Abstract
Aquaporin (AQP) is suggested to be regulated by leptin through the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway. AQP7 and AQP9 are membrane proteins with water and glycerol channels, the latter of which is essential for triglyceride synthesis. We conjectured that the expression of AQP7 and AQP9 would be altered in the skeletal myofibers in obese leptin deficient ob/ob mice as compared with that of wild mice. RNA and protein levels were studied in the quadriceps femoris muscles of ob/ob and wild mice. Real time quantitative RT-PCR analysis showed that mouse AQP7 mRNA levels in skeletal muscles were significantly higher in ob/ob mice than in wild mice (P<0.01), whereas mouse AQP9 mRNA level was not different between the two groups (P>0.05). Histologically the type 1 myofibers of ob/ob mice contained numerous lipid droplets in oil red O stain samples. Immunohistochemical staining of ob/ob mouse muscles revealed enhanced expression of AQP7 at myofiber surface membranes, while AQP9 expression appeared to be similar to that of wild mice. The findings suggest that the upregulated expression of AQP7 in ob/ob mouse muscles facilitates the secretion of glycerol from myocytes.
Keywords: AQP7, AQP9, RNA and protein levels, skeletal muscles, ob/ob and wild mice
I. Introduction
Obese ob/ob mice lack the ob gene, the product of which is a hormone named leptin produced by adipocytes [30]. Leptin regulates several physiological processes such as glucose and lipid metabolism, blood pressure homeostasis, immunity, and reproduction [10, 24]. Recently leptin has been shown to regulate the aquaporin (AQP) function through the phosphatidylinositol 3 kinase/Akt/mammalian target of rapamycin pathway in human visceral adipocytes and hepatocytes [28]. Skeletal muscle is the largest organ in the human body and is an important target for leptin [5, 10]. Several studies of skeletal muscle in ob/ob mice described decreased muscle mass regardless of the degree of obesity [17, 31, 33, 34], although detailed myopathological studies have yet to be performed in these studies. We speculated that neutral lipid deposition in the muscle cells might be increased in obese ob/ob mice. In favor of this, Almond and Enser [2] investigated the fat content of the skeletal muscles from obese ob/ob mice and reported that the content of muscle fat in obese ob/ob mice was three times greater than that of lean wild mice. We studied the neutral lipid content of obese ob/ob myofibers in comparison with lean wild myofibers in the cryosections of muscles stained with oil red O.
AQPs are the small intrinsic channel-forming membrane proteins of epithelial and endothelial cells. They are divided into two groups [1]: AQPs with water-selective transport channel and those with a water channel permeable to neutrally charged small molecules such as glycerol, urea, and purines [15, 16]. Furthermore AQP7 and AQP9 transport arsenite as well [21]. The latter AQPs are called aquaglyceroporins in which AQP3, AQP7, AQP9 and AQP10 are included [1, 26]. Glycerol is an important molecule for lipid metabolism and direct source of glycerol-3 phosphate for the synthesis of triglyceride [4] which is the main substance of neutral lipid.
AQP7 is considered to represent the gateway for the efflux of lipolysis-derived glycerol from adipocytes, whereas AQP9 is considered to play a role in the influx of circulating glycerol into the hepatocytes [10, 24]. Transgenic animals are useful for the analyses of pathophysiological mechanisms of AQPs in the regulation of fat accumulation. AQP7-deficient mice have been reported to lead to obesity [12, 13, 22], while AQP9 deletion leads to the onset of diabetes mellitus [29]. We have so far been analyzing the expression of AQPs3, 7 and 9 in human skeletal muscles and AQP7 in mouse skeletal muscles [14, 38, 39]. These three AQPs in the human skeletal muscles and AQP7 in the mouse skeletal muscles were found to be expressed at the myofiber surface of the skeletal muscle tissues, although the contradictory result has been reported with respect to AQP7 [32]. AQP4 is also highly expressed in fast twitch skeletal muscles [8]. It is reduced in the muscles of Duchenne muscular dystrophy patients [37]; in dystrophic mdx muscles [9, 20]; and syntrophin KO mice [41].
The aim of this study is to examine whether the neutral lipid content of skeletal myofibers of obese ob/ob mice is increased by using the histochemical method and whether the expression of AQP7 and AQP9 in the myofiber surface membranes of obese ob/ob mice is altered at the RNA and protein levels.
II. Materials and Methods
Muscle samples
Ten obese ob/ob mice and 10 age matched lean wild mice two to three months of age were sacrificed by cervical dislocation and the quadriceps femoris muscles were excised from each mouse. All animal experiments were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the ethics committee of Showa University (admission No. 02082).
Quantitative real time reverse transcription polymerase chain reaction (real-time RT-PCR) for AQP7 and AQP9 mRNAs in skeletal muscles of obese ob/ob mice and lean wild mice
Total RNA was extracted from each muscle sample of 10 obese ob/ob mice and 10 lean wild mice using TRIzol (Invitrogen). Concentrations of mouse AQP7 and mouse AQP9 mRNA contents were estimated by real-time RT-PCR. First-strand cDNA was synthesized using AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies) and 100 ng of extracted total RNA. Oligonucleotide primer sets were designed from mouse AQP7 [Mus musculus AQP7, MA101958, TaKaRa], mouse AQP9 [Mus musculus AQP9, MA117402, TaKaRa] sequences: mouse AQP7 mRNA: sense strand, 5'-TGGGTTTTGGATTCGGAGT-3'; antisense strand, 5'-TGTTCTTCTTGTCGGTGATGG-3'; and the mouse AQP9 mRNA: sense strand, 5'-CTCAACTC TGGTTGTGCCATGAA-3'; antisense strand, 5'-ATCATA GGGCCCACGACAGGTA-3'. To compensate for differences in RNA quality or RT efficacy, mRNA expression of mouse 18s ribosomal RNA was calculated in each muscle sample. Oligonucleotide primers for 18s ribosomal RNA were designed as follows: sense strand, 5'-GATCCGAG GGCCTCACTAAAC-3'; antisense strand, 5'-AGTCCCTG CCCTTTGTACACA-3'.
