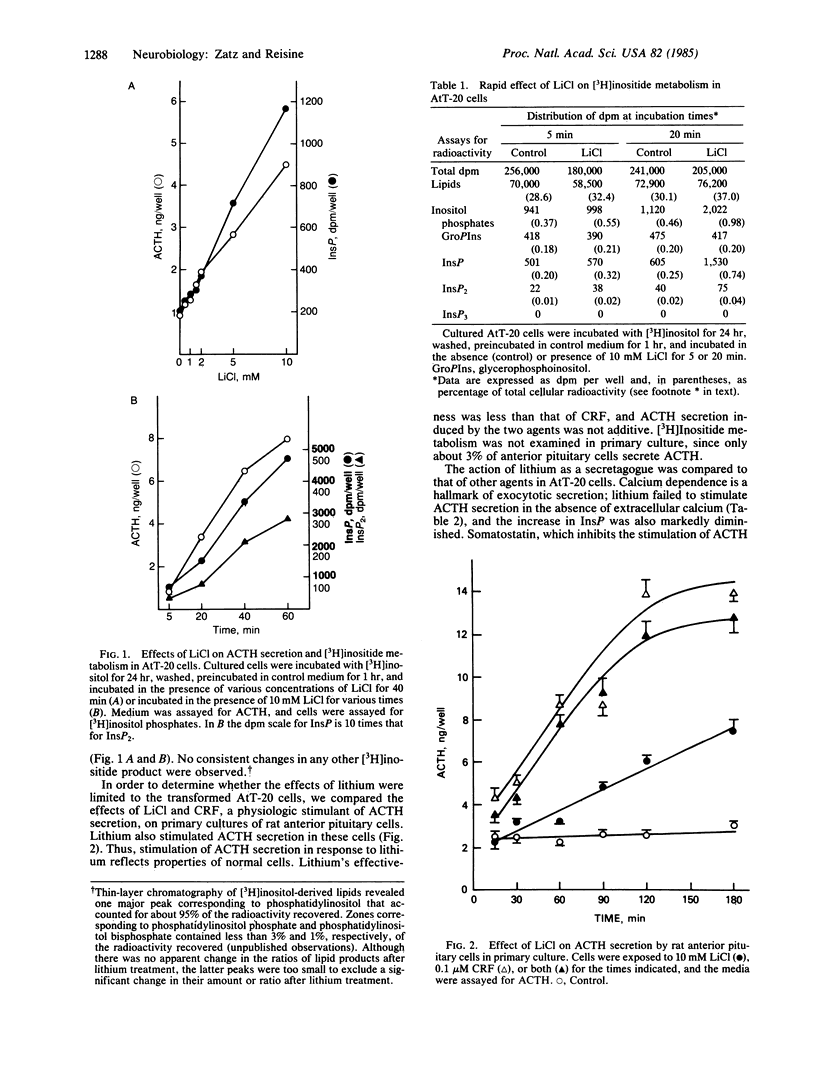
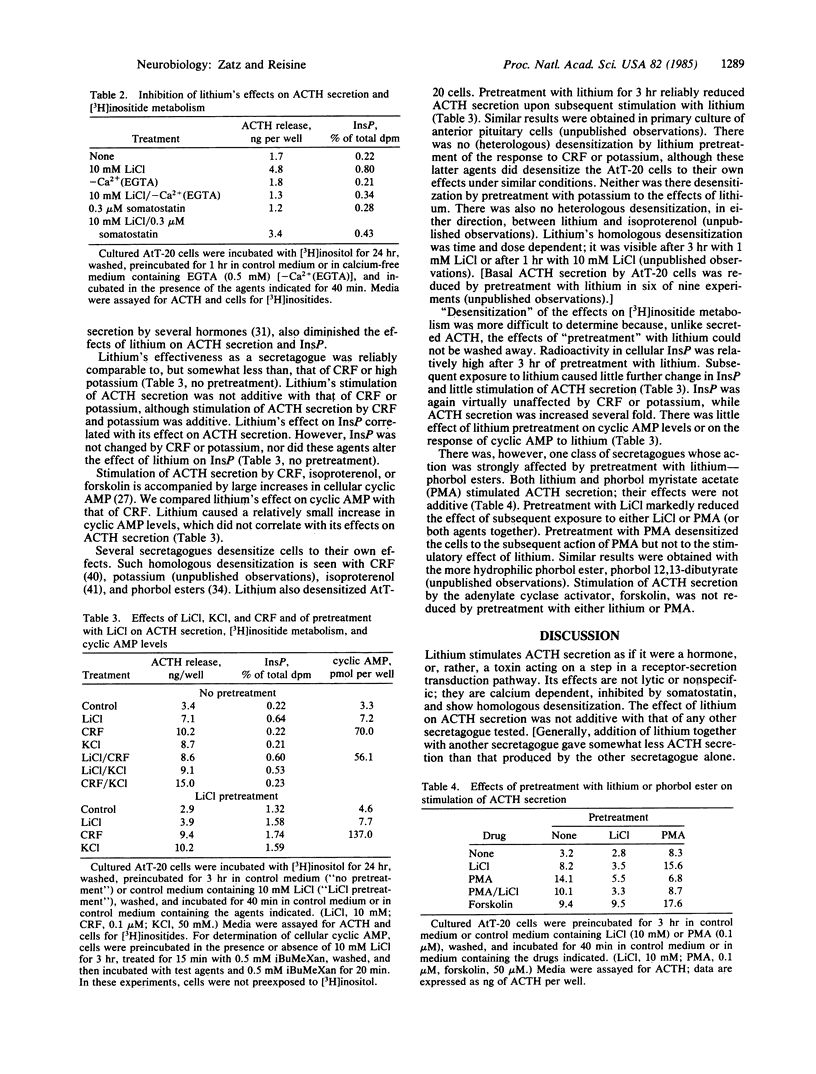
Lithium stimulated corticotropin (ACTH) secretion by mouse pituitary tumor cells (AtT-20/D16-16) and by normal rat anterior pituitary cells in primary culture. Effects were observed at less than 2 mM LiCl. ACTH secretion was comparable in magnitude to that induced by other secretagogues, was calcium dependent, and was inhibited by somatostatin. Lithium also induced changes in [3H]inositide metabolism; these changes accompanied and were correlated with changes in ACTH secretion. The most prominent and reliable effect was to increase [3H]inositol monophosphate. Other secretagogues had no effect on [3H]inositides in the presence or absence of lithium. Pretreatment with lithium for 3 hr desensitized the cells to the effects of subsequent exposure to lithium. The cells were not desensitized to lithium by pretreatment with other secretagogues, nor were they desensitized by lithium to the effects of corticotropin-releasing factor, high potassium, or forskolin. However, pretreatment with lithium did desensitize the cells to stimulation by phorbol esters. The interaction between lithium and phorbol esters suggests the involvement of inositide metabolism and protein kinase C in the regulation of ACTH secretion and possibly of other hormones or neurotransmitters. It also suggests new avenues of research into the basis of lithium's psychopharmacological effects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Lin S. H., Litosch I., Wallace M. Hormonal regulation of phosphatidylinositol breakdown. Life Sci. 1983 May 2;32(18):2055–2067. doi: 10.1016/0024-3205(83)90093-0. [DOI] [PubMed] [Google Scholar]
- Farese R. V. Phosphoinositide metabolism and hormone action. Endocr Rev. 1983 Winter;4(1):78–95. doi: 10.1210/edrv-4-1-78. [DOI] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hauser G., Eichberg J., Gonzalez-Sastre F. Regional distribution of polyphosphoinositides in rat brain. Biochim Biophys Acta. 1971 Oct 5;248(1):87–95. doi: 10.1016/0005-2760(71)90078-6. [DOI] [PubMed] [Google Scholar]
- Hawthorne J. N., White D. A. Myo-inositol lipids. Vitam Horm. 1975;33:529–573. doi: 10.1016/s0083-6729(08)60972-3. [DOI] [PubMed] [Google Scholar]
- Heisler S., Reisine T. D., Hook V. Y., Axelrod J. Somatostatin inhibits multireceptor stimulation of cyclic AMP formation and corticotropin secretion in mouse pituitary tumor cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6502–6506. doi: 10.1073/pnas.79.21.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler S., Reisine T. Forskolin stimulates adenylate cyclase activity, cyclic AMP accumulation, and adrenocorticotropin secretion from mouse anterior pituitary tumor cells. J Neurochem. 1984 Jun;42(6):1659–1666. doi: 10.1111/j.1471-4159.1984.tb12757.x. [DOI] [PubMed] [Google Scholar]
