Abstract
Purpose
This study was designed to evaluate whether magnetic resonance imaging (MRI) is appropriate for detecting early changes in the mandibular bone marrow and pulp tissue of rats after high-dose irradiation.
Materials and Methods
The right mandibles of Sprague-Dawley rats were irradiated with 10 Gy (Group 1, n=5) and 20 Gy (Group 2, n=5). Five non-irradiated animals were used as controls. The MR images of rat mandibles were obtained before irradiation and once a week until week 4 after irradiation. From the MR images, the signal intensity (SI) of the mandibular bone marrow and pulp tissue of the incisor was interpreted. The MR images were compared with the histopathologic findings.
Results
The SI of the mandibular bone marrow had decreased on T2-weighted MR images. There was little difference between Groups 1 and 2. The SI of the irradiated groups appeared to be lower than that of the control group. The histopathologic findings showed that the trabecular bone in the irradiated group had increased. The SI of the irradiated pulp tissue had decreased on T2-weighted MR images. However, the SI of the MR images in Group 2 was high in the atrophic pulp of the incisor apex at week 2 after irradiation.
Conclusion
These patterns seen on MRI in rat bone marrow and pulp tissue were consistent with histopathologic findings. They may be useful to assess radiogenic sclerotic changes in rat mandibular bone marrow.
Keywords: Magnetic Resonance Imaging, Mandible, Bone Marrow, Irradiation
Introduction
Among radiation-induced normal tissue injuries, early side effects appear within a few days to weeks after high-dose irradiation while late effects occur months to years after irradiation with a latent period. Mitosis-linked death is the main cause of the loss of cells in the early period.1,2
The radiation dose and radiosensitivity of the target tissue are two important factors in the degree of impact of irradiation. Target tissues such as bone marrow, lymphoid tissues, and oral mucous membranes that proliferate continuously tend to be sensitive to radiation, while little or no short-term effect of radiation occurs in muscle.2
A dose of 5 to 10 Gy of radiation can decrease the DNA content of osteoblasts in the proliferation, confluence, and post-proliferation stages.3 Furthermore, it has been shown that 10 to 20 Gy irradiation is capable of reducing bone marrow cellularity for a week, although the effect is reversible and gradually returns to normal within 8 weeks.4 The major pathologic changes following fractionated radiotherapy from day 1 to 7 include edema and lack of hematopoiesis, while intense hematopoietic activity and persistent edema are observed from day 10 to 14.5
The effects of dental irradiation, specifically, have also been noted. For example, 20 Gy irradiation leads to cessation of normal dentin deposition,6 and nuclear alterations in the dental pulp tissue of rats can occur after fractionated irradiation with a total dose of 60 Gy.7 Niehoff et al6 reported a significant decline in vertical bone apposition to the outer surface of irradiated mandibles. In children who undergo irradiation, the growth retardation of the facial skeleton occurs primarily in the jaws and teeth. In the mandible, growth retardation specifically occurs in the region of the condylar head.8
Although computed tomography (CT) has been used for bone volume analysis of the irradiated mandible,9 MRI has been known to be useful for detecting signs of radiogenic bone and soft tissue damage. Recognizing mandibular radiogenic bone change early is possible with MRI, allowing for early diagnosis before the onset of clinical symptoms and bone destruction10 and can be used to evaluate early bone marrow changes during radiotherapy.11 Evidence of edema on MR images was found to consist of abnormalities of the bone marrow with a decreased or increased signal intensity (SI).12,13
Comparing among imaging modalities, although CT better illustrates the degree and extent of cortical defects,14 it is not suitable for evaluating bone marrow in the early pathologic stage, while radioisotope scanning has the critical limitation of showing a high diagnostic sensitivity but a low specificity.10
After high-dose irradiation, identifying changes in the mandibular bone marrow and adjacent soft tissues is important in order to evaluate tissue sensitivity and abnormality. The data available on early bone marrow and dental pulp changes of irradiated mandibular bone visible by MRI are limited.