Each primer set for the measurement of mouse AQPs7, 9 and mouse 18s ribosomal RNA levels was mixed with the respective cDNA using SYBR Prime Script RT-PCR Kit II (TaKaRa). Real-time PCR was performed on an ABI Prism 7900 sequence detection system (Applied Biosystems) with thermocycle conditions including initial denaturation at 95°C for 30 sec followed by 45 cycles at 95°C for 5 sec, 60°C for 1 min with dissociative reaction of PCR products from 65°C to 95°C. Threshold cycle was calculated using the Sequence Detector Systems version (Applied Biosystems) by determining the cycle number. Mouse AQP7 and AQP9 mRNA expressions were calculated using the standard curve method normalizing with that of mouse 18s ribosomal RNA internal control. Total RNAs from muscle samples of 10 ob/ob mice and 10 wild mice were analyzed in duplicate by using this method and the mean was calculated in each muscle sample. The difference between ob/ob mouse group and wild mouse group was evaluated using two-tailed t test. A p-value less than 0.05 was considered to be statistically significant.
Peptide syntheses and antibody production
General procedures for peptide syntheses and antibody production were similar to those described previously [14, 39]. Briefly, the peptide (MVQASGHRRSTRGSK-C) of the N-terminal end of the cytoplasmic domain in the human AQP7 molecule (Entrez NM001170) and the peptide (KAEQSEDKPEKYE-C) of the C-terminal end of the cytoplasmic domain in the human AQP9 molecule (Entrez NM020980) were synthesized and the extra cysteine was added to the C-terminus of AQP7 and AQP9 molecules. Bovine thyroglobulin was added at an extra cysteine residue in each peptide. The antibodies against these peptides were generated in rabbits. Solid phase enzyme-linked immunosorbent assay was used to find the rabbit polyclonal antibody titers, which were ×102,400 for both AQP7 and AQP9. These antisera were affinity purified.
The affinity purified rabbit polyclonal antibody against the C-terminal end of the rat AQP4 was previously produced in our laboratory [37].
Histopathological and immunohistochemical studies
The quadriceps femoris muscles were excised from 10 obese ob/ob mice and 10 wild mice after cervical dislocation. The excised muscle samples were immediately frozen in isopentane cooled with liquid nitrogen. Frozen 6-µm-thick cross sections of the muscles were placed on slide glasses and stained with hematoxylin and eosin (HE) and oil red O solutions, respectively. The immunohistochemical stainings with anti-AQP7 and AQP9 antibodies, respectively, were also performed. After the elimination of non-specific reaction by incubating the sectioned muscle samples with 5% normal swine serum in phosphate buffered saline (PBS) for 20 minutes at room temperature, the affinity-purified primary rabbit anti-AQP7 and AQP9 antibodies were diluted to a final IgG concentration of 5 µg/ml, and were applied, respectively, to the sectioned muscle samples during overnight at 4°C. The negative control specimens were prepared by using normal rabbit IgG at a concentration of 5 µg/ml instead of the respective primary antibody. After washing, the sections were incubated for 60 min at room temperature with FITC-conjugated swine anti-rabbit immunoglobulin (DAKO code No. F0205, Denmark) diluted 1:50 in PBS. Serial sections of HE or oil red O stained muscle samples and the immunostained muscle samples from obese or wild mice with anti-AQP7 or AQP9 antibody were also immunostained with the affinity purified rabbit anti-AQP4 antibody at the concentration of 5 µg/ml IgG for the judgment of myofiber type [8]. The immunostained muscles were examined using Nikon H550L fluorescent microscope.
III. Results
Quantitative real time RT-PCR for AQP7 and AQP9 mRNAs in the skeletal muscles of obese ob/ob mice and lean wild mice
The standard curves for the quantification of mouse AQP7 and AQP9 mRNAs were linear across 4 to 5 log ranges of RNA concentration. Correlation coefficients were 0.9642 for mouse AQP7 mRNA, 0.9805 for mouse AQP9 mRNA and 0.9952 for mouse 18s ribosomal RNA. Group mean ratios±standard error of the mean of mouse AQP7 mRNA copy number versus mouse 18s ribosomal RNA mRNA copy number were 216.9±37.1 and 100.0±20.3 in skeletal muscles of 10 obese ob/ob mice and 10 lean wild mice, respectively. These two ratios were statistically significantly different (P<0.01 two tailed t test) (Table 1). On the contrary group mean ratios±standard error of the mean of mouse AQP9 mRNA copy number versus mouse 18s ribosomal RNA mRNA copy number in the 10 obese ob/ob mice and 10 lean wild mice were 114.3±23.6 and 100.0±18.7, respectively. These ratios were statistically non-significant (P>0.05 two tailed t test) (Table 1).
Table 1.
