Abstract
The ciliary zonule in the eye, also known as Zinn’s zonule, is composed of oxytalan fibers, which are bundles of microfibrils consisting mainly of fibrillin-1. However, it is still unclear which of the microfibril-associated molecules present in the ciliary zonule controls oxytalan fibers. Microfibril-associated glycoprotein-1 (MAGP-1) is the only microfibril-associated molecule identified in the human ciliary zonule. In the present study, we used siRNA against MAGP-1 in cultures of human non-pigmented ciliary epithelial cells to examine the extracellular deposition and appearance of fibrillin-1 employing Western blotting and immunofluorescence. MAGP-1 suppression led to a reduction of fibrillin-1 deposition. Immunofluorescence also confirmed that RNAi-mediated down-regulation of MAGP-1 led to suppression of fiber development. These results suggest that MAGP-1 plays a crucial role in the extracellular deposition of fibrillin-1 during formation of the human ciliary zonule.
Keywords: fibrillin, MAGP-1, microfibrils, oxytalan fibers, ciliary zonule
I. Introduction
The elastic system fibers that give tissue resilience and flexibility comprise three types—oxytalan, elaunin and elastic fibers—differing in their relative proportions of microfibrils and elastin [9]. Oxytalan fibers, composed of pure microfibrils, were first identified in periodontal ligaments (PDL) by Fullmer and Lillie [2], and are also distributed in the ciliary zonule of the eye [11]. Among the microfibrillar molecules, fibrillin-1 and fibrillin-2 are the best characterized [8]. We have previously investigated the formation and degradation of oxytalan fibers in PDL using a human PDL cell culture system [18, 19]. Also, using fibroblast culture, we have clearly demonstrated that oxytalan fibers, consisting of fibrillin-1 and fibrillin-2, are formed in cell/matrix layers. The ciliary zonule, also known as Zinn’s zonule, is composed of pure microfibrils [11] and connects the lens to the ciliary body, in order to control the thickness of the lens for focusing. Proteomics analysis has shown that the microfibril-associated molecules present in the human ciliary zonule include fibrillin-1 and microfibril-associated glycoprotein-1 (MAGP-1) [1]. A study using guinea pigs has demonstrated that non-pigmented ciliary epithelial cells in the ciliary body express fibrillin-1 [4]. Therefore, it is thought that non-pigmented ciliary epithelial cells produce the oxytalan fibers of the ciliary zonule. Recently, we showed that human non-pigmented ciliary epithelial cells (HNPCEC) express fibrillin-2 as well as fibrillin-1, and form an oxytalan fiber network in vitro [6, 21]. A more recent study using in situ hybridization and immunofluorescence has revealed that mouse non-pigmented ciliary epithelial cells express both fibrillin-1 and fibrillin-2 mRNA, and that the ciliary zonule is labeled for both fibrillins [12], being consistent with our results. However, the role of MAGP-1 during oxytalan fiber formation in the human ciliary zonule is still unclear.
MAGP-1 has been identified and labeled in the bovine ciliary zonule using immunofluorescence and immunogold-electron microscopy analysis [4]. Up to now, MAGP-1 has received attention as the molecule that controls tropoelastin deposition onto microfibrils for formation of elastic fibers [17], and little has been reported about the role of MAGP-1 in development of the ciliary zonule. To investigate this issue, we generated MAGP-1-knockdown cells using the siRNA technique and performed biochemical and immunofluorescence analysis of fibrillin-1 deposition in HNPCEC cultures. We found that siRNA-mediated gene silencing of human MAGP-1 reduced the extracellular deposition of fibrillin-1 and suppressed the formation of fibrillin-labeled oxytalan fibers.