Thus this study was designed to evaluate whether MRI is appropriate for detecting the early changes in the mandibular bone marrow and pulp tissue of rats after high-dose irradiation. Under different dosages of radiation, the animals were monitored using MR T2-weighted images and the data was confirmed by conventional staining methods with tissues isolated from the rats. Thus, this paper provides new insight into MRI diagnosis to predict early side effects of radiation.
Materials and Methods
Animals
The study was performed in accordance with the Guide for Animal Experimentation by Wonkwang University and approved by the Institutional Animal Care and Use Committee of Wonkwang University.
Fifteen 4-week-old Sprague-Dawley rats were obtained from Hubet Laboratories (Iksan, South Korea). Before the experiment, the rats were allowed 7 days to acclimatize to the conditions.
Five rats were not irradiated to use as controls. Ten rats were randomly divided into two groups of five rats each. Group 1 was given a single dose of external irradiation of 10 Gy, and Group 2 was given a single dose of 20 Gy.
MRI examination
MRI examinations were performed with a 4.7-T animal MRI instrument (Bruker, Ettlingen, Germany) using a linear volume coil for rats. Axial T2-weighted images (T2WI) were obtained with the use of the Turbo RARE T2 technique. The imaging parameters were repetition time (TR) 11776.3 ms, echo time (TE) 36 ms, flip angle 180 degrees, average 6, 256×256 matrix, 0.0117×0.0117 cm pixel size, 0.5 mm slice thickness, and 100 slices. Under inhalational isoflurane anesthesia, an MRI of the rat's head was taken before irradiation and once a week for 4 weeks after irradiation.
Radiation delivery procedure
For X-ray irradiation, the rats were anesthetized with ketamine and xylazine. CT simulator scans (2 mm, Somatom Sensation Open, Siemens, Berlin, Germany) were performed prior to X-ray irradiation, and the target volume was identified by the treatment planning system (Eclipse, Varian Medical Systems Inc, Palo Alto, CA, USA). Irradiation was applied under general anesthesia to the rat's right mandible using a linear accelerator (Clinac iX, Varian Medical Systems) with a 6-MV X-ray beam at a dose-rate of 2 Gy/min. The right mandible was covered by the 95% isodose, and 10 Gy or 20 Gy were prescribed to the isocenter (Fig. 1). Group 1 was irradiated with 10 Gy, and Group 2 was irradiated with 20 Gy, targeted at the right mandible including the condyle.
Fig. 1.
Target volume of the right mandible including the ramus and condyle in CT scans are set prior to high-dose irradiation. 100% isodoses are marked in yellow. Pre-irradiated planning enables setting a one-sided application of radiation and minimizing the change in adjacent tissues, especially the brain.
Histopathologic examination
The rats were sacrificed every week after irradiation for histopathologic study. The mandibles were harvested and fixed in 1.5% paraformaldehyde solution.
The specimens were cut perpendicular to the occlusal plane in the molar region containing three molar teeth and the middle third of the incisor. The explanted bone specimens were fixed for 24 h in a 10% formol-saline solution and then decalcified in a 10% nitric acid bath for 48 h. The decalcified bone specimens were dehydrated with alcohol (100% ethanol solutions) followed by toluene. The specimens of bone were then embedded in paraffin. Serial sections of 5 µm were cut using a microtome. The sections were stained with hematoxylin-eosin (HE) and were then examined under a light microscope.
Image analysis
In order to focus accurately in the same plane of the bone marrow every week, the plane was adjusted. The axial plane was modified to the pulp floor of the molar teeth, the sagittal plane to the midline of the face, and the coronal plane to the first molar including both roots. For incisor pulp evaluation, the sagittal plane was modified to follow the long axis of the incisor.
The MR SI of the region of interest (ROI) in the rat mandible was evaluated using the OnDemand3D program (Cybermed Co., Seoul, Korea) (Fig. 2). The ROI was taken from two regions on the coronal MR image. One was the bone marrow below the first mandibular molar area and the other was from the adjacent muscle. On the modified sagittal plane, the ROI was the incisor pulp below the molar teeth (Fig. 3). The ROI was calculated for the mean value. The SI was calculated using the following equations:
| SI=([mean of ROI])/([mean of ROI] muscle). |
Fig. 2.