Quantitative real time RT-PCR for AQP7 and AQP9 mRNAs in the quadriceps femoris muscles of obese ob/ob mice and lean wild mice
| mouse AQP7 or AQP9 mRNA copy number | |||||
|---|---|---|---|---|---|
| mouse 18s ribosomal RNA mRNA copy number | |||||
| AQP7 | AQP9 | ||||
| obese ob/ob mice (n=10) | 216.9±37.1* | 114.3±23.6* | |||
| P<0.01 | P>0.05 | ||||
| wild mice (n=10) | 100.0±20.3 | 100.0±18.7 | |||
* Group mean±standard error of the mean; P values were calculated by two-tailed t test.
Histopathological and immunohistochemical studies
HE staining samples of the cross-sectioned muscle tissues of wild mice contained polygonal shaped myofibers without variation of myofiber size. Cytoplasm of wild mouse myofibers had two types of appearances: a smooth cytoplasmic appearance and a somewhat granular appearance with slit-like structures (Fig. 1A). HE stained muscles of the obese myofibers revealed somewhat different appearance from those of wild mice. Muscle tissues of obese mice contained more numerous myofibers with granular appearance and slit-like structures (Fig. 1B). Immunostaining of serial muscle section with anti-AQP4 antibody showed that these myofibers were immunonegative and therefore, slow twitch type 1 fibers (Fig. 1C).
Fig. 1.
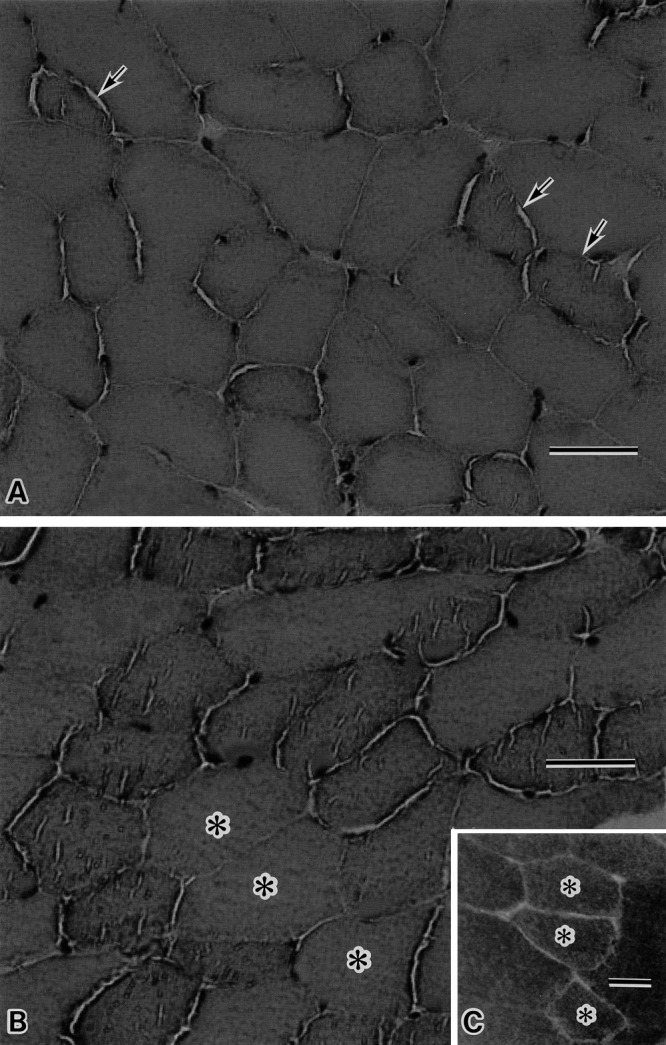
Hematoxylin and eosin (HE) staining of the quadriceps femoris muscles of lean wild mouse (A) and obese ob/ob mouse (B), and immunohistochemical staining with anti-aquaporin 4 (AQP4) antibody in the serial section of ob/ob muscle in figure B (C). In wild mouse muscle (A), the scattered myofibers (arrow) with slight granular sarcoplasmic appearance and slit like structures are seen. In the ob/ob muscle (B), numerous myofibers with more granular sarcoplasmic appearance and the plural number of slit like structures are noted. The myofibers with asterisk show the relatively smooth sarcoplasmic appearance. These myofibers are type 2 fibers, since the serial section reveals that these fibers are well stained with anti-AQP4 antibody (asterisk in C). Bar=50 µm (A, B, C).
Oil red O staining demonstrated that myofibers of obese mice contained marked numerous lipid droplets (Fig. 2A) as compared with those of wild mice (Fig. 2B).
Fig. 2.