II. Materials and Methods
Cells and culture
HNPCEC were purchased from ScienceCell Research Laboratories (Carlsbad, CA, USA) and cultured in Dulbecco’s modified Eagle Medium (DMEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% newborn calf serum (NCS; Invitrogen) and 100 units/ml penicillin and 100 µg/ml streptomycin (Roche Diagnostics, Mannheim, Germany) at 37°C in humidified air containing 5% CO2. When outgrowth of the cells reached confluence, they were harvested with 0.025% trypsin (Invitrogen) in PBS, and transferred to plastic culture dishes at a 1:4 split ratio. For experiments, the cells were trypsinized and seeded at 1×106 cells/ml per 35-mm culture dish (Corning Inc., Corning, NY, USA). The HNPCEC were found to be confluent after 72 hr (this day being set as day 0). Three different lots of HNPCEC were used (lot no. 0564, 0579, 5968), and employed in experiments from the 3rd to 6th passages in this study.
PCR analysis
For PCR amplification, specific oligonucleotide primers for human MAGP-1 and MAGP-2 sequences were designed on the basis of the GeneBank sequences NM_ 017459 and NM_003480, respectively.
MAGP-1: Forward: 5'-CCCAAGCTTGTGAGGAAC AGTACCCGT-3', Reverse: 5'-CGGAATTCGATACTCCC CCAACCCGA-3' (331–828 bp)
MAGP-2: Forward: 5'-CCCAAGCTTGCCAAAGGA CTCGGTGGA-3', Reverse: 5'-CGGAATTCCAGGAGGA AGTCGGAAG-3' (173–698 bp)
GAPDH: Forward: 5'-GCGGATCCCTCTGCTCCTC CTGTTCGAC-3', Reverse: 5'-GGAATTCTGACAAAGTG GTCGTTGAGG-3'
The product sizes of MAGP-1, MAGP-2 and GAPDH were 498, 526, and 998 bp, respectively.
The reaction was performed using the Taq PCR Master Mix Kit (Qiagen, Hilden, Germany) as follows: 1 µl of cDNA was used as the template in a 20-µl amplification mixture containing of 1 U of Taq DNA polymerase, each of the 5' and 3' primers at 0.5 mM, and distilled water. Experiments were performed to determine the optimal number of cycles that would yield a linear phase of amplification. PCR was performed in a Gene AMP® PCR System 9700 (Applied Biosystems Japan Ltd., Tokyo, Japan) with an initial denaturation at 94°C for 2 min, followed by a cycle of 20 sec at 94°C, 30 sec at 60°C, extension at 72°C for 90 sec, and an additional extension step at 72°C for 10 min. Amplification products were resolved by electrophoresis on a 2% w/v agarose gel. Ethidium bromide-stained agarose gels with separated PCR products were photographed under UV light.
Immunofluorescence
At 3, 5, and 7 days of culture, HNPCEC were fixed in ice-cold 4% paraformaldehyde for 15 min, followed by washing with PBS. Nonspecific immunoreactivity was blocked with 1% goat serum (Sigma-Aldrich, Saint Louis, MO, USA) in PBS for 1 hr at room temperature. The cell/matrix layers were then incubated for 2 hr at room temperature with the appropriate primary antibodies (clone 11C1.3, monoclonal antibody against human fibrillin-1 diluted 1:1000; Thermo Fisher Scientific Anatomical Pathology, Fremont, CA, USA, rabbit antibody against human MAGP-1 diluted 1:1000; Sigma-Aldrich). Controls included the use of preimmune normal mouse and rabbit IgG for incubation with the primary antibody. After being rinsed in PBS, the cells were incubated with Alexa Fluor® 488-labeled goat anti-mouse IgG antibody or Alexa Fluor® 568-labeled goat anti-rabbit IgG antibody (Molecular Probes, Eugene, OR, USA), diluted 1:2000 with blocking buffer, for 1 hr at room temperature. After a final wash, the cells were stained with DAPI and viewed using a fluorescence microscope (BZ-8100; Keyence, Osaka, Japan).