Coronal fat-suppressed T2-weighted MR image. In order to locate the same plane every week, the axial (pulp floor of molar teeth), sagittal (midline of face), and coronal (first molar including two roots) planes were adjusted. The location of the region of interest (ROI) was used to measure the signal intensity in the mandibular first molar area. The ROIs included the alveolar bone marrow between the first molar and the incisor, and the adjacent muscles.
Fig. 3.
The ROI of the incisor pulp tissue below the molar teeth on the modified sagittal section is obtained along the long axis of the incisor.
The muscle has a lower sensitivity to irradiation than does the bone marrow. In addition, the height of the mandibular ramus was measured from the condylar head to the mandibular angle on the MR images using a Tapeline measuring tool in the OnDemand 3D program (Fig. 4).
Fig. 4.

Coronal fat-suppressed T2-weighted MR image. The image shows an example of a mandibular ramus height (condyle to mandibular angle) measurement using the Tapeline tool in the OnDemand3D program.
The MR SI and mandibular ramus height of the irradiated side were compared with those of the control side. The MRI findings were compared with the histopathologic observations.
Results
In the control group, the pulp tissue showed a high SI and the bone marrow between the first molar and incisor presented an intermediate SI compared with that of the muscle. Although the size of the first molar pulp cavity had decreased, the height of the mandibular bone had increased during the period. The SI of the bone marrow had fallen continuously from week to week after irradiation.
In the irradiated groups, both Groups 1 and 2, the patterns of SI in the pulp and the bone marrow were very similar to those of the control group. The SI of the right irradiated bone marrow had decreased. The SI of the irradiated groups was lower than that of the control group. These differences occurred in the first week after irradiation. Based on these results, it can be concluded that bone marrow is very sensitive to X-rays. However, only a small difference was found between Groups 1 and 2.
The height of the mandibular bone and size of the pulp had decreased more than those in the control group, but the SI in the muscle was slightly higher than that in the control and contralateral side.
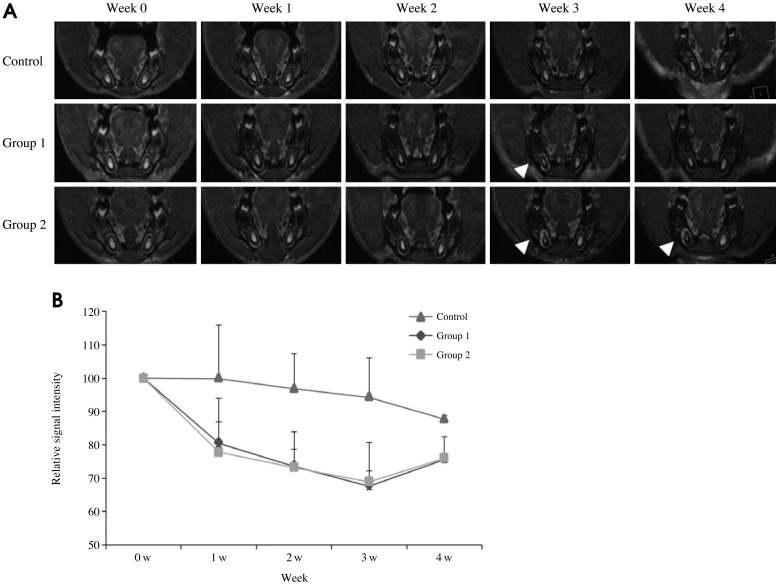
The SI of the irradiated tissues around the right incisor pulp was increased. The changes in the MR images in Group 2 were prominent (Fig. 5).
Fig. 5.
A. The MR images show the changes during the 4 weeks after irradiation. The SI of the irradiated tissues around the right incisor pulp has increased (arrowhead). The changes in the MR images in Group 2 are prominent. B. The diagram shows the SI of the bone marrow versus the time curve. In the control group, the SI of the bone marrow has decreased. The SI of the irradiated right bone marrow has also decreased. The irradiated groups show a lower level of SI than that of the control group. These differences appear from the first week. However, a small difference can be seen between the Groups 1 and 2.