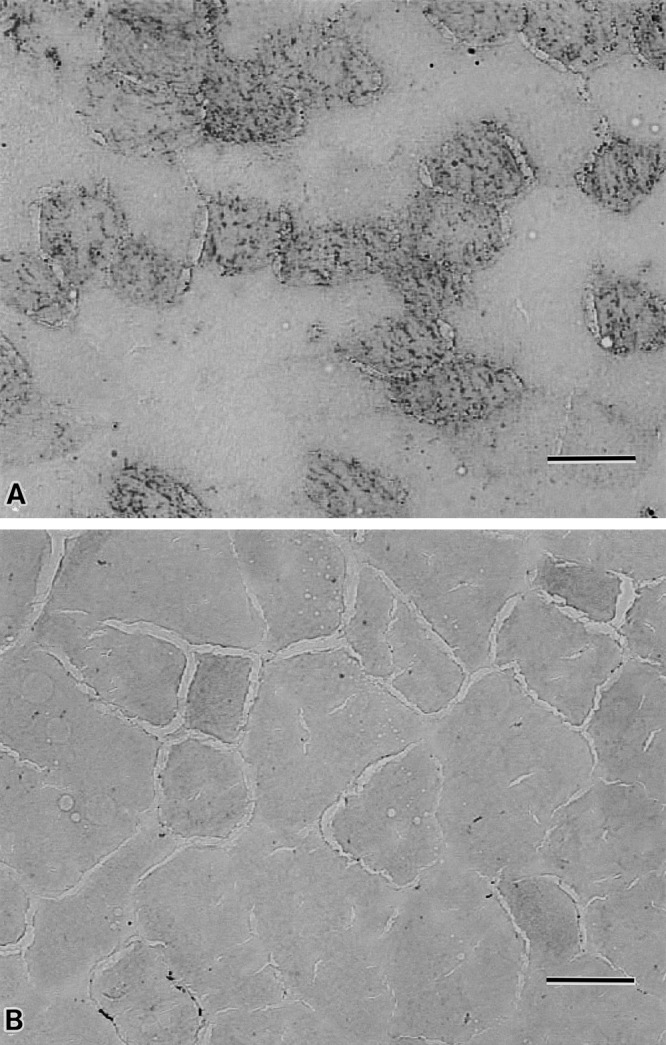
Oil red O staining muscle samples of obese ob/ob mouse (A) and lean wild mouse (B). The ob/ob muscle (A) contains a lot of myofibers with numerous lipid droplets in their sarcoplasm; while the myofibers of lean wild mouse scarcely contains the lipid droplets (B). Bar=50 µm (A, B).
Immunohistochemical staining of wild mouse skeletal muscle with primary anti-AQP7 antibody revealed the presence of both immunopositive and immunonegative myofibers at the cell surface (Fig. 3A). Immunostained serial muscle section with primary anti-AQP4 antibody showed that AQP7 positive myofibers were also AQP4 immunopositive and, therefore, these myofibers were type 2 fibers (Fig. 3B). Immunohistochemistry of skeletal myofibers in wild mice with the primary anti-AQP9 antibody contained both immunopositive and immunonegative myofibers (Fig. 3C), the former of which were type2 fibers as judged by the anti-AQP4 antibody staining of serial muscle section (Fig. 3B). Negative control muscle samples of the wild mice without primary antibody showed no immunoreaction at muscle cell surfaces (Fig. 3D).
Fig. 3.
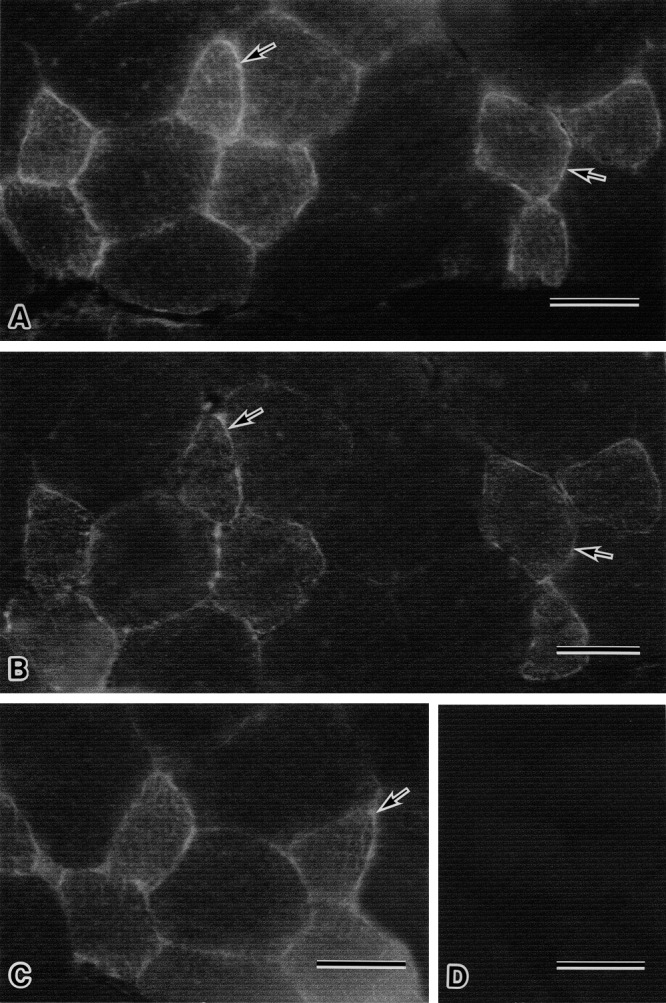
Immunohistochemical stainings with anti-AQP7, AQP4 and AQP9 antibodies of serial sections of muscle from wild mouse (A, B, C), respectively, and immunocontrol staining of wild mouse muscle (D). The immunostaining sample with anti-AQP7 antibody from wild muscle contains the myofibers reacted with anti-AQP7 antibody at their cell surface (arrows in A) and the myofibers without immunoreactivity by anti-AQP7 antibody (A). The serial muscle section immunostained with anti-AQP4 antibody (B) demonstrates that the fairly well stained myofibers at their cell surface with anti-AQP7 antibody observed in A (arrows) are also obviously immunoreactive with anti-AQP4 antibody (arrows in B), and therefore AQP7 positive myofibers are type 2 fibers. The serial muscle section with immunostaining by anti-AQP9 antibody reveals that the fairly well stained myofibers with this antibody observed in C (arrow) are also type 2 myofibers on the basis of evidence provided with figure B. The immunocontrol sample of wild muscle reveals no immunoreaction at the myofiber surface (D). Bar=50 µm (A–D).