Northern blot analysis
Total RNA was prepared from the cultured HNPCEC at 7 days using an RNeasy Mini Kit (Qiagen). One microgram of RNA was subjected to Northern blot analysis, as described previously [15]. The probes for recognition of human fibrillin-1 were generated as described previously [16]. Briefly, templates were obtained using the reverse-transcription polymerase chain reaction (RT-PCR) with RNA extracted from human gingival fibroblasts, an approach that has previously been shown to produce significant amounts of both fibrillins [15]. The PCR products were ligated into the pT7/T3-α18 vector (Life Technologies, Grand Island, NY, USA), and then the plasmid was linearized with Hind III followed by T7 RNA polymerase mixed with digoxigenin (DIG)-labeled nucleotides (Roche Molecular Biochemicals) to generate the DIG-labeled 698-bp fibrillin-1 RNA probe. The RNA probe for β-actin was from Roche Molecular Biochemicals.
Western blot analysis
At 3, 5 and 7 days of HNPCEC culture, cell/matrix samples were prepared as described previously [16]. The proteins (5 µg) were subjected to electrophoresis on 4–12% NuPAGE Bis-Tris gel (Invitrogen) for Western blot analysis, as described previously [10]. The primary antibodies used were those against human fibrillin-1 (mouse monoclonal antibody, clone 11C1.3, Thermo Fisher Scientific Anatomical Pathology, Fremont, CA, USA), MAGP-1 (rabbit polyclonal antibody, Abcam, Cambridge, MA, USA) and β-actin (Sigma-Aldrich) at 1:5000 dilution. Prestained molecular weight markers (Invitrogen) were also run on each blot. Densitometric analysis of the signals was performed using the Image J program (National Institutes of Health, Bethesda, MD, USA) after finding the linear portion by sequential dilution of the proteins. Small variations in protein loading were corrected by normalization relative to the intensity of the corresponding band of β-actin.
Small interfering RNA (siRNA) design and transient transfection
siRNAs for human MAGP-1 (accession # NM_ 017459) was designed and synthesized by Sigma Aldrich Corp. The synthesized siRNA for MAGP-1 corresponded to bases 462–484 in mRNA. The siRNA sequence was: sense 5'-GUCUCAACGAGGUCUGCUUCU-3', antisense 3'-AAGCAGACCUCGUUGAGACAC-5'. The negative control (scrambled order) was: sense 5'-CCUUAUGGCGG CUUUAGCGAU-3', antisense 3'-CGCUAAAGCCGCCAU AAGGAG-5'. The sequence of the negative control was designed as a randomized version of bases of MAGP-1. BLAST searches indicated that this siRNA was specific for MAGP-1, and had no homology with other proteins.
Transfection was performed continuously on days 1 and 4 of culture. The siRNA was transfected into HNPCEC using HiPerFect Transfection Reagent (Qiagen, Hilden, Germany). First, 237.5 µl of OptiMEM medium/dish (Invitrogen, Grand Island, NY, USA) and 12.5 µl of the transfection reagent were preincubated for 10 min at room temperature. During this time, 748 µl of OptiMEM medium was mixed with 2 µl of 100 µM siRNA. The two mixtures were then combined and incubated for 20 min at room temperature to allow formation of their complex. The entire mixture was added to the cells in one dish, resulting in a final concentration of 200 nM for the siRNAs. After 12 hr of incubation, the transfection medium was replaced with fresh complete medium (DMEM with 10% NCS). Mock transfection of cultures with the transfection reagent alone was used as a control. HNPCEC were transfected three times with the siRNA duplex (0, 200 nM), with a 72-hr interval between, and analyzed on day 7.