The SI of the irradiated pulp tissue showed a pattern of reduction until week 3. There were differences among the groups at week 3. The SI of the MR images in Group 2 was increased at the apex of the incisor beginning at week 2 after irradiation (Fig. 6).
Fig. 6.
A. The MR images show the changes during the 4 weeks after irradiation. A modified sagittal view is obtained along the long axis of the incisor. The size and SI of pulp of incisor have decreased (arrow) in Group 2. However, the SI of the MR images in the Group 2 is high at the apex of the incisor from week 2 after irradiation (arrowhead). B. The diagram shows the SI of the pulp tissue versus the time curve. The SI of the irradiated pulp tissue has decreased until week 3 after irradiation. There are differences among the groups at week 3.
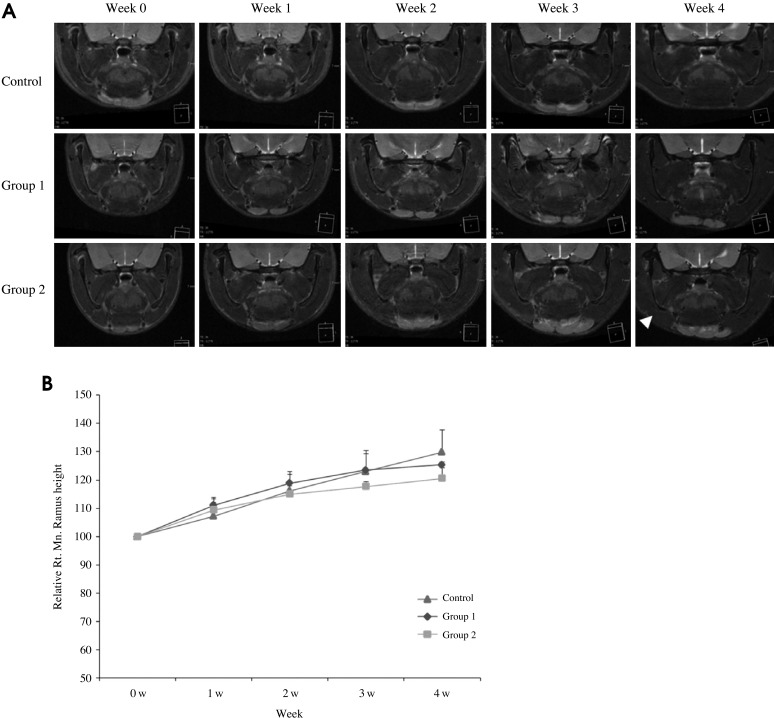
The height of the mandibular ramus was measured from the condylar head to the mandibular angle on the coronal MR image. Interestingly, it showed a reduced pattern in the right mandible of the irradiated groups and showed a marked decreased in Group 2 compared with the control group. There was a mandibular asymmetry due to the retardation of the growth on the right side (Fig. 7).
Fig. 7.
A. The MR images show the changes during the 4 weeks after irradiation. There is a mandibular asymmetry due to the retardation of the growth on the right side (arrowhead). B. The diagram shows the mandibular ramus height versus the time curve. The height of the irradiated ramus is shorter than the contralateral and control side.
There was no inflammatory reaction in any of the groups. In the irradiated sites, histopathologic changes, specifically increasing trabecular formation and bone sclerosis were seen in the irradiated groups, most prominently in the molar region of Group 2 (Fig. 8).
Fig. 8.
Histopathologic examination of the alveolar bone below molar tooth (H&E stain, 100×). At the irradiated sites, the histopathologic changes show increasing trabecular bone formation and bone sclerosis in the irradiated groups, prominent in the molar region in Group 2.
Atrophic incisor pulp tissue due to irradiation corresponded with a low SI on MR images. However, enlarged vessels and hemorrhagic changes at the apex of the incisor showed a high SI. The formation of dental hard tissue was inhibited, and there was new bone formation at the site (Fig. 9).
Fig. 9.