Immunohistochemical staining of obese ob/ob mouse skeletal muscles with anti-AQP7 antibody demonstrated more intense immunostaining at muscle cell surface of almost all the myofibers (Fig. 4A) than that of wild mouse skeletal muscles (Fig. 4B). On the contrary, immunostaining samples of wild mouse skeletal muscles with anti-AQP7 antibody contained fairly well stained myofibers at fiber surfaces and faint to no immunoreactive myofibers (Fig. 4B).
Fig. 4.
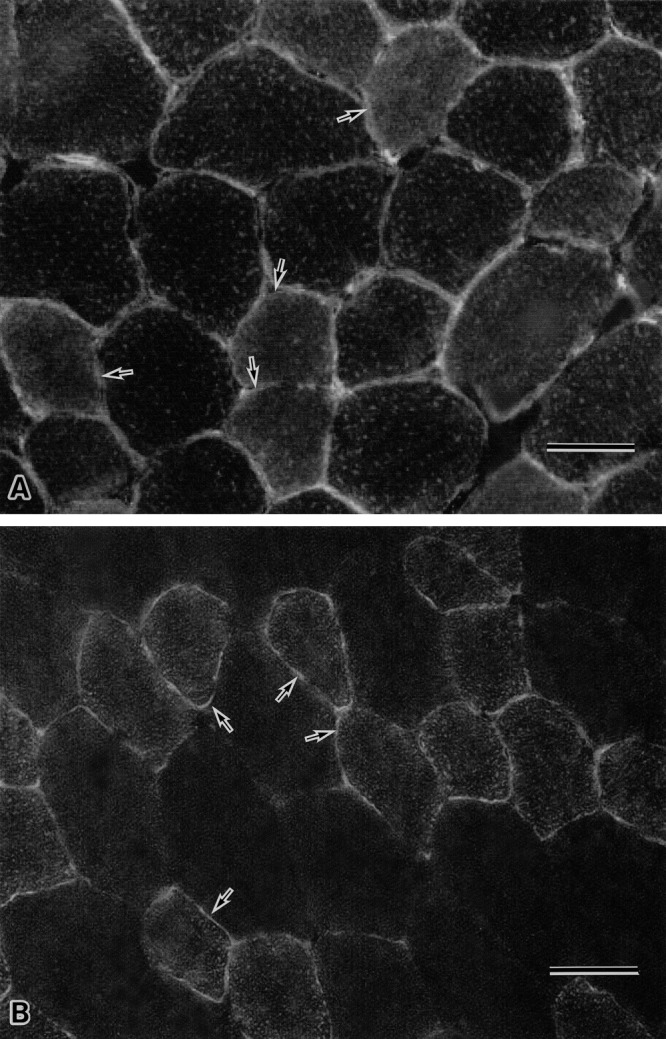
Immunohistochemical staining with anti-AQP7 antibody of muscles from ob/ob mouse (A) and wild mouse (B). The immunostaining sample of ob/ob muscle shows that apparently all the myofibers including the seemingly type 2 fibers (arrow in A) react with anti-AQP7 antibody at their cell surface (A); while the immunostaining sample of wild muscle with anti-AQP7 antibody reveals the presence of myofibers with relatively well stained surface membrane (arrow in B) (type2 fiber) and the myofibers with weakly immunopositive to apparently immunonegative cell surface (B). Bar=50 µm (A, B).
Immunohistochemistry with anti-AQP9 antibody of the ob/ob mouse skeletal muscles showed that immunoreaction of the antibody was occurred at the muscle cell surface (Fig. 5A) and that the immunoreactivity was apparently similar to that of wild mouse muscles (Fig. 5B).
Fig. 5.
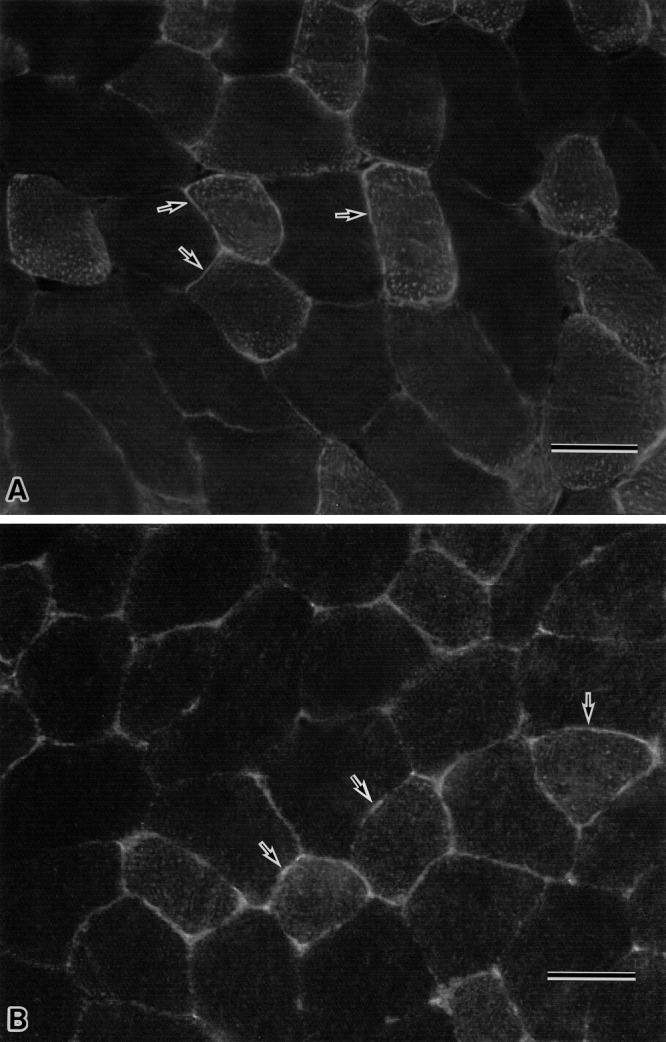
Immunohistochemical staining with anti-AQP9 antibody in the muscle from ob/ob mouse (A) and wild mouse (B). In the figures of both A and B, the relatively strong immunoreactive myofibers at their cell surface (arrow) and the faintly to scarcely immunostained myofibers are similarly observed, and the immunoreactivity of anti-AQP9 antibody in the muscles of A and B looks similar. Bar=50 µm (A, B).