III. Results
HNPCEC express MAGP-1 gene and protein
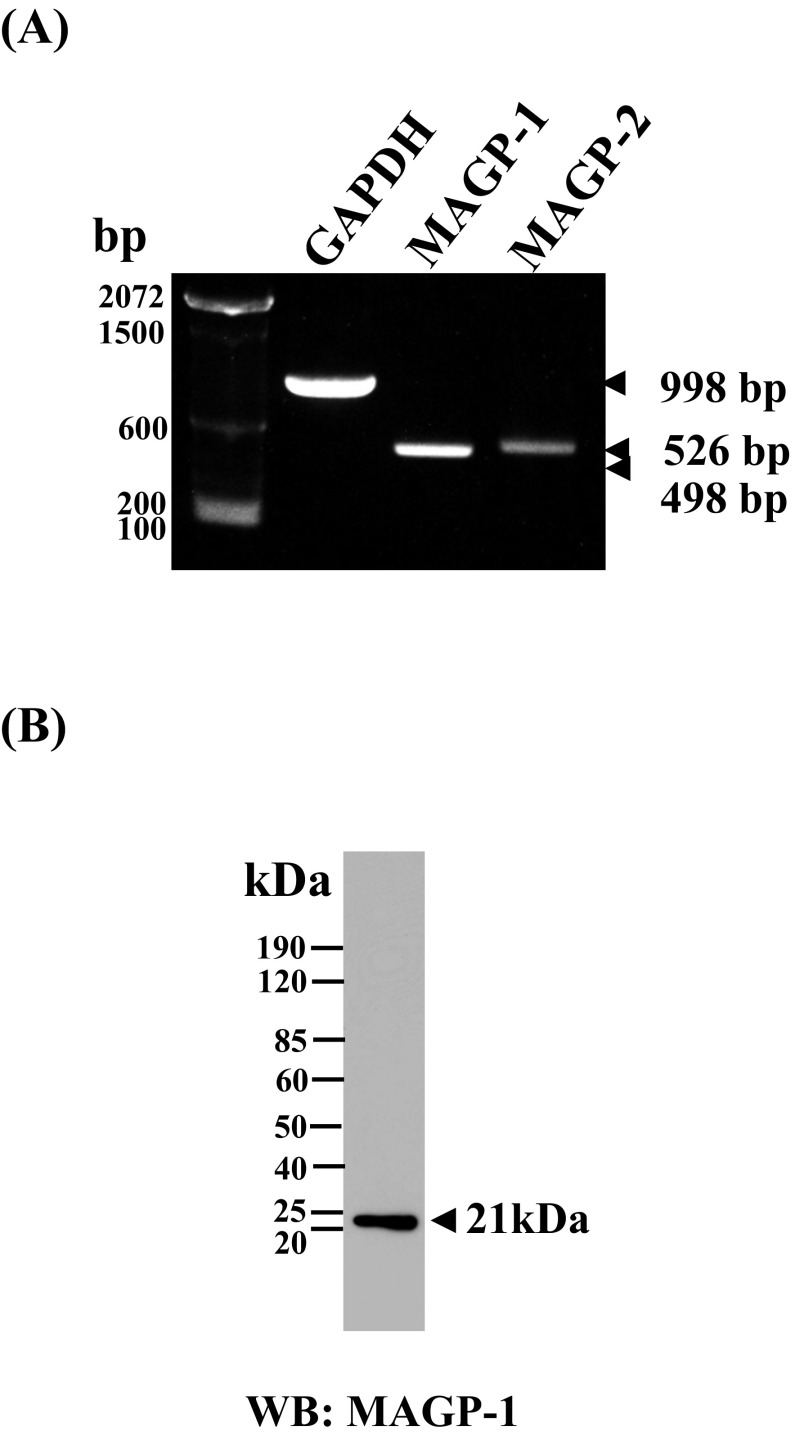
Using PCR analysis and Western blotting, respectively, we first examined whether HNPCEC express MAGP-1 mRNA and protein (Fig. 1). At the same time, we similarly examined MAGP-2 mRNA, which is the other isoform in the MAGP family. PCR analysis clearly detected the MAGP-1 product at the predicted size of 498 bp and MAGP-2 faintly at 526 bp (Fig. 1A). We then examined protein deposition in the HNPCEC cell/matrix layer of the two proteins by Western blotting. The MAGP-1 antibody detected a single band at an appropriate molecular mass of 21 kDa (Fig. 1B). MAGP-2 was not detected at the protein level (data not shown).
Fig. 1.