Histopathologic changes show the atrophic incisor pulp tissue due to irradiation. The enlarged vessels and hemorrhagic changes are visible. The formation of dental hard tissue is inhibited, but it shows new bone formation (Group 2 at week 4 after irradiation. H&E stain, 100×).
Discussion
MRI provides high diagnostic sensitivity in the detection of normal and pathologic development in bone marrow. Normal mandibular marrow has a relatively sparse vascular supply and yellow marrow appearance on MR such as a high SI on T1WI and intermediate SI on T2WI.15
In this study, 5-week-old rats were allowed to grow up to week 9. There were two reasons for using juvenile rats as an animal model. One was to identify the response of the growing mandible to irradiation and the other was the relatively small size of the MR magnet for an adult rat head.
The MRI fat-suppressed option was chosen to compensate for the amount of fat around the neck of the rat. It was thought that the MR fat-suppressed T2WI SI of the control group decreased due to an increase in the fat content. The basic components of bone marrow that correspond with the formation of each of the SIs are fat, water, and minerals.16 As the bone grows, the red bone marrow of the mandible becomes yellow bone marrow with increased fat composition.17 Thus the results of this study appear to be based on changes in the ratio of bone marrow components.
Generally on MR T2WI, inflammation increases the SI in bone marrow, which probably reflects the increased water content within the bone marrow.18 MR T2WI may help identify the sites of inflammation corresponding to the sites of active infection from the perspective of histopathologic examination.19
MR T2WI was utilized to detect radiation-induced acute inflammation or an edematous reaction after irradiation. In this study, although it was expected that the SI would be elevated in the bone marrow, it was, rather, found to have decreased significantly, probably due to the formation of new trabecular bone.
Matsumura et al3 reported that the alkaline phosphatase activity was increased by irradiation and irradiation influenced the differentiation of osteoblasts and the calcification process.
However, it was observed that the SI had increased in the muscle due to edematous change20 and the apex of the incisor, reflecting vascular congestion.21
Although T1WI did not show pathognomonic changes in active infection, they have also been noted to depict the pathologic lesions in bone marrow and adipose tissue with a high sensitivity.22
Unfortunately, it was difficult to acquire both T1WI and T2WI together because some rats underwent hypothermia during the MRI examination. Thus T1WI were not collected in this study and the findings of T1WI could not be compared.
In this study, one of the differences between the irradiated groups was the degree of sclerosis. Osteosclerotic lesions in bone sclerosis appeared hypointense by an MR signal.23 In this study, a difference was observed between the control and irradiated groups on day 7. It may be meaningful to perform further research on these sclerotic bone qualities and bone strength to determine the cause-effect relationship.
In the course of the development of the maxillo-facial complex, the mandibular condyle showed the most significant growth. Niehoff et al6 reported that the mandibular condylar diameter was reduced significantly in mandibles irradiated with 20 Gy.
In this study, the growth retardation of the irradiated mandibular condyle using 5-week-old developing rats was observed.
The dental papilla of the rodent incisor continuously develops. Research has also found that rodent incisors underwent growth retardation after high-dose irradation.24 When the initial change in the pulp was analyzed with an MR coronal section, the data repeatedly fluctuated and did not stabilize. This was assumed to be a result of the continuous development of the incisors. The cells of this observation area could have been replaced.7 It was very difficult to repeatedly focus on the same pulp tissue every week. It was thus estimated based on the SI from the pulp below the molar teeth on a modified sagittal section along the long axis of the incisor.
In this study, the dentinogenesis of the incisor apex area in Group 2 was disrupted. In the irradiated groups, the vessels were enlarged and congested in the odontoblastic layer and pulp tissue.
Because of the short period of observation, it was difficult to monitor the late effects of irradiation. Rodents' metabolic rates are higher than that of humans.25,26 Four weeks seems to be an adequate period for determining early changes.
Among the irradiated rats, all survived the study period. A dose of irradiation to the brain higher than 40 Gy, which would have been included in the field of mandibular irradiation, would not have allowed the animals to survive.27 This study used a single dose of 10 Gy or 20 Gy of radiation based on the volume calculated from CT imaging, which allows accurate localization of the right mandible and shielding of the brain.