IV. Discussion
Intracellular triglycerides in liver and skeletal muscle are metabolically important [6, 7]. Glycerol is a direct source of glycerol-3 phosphate for triglyceride synthesis [4]. It is established that AQP 7 and 9, both of which transport glycerol as well as water, are present in the adipocytes and the liver, respectively [23].
AQP7 is now considered the primary aquaglyceroporin facilitating the secretion of glycerol from adipocytes [18, 19, 36], although the presence of additional glycerol channels has been postulated in adipose tissue [12, 22]. The present study provides us with the evidence that AQP7 is expressed at the surface membrane of normal murine skeletal myofibers especially in type 2 myofibers [39], and that the skeletal muscles constitute an important source of plasma glycerol in addition to adipose tissue [4]. We also observed upregulated expression of AQP7 on the surface membrane of obese ob/ob skeletal myofibers. In contrast with this, decreased AQP7 gene expression in adipose tissue of human obese high fat consumers has been reported [25]. With regard to the adipose tissue and liver, our study showed that the mRNA levels of mouse AQP7 and AQP9 in white adipose tissue of obese ob/ob mice were not different from those of lean wild mice, whereas those of mouse AQP9 in the obese mouse livers were upregulated (unpublished observation). Why the AQP7 expression is different in the obese mouse skeletal muscle and obese human adipose tissue is unknown. Human diet induced obese subjects do not have leptin deficiency and leptin downregulates AQP7 expression through the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway in visceral adipocytes [28]. The discrepant expression of AQP7 in the ob/ob mouse skeletal muscle and the adipose tissue of human diet induced obese subjects may be explained by the action of leptin which downregulates AQP7. The present studies confirmed our previous study [14] that the AQP9 was also expressed on the surface membrane of murine skeletal myofibers, especially in type 2 myofibers, although the expression level was not significantly different in the skeletal muscles between obese ob/ob mice and wild mice. Although AQP is suggested to be regulated by leptin through the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway in visceral adipocytes and hepatocytes [28], AQP7 may be more influenced by this pathway than AQP9 in the skeletal muscle.
There are only limited studies regarding the morphological and biochemical alterations occurring in skeletal muscles of the obese mice. Almond and Enser [2] reported that the fat content of muscles from obese mice was three times greater than in lean mice, and that a reduction in the population of type 2 fast twitch glycolytic fibers was noted in ob/ob mice. In human skeletal muscle, it was reported that intramuscular lipid content was increased in obesity and decreased by weight loss [11]. Several groups of investigators [17, 31, 33, 34] showed reduced skeletal muscle mass and small myofiber diameter in obese ob/ob mice. Moreover, among the skeletal muscles of sternomastoid (SM; fast twitch), extensor digitorum longus (EDL; fast twitch) and soleus (SOL; mixed) muscles of ob/ob mice and lean mice, Kemp et al. [17] found that all three muscles from ob/ob mice were smaller in size, with the type 2 muscle mass (SM and EDL) being the most affected in comparison with muscles from lean mice. Such type 2 myofiber atrophy may be due to less active feature (functional disuse) of obese mice [30]. Warmington et al. [40] histochemically investigated ob/ob skeletal muscles which displayed a slower type profile from the viewpoint of fiber proportions. We found an increased amount of neutral lipid droplets in the type 1 skeletal myofiber sarcoplasm of the genetically obese ob/ob mice. In obese ob/ob mice, the plasma glycerol [3] and free fatty acid [35] levels are elevated. Elevated glycerol enters the myocyte through AQP9 in the sarcolemmal plasma membrane. Glycerol is a precursor of gluconeogenesis and a direct source of glycerol-3 phosphate for the synthesis of triglycerides which are the main constituent of neutral lipid droplets observed in obese ob/ob mice in this study [4, 27]. A similar finding was reported in obese human subject muscle [11].
Finally this study showed that the expression of AQP7, but not AQP9, in the skeletal muscles of obese ob/ob mice was upregulated at RNA and protein levels. Based on this result the skeletal muscle tissue is the source of circulating glycerol in addition to the hitherto mentioned adipose tissue, although triglyceride is also important as the energy source for muscle cell contraction.