Human non-pigmented ciliary epithelial cells express MAGP-1 mRNA and protein. Human non-pigmented ciliary epithelial cells were cultured for 7 days, and cDNA was amplified with specific primers for MAGP-1, MAGP-2, and the housekeeping gene GAPDH. A MAGP-1 band corresponding to 498 bp was clearly detected, whereas the MAGP-2 band corresponding to 526 bp was fainter than the MAGP-1 band (A). Western blot of cell/matrix lysates (5 µg) of non-pigmented ciliary epithelial cells cultured for 7 days (B). The blots were obtained using anti-MAGP-1 and MAGP-2 antibodies. The two probes recognized bands of 21 and 19 kDa, respectively. MAGP-1 was detected at an estimated 21 kDa, whereas MAGP-2 was not detected (not shown).
MAGP-1 deposition in cell/matrix layers of HNPCEC culture
Deposition of MAGP-1 in HNPCEC cultures was investigated on days 3, 5, and 7 by immunohistochemistry and Western blotting (Fig. 2). As we had demonstrated previously [21], on day 3 of culture, positive staining for fibrillin-1 was observed on oxytalan fibers, which appeared as networks of thin fibers (Fig. 2A). On day 5 of culture, the thick oxytalan fibers positive for fibrillin-1 appeared to have become wider and were associated with thinner fibers. On day 7 of culture, the thick fibrillin-1-positive fibers occupied most of the area. On the other hand, on day 3, the MAGP-1-positive portion was not detected (Fig. 2A). On day 5, the MAGP-1-positive area had a dendrite-like appearance, and was located on fibers where fibrillin-1 was positive. Subsequently on day 7, the dendrite zone positive for MAGP-1 was enlarged, and its positivity for fibrillin-1 was greater than that on day 5.
Fig. 2.
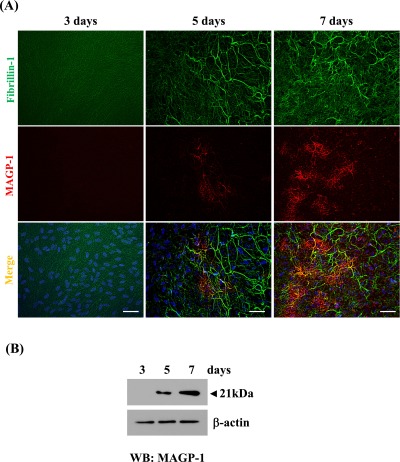
Immunolocalization and deposition of MAGP-1 to oxytalan fibers. (A) Double immunofluorescence for fibrillin-1 (upper lane) and MAGP-1 (middle lane) in cultures of human non-pigmented ciliary epithelial cells. Human non-pigmented ciliary epithelial cells were cultured, then simultaneously labeled for fibrillin-1 (green) (upper panels), MAGP-1 (red) (middle panels), and superimposition of both labels (lower panels) on days 3, 5 and 7. DAPI was used for nuclear staining (blue). Bar=20 µm. (B) Western blot analysis: Total cell/matrix proteins were extracted and 5 µg was subjected to Western blot analysis, as described in “Materials and Methods”. A 21-kDa band corresponding to MAGP-1 was detected on day 5 and the level of intensity increased up to day 7.
Deposition of MAGP-1 in HNPCEC cultures was investigated on days 3, 5 and 7 by Western blotting of cell/matrix layer lysates (Fig. 2B). Western blot analysis detected the 21-kDa band reactive with the MAGP-1 antibody on day 5, and the signal increased up to day 7, being consistent with the immunofluorescence data.
Effect of MAGP-1 knockdown on oxytalan fiber formation
The effect of suppression of each of the genes for MAGP-1 on oxytalan fiber formation in HNPCEC culture was then investigated (Fig. 3). Transient transfection with siRNA for MAGP-1 was performed on day 7. The immunohistochemistry data showed that HNPCEC transfected with scrambled MAGP-1 siRNA had features similar to those of the control (mock-transfected) cells (Fig. 3A). HNPCEC transfected with MAGP-1 siRNA produced fibrillin-1-positive networks of thin fibers, resembling those of the control at day 3, as shown in Fig. 1. In cells transfected with MAGP-1 siRNA, fibrillin-1 staining was diminished in comparison with the control and scrambled siRNA-transfected HNPCEC.