Exposure to high-dose X-rays can cause the bone marrow and pulp tissue to become less resistant to infection. The bone marrow and periapical tissues could become inflamed more easily as a result of subsequent infection, and osteoradionecrosis could follow. Thus the results of this study underscored that irradiating a patient with MRI requires early diagnosis of the condition of the bone marrow and pulp tissue.
In conclusion, the bone marrow is properly represented through MRI, and these images correlate well with the pathologic changes seen upon microscopic examination. MRI could be used to evaluate early radiogenic sclerotic changes to the bone marrow and atrophic changes to the pulp tissue.
Footnotes
This paper was supported by Wonkwang University in 2013.
References
- 1.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.White SC, Pharoah MJ. Oral radiology; principles and interpretation. 5th ed. St. Louis: Mosby-Year Book; 2004. pp. 25–44. [Google Scholar]
- 3.Matsumura S, Jikko A, Hiranuma H, Deguchi A, Fuchihata H. Effect of X-ray irradiation on proliferation and differentiation of osteoblast. Calcif Tissue Int. 1996;59:307–308. doi: 10.1007/s002239900129. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto M, Takahashi S, Toguchida J, Kotoura Y, Shibamoto Y, Yamamuro T. Changes in bone after high-dose irradiation. Biomechanics and histomorphology. J Bone Joint Surg Br. 1991;73:492–497. doi: 10.1302/0301-620X.73B3.1670456. [DOI] [PubMed] [Google Scholar]
- 5.Moore GS. Pediatric musculoskeletal imaging. In: Stark DS, Bradley WG, editors. Magnetic resonance imaging. 2nd ed. St. Louis: Mosby-Year Book; 1992. pp. 2223–2274. [Google Scholar]
- 6.Niehoff P, Springer IN, Açil Y, Lange A, Marget M, Roldán JC, et al. HDR brachytherapy irradiation of the jaw - as a new experimental model of radiogenic bone damage. J Craniomaxillofac Surg. 2008;36:203–209. doi: 10.1016/j.jcms.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Vier-Pelisser FV, Figueiredo MA, Cherubini K, Braga Filho A, Figueiredo JA. The effect of head-fractioned teletherapy on pulp tissue. Int Endod J. 2007;40:859–865. doi: 10.1111/j.1365-2591.2007.01294.x. [DOI] [PubMed] [Google Scholar]
- 8.Furstman LL. Effect of X irradiation on the mandibular condyle. J Dent Res. 1970;49:419–427. doi: 10.1177/00220345700490023701. [DOI] [PubMed] [Google Scholar]
- 9.Tamplen M, Trapp K, Nishimura I, Armin B, Steinberg M, Beumer J, et al. Standardized analysis of mandibular osteoradionecrosis in a rat model. Otolaryngol Head Neck Surg. 2011;145:404–410. doi: 10.1177/0194599811400576. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann G, Rössler R, Klett R, Rau WS, Bauer R. The role of magnetic resonance imaging and scintigraphy in the diagnosis of pathologic changes of the mandible after radiation therapy. Int J Oral Maxillofac Surg. 1996;25:189–195. doi: 10.1016/s0901-5027(96)80027-0. [DOI] [PubMed] [Google Scholar]
- 11.Onu M, Savu M, Lungu-Solomonescu C, Harabagiu I, Pop T. Early MR changes in vertebral bone marrow for patients following radiotherapy. Eur Radiol. 2001;11:1463–1469. doi: 10.1007/s003300000804. [DOI] [PubMed] [Google Scholar]
- 12.Stevens SK, Moore SG, Kaplan ID. Early and late bone-marrow changes after irradiation: MR evaluation. AJR Am J Roentgenol. 1990;154:745–750. doi: 10.2214/ajr.154.4.2107669. [DOI] [PubMed] [Google Scholar]
- 13.Blomlie V, Rofstad EK, Skjønsberg A, Tverå K, Lien HH. Female pelvic bone marrow: serial MR imaging before, during, and after radiation therapy. Radiology. 1995;194:537–543. doi: 10.1148/radiology.194.2.7824737. [DOI] [PubMed] [Google Scholar]