- Honchar M. P., Olney J. W., Sherman W. R. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983 Apr 15;220(4594):323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Marx J. L. A new view of receptor action. Science. 1984 Apr 20;224(4646):271–274. doi: 10.1126/science.6143399. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Reisine T., Kebabian J. W. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase activity in rodent pituitary tissue: possible role in cAMP-dependent hormone secretion. Endocrinology. 1984 Nov;115(5):1933–1945. doi: 10.1210/endo-115-5-1933. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Jaken S. Specific desensitization to tumor-promoting phorbol esters in mouse pituitary cells. Evidence that desensitization is a two-step process. J Biol Chem. 1983 Mar 10;258(5):2875–2881. [PubMed] [Google Scholar]
- Phillips M., Tashjian A. H., Jr Characterization of an early inhibitory effect of glucocorticoids on stimulated adrenocorticotropin and endorphin release from a clonal strain of mouse pituitary cells. Endocrinology. 1982 Mar;110(3):892–900. doi: 10.1210/endo-110-3-892. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol lipids and cell stimulation in mammalian salivary gland. Cell Calcium. 1982 Oct;3(4-5):369–383. doi: 10.1016/0143-4160(82)90024-0. [DOI] [PubMed] [Google Scholar]
- Reisine T. D., Heisler S., Hook V. Y., Axelrod J. Activation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells increases cyclic adenosine 3':5'-monophosphate synthesis and adrenocorticotropin release. J Neurosci. 1983 Apr;3(4):725–732. doi: 10.1523/JNEUROSCI.03-04-00725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine T., Heisler S. Desensitization of beta adrenergic receptors linked to adrenocorticotropin secretion. J Pharmacol Exp Ther. 1983 Oct;227(1):107–114. [PubMed] [Google Scholar]
- Reisine T., Hoffman A. Desensitization of corticotropin-releasing factor receptors. Biochem Biophys Res Commun. 1983 Mar 29;111(3):919–925. doi: 10.1016/0006-291x(83)91387-6. [DOI] [PubMed] [Google Scholar]
- Richardson U. I., Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology. 1981 Jan;108(1):281–290. doi: 10.1210/endo-108-1-281. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Sabol S. L. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980 Aug;203(1):37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Leavitt A. L., Honchar M. P., Hallcher L. M., Phillips B. E. Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem. 1981 Jun;36(6):1947–1951. doi: 10.1111/j.1471-4159.1981.tb10819.x. [DOI] [PubMed] [Google Scholar]