Fig. 3.
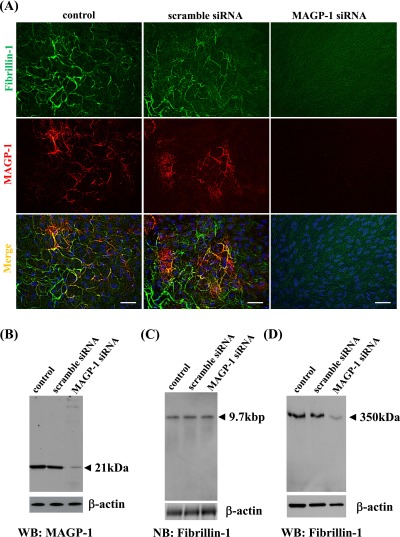
Effect of MAGP-1 suppression on oxytalan fiber formation. (A) Human non-pigmented ciliary epithelial cells were transiently transfected with 200 nM scrambled siRNA for MAGP-1 (middle column), or with 200 nM siRNA for MAGP-1 (right column). Mock-transfected cells are shown in the left column. Immunofluorescence staining was performed with antibodies against fibrillin-1 (upper column) and MAGP-1 (middle column), and superimposition of both labels (lower column) on day 7. DAPI was used for nuclear staining (blue). Bar=20 µm. (B) Western blot analysis using MAGP-1 antibody. Human non-pigmented ciliary epithelial cells were mock-transfected (left lane) or transfected with scrambled siRNA duplex for MAGP-1 (middle lane) and MAGP-1 (right lane). Equal amounts of proteins (5 µg) were subjected to Western blot analysis. (C) Northern blot analysis using the fibrillin-1 probe. Human non-pigmented ciliary epithelial cells were mock-transfected (left lane) or transfected with scrambled siRNA duplex for MAGP-1 (middle lane) and MAGP-1 (right lane). Equal amounts of RNA (1 µg) on day 7 were subjected to Northern blot analysis. (D) Western blot analysis using fibrillin-1 antibody. Human non-pigmented ciliary epithelial cells were mock-transfected (left lane) or transfected with scrambled siRNA duplex for MAGP-1 (middle lane) and MAGP-1 (right lane). Equal amounts of proteins (5 µg) were subjected to Western blot analysis.
Densitometric analysis based on the western blot showed that MAGP-1 siRNA effectively reduced the level of MAGP-1 deposition to about 6% level of the control (vehicle only) in the HNPCEC at 7 days (Fig. 3B). Moreover, scrambled siRNA had no effect on MAGP-1 deposition, because of no difference from the control. Next, we investigated whether MAGP-1 siRNA would affect fibrillin-1 gene expression and protein deposition. Northern blotting showed that fibrillin-1 gene expression after transfection with MAGP-1 siRNA was similar to that after transfection with mock and scrambled siRNA, showing that MAGP-1 siRNA had no effect on expression of the fibrillin-1 gene (Fig. 3C). However, Western blot data showed that MAGP-1 siRNA reduced the deposition of fibrillin-1 relative to control and scrambled MAGP-1 siRNA-transfected cells, being consistent with the immunofluorescence data (Fig. 3D).