- 14.Store G, Larheim TA. Mandibular osteoradionecrosis: a comparison of computed tomography with panoramic radiography. Dentomaxillofac Radiol. 1999;28:295–300. doi: 10.1038/sj/dmfr/4600461. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan PA, Dussault RG. Magnetic resonance imaging of the bone marrow. In: Higgins CB, Hricak H, Helms CA, editors. Magnetic resonance imaging of the body. 3rd ed. Philadelphia: Lippincott-Raven; 1997. pp. 101–126. [Google Scholar]
- 16.Vogler JB, 3rd, Murphy WA. Bone marrow imaging. Radiology. 1988;168:679–693. doi: 10.1148/radiology.168.3.3043546. [DOI] [PubMed] [Google Scholar]
- 17.Kaneda T, Minami M, Ozawa K, Akimoto Y, Okada H, Yamamoto H, et al. Magnetic resonance appearance of bone marrow in the mandible at different ages. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:229–233. doi: 10.1016/s1079-2104(96)80262-9. [DOI] [PubMed] [Google Scholar]
- 18.Unger E, Moldofsky P, Gatenby R, Hartz W, Broder G. Diagnosis of osteomyelitis by MR imaging. AJR Am J Roentgenol. 1988;150:605–610. doi: 10.2214/ajr.150.3.605. [DOI] [PubMed] [Google Scholar]
- 19.Kaneda T, Minami M, Ozawa K, Akimoto Y, Utsunomiya T, Yamamoto H, et al. Magnetic resonance imaging of osteomyelitis in the mandible. Comparative study with other radiologic modalities. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:634–640. doi: 10.1016/s1079-2104(05)80107-6. [DOI] [PubMed] [Google Scholar]
- 20.Weber-Donat G, Amabile JC, Lahutte-Auboin M, Potet J, Baccialone J, Bey E, et al. MRI assessment of local acute radiation syndrome. Eur Radiol. 2012;22:2814–2821. doi: 10.1007/s00330-012-2549-4. [DOI] [PubMed] [Google Scholar]
- 21.Sugimura H, Kisanuki A, Tamura S, Kihara Y, Watanabe K, Sumiyoshi A. Magnetic resonance imaging of bone marrow changes after irradiation. Invest Radiol. 1994;29:35–41. doi: 10.1097/00004424-199401000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Tang JS, Gold RH, Bassett LW, Seeger LL. Musculoskeletal infection of the extremities: evaluation with MR imaging. Radiology. 1988;166:205–209. doi: 10.1148/radiology.166.1.3336680. [DOI] [PubMed] [Google Scholar]
- 23.Nolte-Ernsting CC, Adam G, Bühne M, Prescher A, Günther RW. MRI of degenerative bone marrow lesions in experimental osteoarthritis of canine knee joints. Skeletal Radiol. 1996;25:413–420. doi: 10.1007/s002560050108. [DOI] [PubMed] [Google Scholar]
- 24.Springer IN, Niehoff P, Açil Y, Marget M, Lange A, Warnke PH, et al. BMP-2 and bFGF in an irradiated bone model. J Craniomaxillofac Surg. 2008;36:210–217. doi: 10.1016/j.jcms.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Schultze-Mosgau S, Lehner B, Rödel F, Wehrhan F, Amann K, Kopp J, et al. Expression of bone morphogenic protein 2/4, transforming growth factor-beta1, and bone matrix protein expression in healing area between vascular tibia grafts and irradiated bone-experimental model of osteonecrosis. Int J Radiat Oncol Biol Phys. 2005;61:1189–1196. doi: 10.1016/j.ijrobp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Cohen M, Nishimura I, Tamplen M, Hokugo A, Beumer J, Steinberg ML, et al. Animal model of radiogenic bone damage to study mandibular osteoradionecrosis. Am J Otolaryngol. 2011;32:291–300. doi: 10.1016/j.amjoto.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Little JB. Cellular, molecular, and carcinogenic effects of radiation. Hematol Oncol Clin North Am. 1993;7:337–352. [PubMed] [Google Scholar]