V. Acknowledgments
We wish to thank Ms. Ai Kimura for technical help and Ms. H. Ozawa for assistance with manuscript preparation. This work was supported in part by a Research Grant from the Asahi Group Foundation to Y. W. and a Grant-in-Aid for Scientific Research on Innovative Areas (to SS no. 22126004) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
VI. References
- 1.Agre P. Aquaporin water channels. Biosci. Rep. 2004;24:127–148. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- 2.Almond R. E., Enser M. A histochemical and morphological study of skeletal muscle from obese hyperglycaemic ob/ob mice. Diabetologia. 1984;27:407–413. doi: 10.1007/BF00304859. [DOI] [PubMed] [Google Scholar]
- 3.Almond R. E., Enser M. Effects of adrenalectomy on muscle fibre growth and fibre-type composition in obesehyperglycaemic (ob/ob) and lean mice. Int. J. Obes. 1989;13:791–800. [PubMed] [Google Scholar]
- 4.Baba H., Zhang X. J., Wolfe R. R. Glycerol gluconeogenesis in fasting humans. Nutrition. 1995;11:149–153. [PubMed] [Google Scholar]
- 5.Ceddia R. B., William W. N., Jr., Curi R. The response of skeletal muscle to leptin. Front. Biosci. 2001;6:D90–97. doi: 10.2741/ceddia. [DOI] [PubMed] [Google Scholar]
- 6.Coppack S. W., Jensen M. D., Miles J. M. In vivo regulation of lipolysis in humans. J. Lipid. Res. 1994;35:177–193. [PubMed] [Google Scholar]
- 7.Dagenais G. R., Tancredi R. G., Zierler K. L. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J. Clin. Invest. 1976;58:421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frigeri A., Nicchia G. P., Verbavatz J. M., Valenti G., Svelto M. Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J. Clin. Invest. 1998;102:695–703. doi: 10.1172/JCI2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frigeri A., Nicchia G. P., Nico B., Quondamatteo F., Herken R., Roncali L., Svelto M. Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J. 2001;15:90–98. doi: 10.1096/fj.00-0260com. [DOI] [PubMed] [Google Scholar]
- 10.Frühbeck G. A heliocentric view of leptin. Proc. Nutr. Soc. 2001;60:301–318. doi: 10.1079/pns200196. [DOI] [PubMed] [Google Scholar]
- 11.Goodpaster B. H., Theriault R., Watkins S. C., Kelley D. E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 12.Hara-Chikuma M., Sohara E., Rai T., Ikawa M., Okabe M., Sasaki S., Uchida S., Verkman A. S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005;280:15493–15496. doi: 10.1074/jbc.C500028200. [DOI] [PubMed] [Google Scholar]
- 13.Hibuse T., Maeda N., Funahashi T., Yamamoto K., Nagasawa A., Mizunoya W., Kishida K., Inoue K., Kuriyama H., Nakamura T., Fushiki T., Kihara S., Shimomura I. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. U S A. 2005;102:10993–10998. doi: 10.1073/pnas.0503291102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue M., Wakayama Y., Kojima H., Shibuya S., Jimi T., Hara H., Iijima S., Masaki H., Oniki H., Matsuzaki Y. Aquaporin 9 expression and its localization in normal skeletal myofiber. J. Mol. Histol. 2009;40:165–170. doi: 10.1007/s10735-009-9226-1. [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi K., Kuwahara M., Gu Y., Kageyama Y., Tohsaka A., Suzuki F., Marumo F., Sasaki S. Cloning and functional expression of a new water channel abundantly expressed in the testis permeable to water, glycerol, and urea. J. Biol. Chem. 1997;272:20782–20786. doi: 10.1074/jbc.272.33.20782. [DOI] [PubMed] [Google Scholar]
- 16.Ishibashi K., Kuwahara M., Gu Y., Tanaka Y., Marumo F., Sasaki S. Cloning and functional expression of a new aquaporin (AQP9) abundantly expressed in the peripheral leukocytes permeable to water and urea, but not to glycerol. Biochem. Biophys. Res. Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- 17.Kemp J. G., Blazev R., Stephenson D. G., Stephenson G. M. Morphological and biochemical alterations of skeletal muscles from the genetically obese (ob/ob) mouse. Int. J. Obes. (Lond) 2009;33:831–841. doi: 10.1038/ijo.2009.100. [DOI] [PubMed] [Google Scholar]
- 18.Kishida K., Kuriyama H., Funahashi T., Shimomura I., Kihara S., Ouchi N., Nishida M., Nishizawa H., Matsuda M., Takahashi M., Hotta K., Nakamura T., Yamashita S., Tochino Y., Matsuzawa Y. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000;275:20896–20902. doi: 10.1074/jbc.M001119200. [DOI] [PubMed] [Google Scholar]
- 19.Kondo H., Shimomura I., Kishida K., Kuriyama H., Makino Y., Nishizawa H., Matsuda M., Maeda N., Nagaretani H., Kihara S., Kurachi Y., Nakamura T., Funahashi T., Matsuzawa Y. Human aquaporin adipose (AQPap) gene. Genomic structure, promoter analysis and functional mutation. Eur. J. Biochem. 2002;269:1814–1826. doi: 10.1046/j.1432-1033.2002.02821.x. [DOI] [PubMed] [Google Scholar]