IV. Discussion
In the present study, we have demonstrated for the first time that HNPCEC express MAGP-1, which controls the development of oxytalan fibers. MAGP-2 was not detected under protein level. This result is thought to be reasonable, for MAGP-2 also did not be identified in human ciliary zonule by proteomics analysis [1] and in bovine ciliary zonule by immunofluorescence [3]. MAGP-1 is localized on the bead regions of microfibrils [5], and has been the best candidate for a regulator of elastin deposition on microfibrils [7]. In fact, we previously demonstrated that MAGP-1 controls the deposition of elastin on microfibrils in elastin-producing cell culture [17]. However, the role of MAGP-1 in the metabolism of oxytalan fibers has remained unclear. Since the ciliary zonule consists of pure microfibrils, it is a good structure representative of oxytalan fibers distributed elsewhere in the human body. MAGP-1 was first localized on the bovine ciliary zonule. Since in situ hybridization has shown that non-pigmented ciliary epithelial cells express fibrillin-1 [4, 12], it has been thought that non-pigmented ciliary epithelial cells form the ciliary zonule. We previously demonstrated in vitro that HNPCEC express fibrillin-1 and form an oxytalan fiber network [21]. Although MAGP-1 is able to bind with fibrillin-1 [14], it is unknown how it contributes to development of the ciliary zonule. Skin fibroblasts from MAGP-1-knockout mice form a normal oxytalan fiber network labeled with fibrillin-2 [20]. They did not refer to a phenotype of the eye. The fact that fibroblasts from MAGP-1 knockout mice form normal oxytalan fibers may be due to the difference of cell type; epithelial cells and fibroblasts, as well as that of species. In the present study, however, HNPCEC formed a fibrillin-1-positive thin oxytalan fiber network, but did not form thick fibers in culture after MAGP-1 silencing. As is the case in MAGP-1-knockout mice, MAGP-2 may compensate for MAGP-1. In our present study, however, MAGP-2 was not detected even under MAGP-1-suppressed conditions (data not shown). We have also previously demonstrated that both fibrillin-1 and fibrillin-2 are required for fiber development in HNPCEC culture [21]. More recently, Shi et al. showed that the fibrillin-2-null mouse had a disordered ciliary zonule [13]. At least, in this study, MAGP-1 was associated with oxytalan fiber development. Further study will be necessary to clarify the association of MAGP-1 with fibrillin-1 and fibrillin-2.
Shi et al. also showed that MAGP-1 was a representative molecule for detection of the mouse ciliary zonule [12]. However, in our in vitro study, immunofluorescence for MAGP-1 was partially merged with fibrillin-1-positive oxytalan fibers, although MAGP-1 is essential for the development of oxytalan fibers. MAGP-1 may therefore be a trigger for fiber development, or MAGP-1 may bind with other microfibril-associated molecules and control fiber development indirectly.
In summary, we have demonstrated that MAGP-1 suppression in cultured HNPCEC resulted in a reduction of extracellular deposition and development of oxytalan fibers. These results suggest that MAGP-1 plays a role in the extracellular deposition of fibrillin-1 during formation of the human ciliary zonule.
V. References
- 1.Cain S. A., Morgan A., Sherratt M. J., Ball S. G., Shuttleworth C. A., Kielty C. M. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6:111–122. doi: 10.1002/pmic.200401340. [DOI] [PubMed] [Google Scholar]
- 2.Fullmer H. M., Lillie R. D. The oxytalan fiber: a previously undescribed connective tissue fiber. J. Histochem. Cytochem. 1958;6:425–430. doi: 10.1177/6.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Gibson M. A., Finnis M. L., Kumaratilake J. S., Cleary E. G. Microfibril-associated glycoprotein-2 (MAGP-2) is specifically associated with fibrillin-containing microfibrils but exhibits more restricted patterns of tissue localization and developmental expression than its structural relative MAGP-1. J. Histochem. Cytochem. 1998;46:871–886. doi: 10.1177/002215549804600802. [DOI] [PubMed] [Google Scholar]
- 4.Hanssen E., Franc S., Garrone R. Synthesis and structural organization of zonular fibers during development and aging. Matrix Biol. 2001;20:77–85. doi: 10.1016/s0945-053x(01)00122-6. [DOI] [PubMed] [Google Scholar]
- 5.Henderson M., Polewski R., Fanning J. C., Gibson M. A. Microfibril-associated glycoprotein-1 (MAGP-1) is specifically located on the beads of the beaded-filament structure for fibrillin-containing microfibrils as visualized by the rotary shadowing technique. J. Histochem. Cytochem. 1996;44:1389–1397. doi: 10.1177/44.12.8985131. [DOI] [PubMed] [Google Scholar]
- 6.Kawagoe M., Tsuruga E., Oka K., Sawa Y., Ishikawa H. Matrix metalloproteinase-2 degrades fibrillin-1 and fibrillin-2 of oxytalan fibers in the human eye and periodontal ligaments in vitro. Acta Histochem. Cytochem. 2013;46:153–159. doi: 10.1267/ahc.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kielty C. M., Sherratt M. J., Shuttleworth C. A. Elastic fibres. J. Cell Sci. 2002;115:2817–2828. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- 8.Kielty C. M. Elastic fibres in health and disease. Expert Rev. Mol. Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 9.Mecham R. P., Davis E. C. In “Extracellular Matrix Assembly and Structure” ed. by P. D. Yurchenco, D. E. Birk and R. P. Mecham. Academic Press; New York: 1994. Elastic fiber structure and assembly; pp. 281–314. [Google Scholar]
- 10.Nakatomi Y., Tsuruga E., Nakashima K., Sawa Y., Ishikawa H. EMILIN-1 regulates the amount of oxytalan fiber formation in periodontal ligaments in vitro. Connect. Tissue Res. 2011;52:30–35. doi: 10.3109/03008207.2010.502982. [DOI] [PubMed] [Google Scholar]
- 11.Raviola G. The fine structure of the ciliary zonule and ciliary epithelium. With special regard to the organization and insertion of the zonular fibrils. Invest. Ophthalmol. 1971;10:851–869. [PubMed] [Google Scholar]
- 12.Shi Y., Tu Y., De Maria A., Mecham R. P., Bassnett S. Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest. Ophthalmol. Vis. Sci. 2013;54:2504–2515. doi: 10.1167/iovs.13-11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y., Tu Y., Mecham R. P., Bassnett S. Ocular phenotype of fbn2-null mice. Invest. Ophthalmol. Vis. Sci. 2013;54:7163–7173. doi: 10.1167/iovs.13-12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trask B. C., Trask T. M., Broekelmann T., Mecham R. P. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol. Biol. Cell. 2000;11:1499–1507. doi: 10.1091/mbc.11.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuruga E., Irie K., Sakakura Y., Yajima T. Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J. Periodont. Res. 2002;37:23–28. doi: 10.1034/j.1600-0765.2002.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsuruga E., Irie K., Yajima T. Gene expression and accumulation of fibrillin-1, fibrillin-2, and tropoelastin in cultured periodontal fibroblasts. J. Dent. Res. 2002;81:771–775. doi: 10.1177/0810771. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruga E., Yajima T., Irie K. Microfibril-associated glycoprotein-1 and fibrillin-2 are associated with tropoelastin deposition in vitro. Int. J. Biochem. Cell Biol. 2005;37:120–129. doi: 10.1016/j.biocel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Tsuruga E., Irie K., Yajima T. Fibrillin-2 degradation by matrix metalloproteinase-2 in periodontium. J. Dent. Res. 2007;86:352–356. doi: 10.1177/154405910708600410. [DOI] [PubMed] [Google Scholar]
- 19.Tsuruga E., Nakashima K., Ishikawa H., Yajima T., Sawa Y. Stretching modulates oxytalan fibers in human periodontal ligament cells. J. Periodont. Res. 2009;44:170–174. doi: 10.1111/j.1600-0765.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 20.Weinbaum J. S., Broekelmann T. J., Pierce R. A., Werneck C. C., Segade F., Craft C. S., Knutsen R. H., Mecham R. P. Deficiency in microfibril-associated glycoprotein-1 leads to complex phenotypes in multiple organ systems. J. Biol. Chem. 2008;283:25533–25543. doi: 10.1074/jbc.M709962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamanouchi K., Tsuruga E., Oka K., Sawa Y., Ishikawa H. Fibrillin-1 and fibrillin-2 are essential for formation of thick oxytalan fibers in human nonpigmented ciliary epithelial cells in vitro. Connect. Tissue Res. 2012;53:14–20. doi: 10.3109/03008207.2011.602767. [DOI] [PubMed] [Google Scholar]