- 20.Liu J. W., Wakayama Y., Inoue M., Shibuya S., Kojima H., Jimi T., Oniki H. Immunocytochemical studies of aquaporin 4 in the skeletal muscle of mdx mouse. J. Neurol. Sci. 1999;164:24–28. doi: 10.1016/s0022-510x(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z., Shen J., Carbrey J. M., Mukhopadhyay R., Agre P., Rosen B. P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc. Natl. Acad. Sci. U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda N., Funahashi T., Hibuse T., Nagasawa A., Kishida K., Kuriyama H., Nakamura T., Kihara S., Shimomura I., Matsuzawa Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. U S A. 2004;101:17801–17806. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeda N., Funahashi T., Shimomura I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:627–634. doi: 10.1038/ncpendmet0980. [DOI] [PubMed] [Google Scholar]
- 24.Margetic S., Gazzola C., Pegg G. G., Hill R. A. Leptin: a review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 25.Marrades M. P., Milagro F. I., Martinez J. A., Moreno-Aliaga M. J. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem. Biophys. Res. Commun. 2006;339:785–789. doi: 10.1016/j.bbrc.2005.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki T., Hata H., Ozawa H., Takata K. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem. Cytochem. 2009;42:159–169. doi: 10.1267/ahc.09023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C. M., Kalhan S. C., Tilghman S. M., Hanson R. W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003;278:30413–30416. doi: 10.1074/jbc.R300017200. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez A., Catalan V., Gomez-Ambrosi J., Garcia-Navarro S., Rotellar F., Valenti V., Silva C., Gil M. J., Salvador J., Burrell M. A., Calamita G., Malagon M. M., Fruhbeck G. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011;96:E586–E597. doi: 10.1210/jc.2010-1408. [DOI] [PubMed] [Google Scholar]
- 29.Rojek A. M., Skowronski M. T., Fuchtbauer E. M., Fuchtbauer A. C., Fenton R. A., Agre P., Frokiaer J., Nielsen S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. U S A. 2007;104:3609–3614. doi: 10.1073/pnas.0610894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145:2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- 31.Sáinz N., Rodriguez A., Catalan V., Becerril S., Ramirez B., Gomez-Ambrosi J., Fruhbeck G. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1α in ob/ob mice. PLoS One. 2009;4:e6808. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skowronski M. T., Lebeck J., Rojek A., Praetorius J., Fuchtbauer E. M., Frokiaer J., Nielsen S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: implications in glycerol metabolism. Am. J. Physiol. Renal. Physiol. 2007;292:F956–F965. doi: 10.1152/ajprenal.00314.2006. [DOI] [PubMed] [Google Scholar]
- 33.Stickland N. C., Batt R. A., Crook A. R., Sutton C. M. Inability of muscles in the obese mouse (ob/ob) to respond to changes in body weight and activity. J. Anat. 1994;184:527–533. [PMC free article] [PubMed] [Google Scholar]
- 34.Trostler N., Romsos D. R., Bergen W. G., Leveille G. A. Skeletal muscle accretion and turnover in lean and obese (ob/ob) mice. Metabolism. 1979;28:928–933. doi: 10.1016/0026-0495(79)90093-3. [DOI] [PubMed] [Google Scholar]
- 35.Turpin S. M., Ryall J. G., Southgate R., Darby I., Hevener A. L., Febbraio M. A., Kemp B. E., Lynch G. S., Watt M. J. Examination of ‘lipotoxicity’ in skeletal muscle of high-fat fed and ob/ob mice. J. Physiol. 2009;587:1593–1605. doi: 10.1113/jphysiol.2008.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verkman A. S. Mammalian aquaporins: diverse physiological roles and potential clinical significance. Expert Rev. Mol. Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakayama Y., Jimi T., Inoue M., Kojima H., Murahashi M., Kumagai T., Yamashita S., Hara H., Shibuya S. Reduced aquaporin 4 expression in the muscle plasma membrane of patients with Duchenne muscular dystrophy. Arch. Neurol. 2002;59:431–437. doi: 10.1001/archneur.59.3.431. [DOI] [PubMed] [Google Scholar]
- 38.Wakayama Y., Jimi T., Inoue M., Kojima H., Shibuya S., Murahashi M., Hara H., Oniki H. Expression of aquaporin 3 and its localization in normal skeletal myofibres. Histochem. J. 2002;34:331–337. doi: 10.1023/a:1023382609541. [DOI] [PubMed] [Google Scholar]
- 39.Wakayama Y., Inoue M., Kojima H., Jimi T., Shibuya S., Hara H., Oniki H. Expression and localization of aquaporin 7 in normal skeletal myofiber. Cell Tissue Res. 2004;316:123–129. doi: 10.1007/s00441-004-0857-y. [DOI] [PubMed] [Google Scholar]
- 40.Warmington S. A., Tolan R., McBennetts S. Functional and histological characteristics of skeletal muscles and the effects of leptin in the genetically obese (ob/ob) mouse. Int. J. Obes. Relat. Metab. Disord. 2000;24:1040–1050. doi: 10.1038/sj.ijo.0801357. [DOI] [PubMed] [Google Scholar]
- 41.Yokota T., Miyagoe Y., Hosaka Y., Tsukita K., Kameya S., Shibuya S., Matsuda R., Wakayama Y., Takeda S. Aquaporin-4 is absent at the sarcolemma and at perivascular astrocyte endfeet in α1-syntrophin knockout mice. Proc. Jpn. Acad. Ser. B. 2000;76:22–27. [Google Scholar]


