Abstract
Pregnancy stimulates induced Foxp3 expression among maternal CD4+ T cells with fetal specificity. Although sustained maternal Treg expansion is essential for maintaining fetal tolerance during pregnancy, the necessity for Foxp3+ cells with fetal specificity remains undefined. Here we demonstrate that mitigating Treg differentiation among maternal CD4+ T cells with a single surrogate fetal specificity elicits antigen specific fetal loss. Using recombinant Listeria monocytogenes (Lm) to prime stably differentiated Th1 CD4+ T cells with fetal IAb: 2W1S55-68 specificity refractory to pregnancy induced Foxp3 expression, we show antigen delivery by cytoplasmic Lm causes selective loss of 2W1S+ offspring through CD4 cell and IFN-γ dependent pathways. By contrast, CD4+ T cells primed by Lm restricted from the cell cytoplasm are markedly more plastic for induced Foxp3 expression with normal pregnancy outcomes. Thus, committed Th1 polarization blocks pregnancy induced Treg differentiation among maternal CD4+ T cells with fetal specificity and triggers antigen specific fetal loss.
INTRODUCTION
Pregnancy requires expanded tolerance to encompass foreign paternal antigens expressed by the developing fetus (1, 2). The accumulation of maternal Foxp3+ regulatory CD4+ T cells (Tregs) occurs in healthy human pregnancy, while complications such as spontaneous abortion or preeclampsia that likely stem from disrupted fetal tolerance have been linked with blunted maternal Treg expansion (3-8). In particular, in uncomplicated human pregnancy, the natural heterogeneity between maternal and paternal HLA-C allo-antigens has been shown to recruit Tregs to the maternal-fetal interface that is associated with silencing effector T cell inflammatory responses (9-12). In turn, complementary animal studies allowing for experimental Treg manipulation have established maternal Tregs begin accumulating within the uterine draining lymph nodes shortly after conception in response to seminal fluid, and their necessity for sustaining fetal tolerance during allogeneic pregnancy (13-17). Thus, expanded maternal Tregs protect immunologically foreign fetal tissue from rejection.
With increasingly recognized heterogeneity among Foxp3+ cells, the necessity for unique maternal Treg subsets based on origin and specificity has been proposed (18-20). For example, the accumulation of Foxp3+ CD4+ T cells with specificity to fetal-expressed antigen, and fetal resorption induced by prior stimulation with surrogate fetal antigens each suggests maternal Tregs with fetal specificity play important protective roles (18-21). Induced Foxp3 expression is also likely essential since a majority of maternal Tregs with fetal specificity arise from Foxp3- CD4+ T cells during primary pregnancy, and fetal resorption occurs when peripheral Treg conversion is circumvented in mice with disruption of the foxp3 enhancer conserved noncoding sequence-1 (18, 19). However, despite accumulation of maternal Tregs with fetal specificity, their role in sustaining pregnancy remains uncertain given the lack of tools for manipulating Tregs in an antigen specific fashion. To investigate the necessity for maternal Tregs with fetal specificity, pregnancy outcomes were evaluated in mice containing CD4+ T cells with surrogate fetal specificity stably differentiated into non-Treg effectors prior to mating. Collectively, these studies show committed Th1 CD4+ T cell differentiation blocks pregnancy induced Foxp3 expression causing antigen specific fetal loss.
MATERIALS AND METHODS
Mice, infection, and adoptive cell transfer
C57Bl/6, congenic CD45.1+ and CD90.1+ mice (all H-2b), and mice expressing 2W1S55-68 peptide behind the ubiquitously active β-actin promoter backcrossed to Balb/c (H-2d) or C57Bl/6 mice have been described (19, 22). Expression of the 2W1S transgene was screened using 2W1S primers: 5′-CCAATCTGTCTGGCATCTCC-3′; and 5′-ATGATGGCCATAGCTCCAAG-3′ (22). For infection, Lm were grown to early log-phase (OD600 0.1), washed and suspended in PBS and inoculated i.v. at the following dosages: ΔACTA Lm-2W1S (106 CFUs), ΔLLOΔPLC Lm-2W1S (107 CFUs), or non-recombinant ΔACTA Lm (106 CFUs) (23-25). For adoptive transfer, CD4+ T cells from the spleen and lymph nodes were purified by negative selection, and one mouse equivalent of CD45.1+ and CD90.1+ cells at an 1:1 ratio were inoculated i.v. into CD45.2+ CD90.2+ recipient mice before mating. For depletion, anti-CD4 (GK1.5) or anti-IFN-γ (XMG1.2) antibodies were administered i.p. one day prior to mating and weekly thereafter (500 μg/dose). All experiments were performed in accordance with institutional IACUC approved protocols.
Tetramer staining and enrichment
Mononuclear cells from the spleen, axillary, brachial, cervical, inguinal, mesenteric, pancreatic, para-aortic/uterine lymph nodes were collected, enriched with PE conjugated I-Ab 2W1S55-68 tetramer (19, 26), followed by cell-surface (CD4, CD44, CD25, CD8, CD11b, CD11c, B220, F4/80), intracellular (IFN-γ, IL-17), or intranuclear (Foxp3, T-bet) staining. For stimulation, PMA (100 ng/ml) and ionomycin (1 μg/ml) was added for 5 hours in media supplemented with Brefeldin A (22).
Treg and Th17 differentiation
For Treg differentiation, purified CD4+ T cells were stimulated with syngeneic APCs, 2W1S55-68 peptide (10 μM), IL-2 (20 ng/ml), and TGF-β (up to 1.6 ng/ml). For Th17 polarization, CD4+ T cells were stimulated with syngeneic APCs, 2W1S55-68 peptide (10 μM), IL-6 (20 ng/ml), IL-23 (10 ng/ml), and TGF-β (1 ng/ml) in media supplemented with anti-IFN-γ and anti-IL-4 antibodies (10 μg/ml each). Five days after stimulation, Foxp3 expression and cytokine production were analyzed by intranuclear and intracellular staining.
Statistics
Differences in percent cells, resorption frequency, and number of pups were analyzed using an unpaired Student’s t test (two groups) or ANOVA with Dunnett correction for multiple comparisons (more than two groups) with P < 0.05 taken as statistical significance.
RESULTS AND DISCUSSION
Lm selectively expands non-Treg CD4+ T cells regardless of cytoplasmic entry
Recombinant strains of the intracellular bacterium, Listeria monocytogenes (Lm), have been widely used to characterize the T cell response to infection. We previously described Lm cytoplasmic entry primes Th1 CD4+ T cell polarization and differentiation stability for cells responsive to Lm expressed antigen (23). However, the use of monoclonal cells from TCR transgenic mice with fixed specificity may limit their applicability for Treg differentiation given the discordance in affinity and TCR repertories between Foxp3+ and Foxp3- CD4+ T cells (27). These drawbacks are bypassed by using MHC tetramers to identify endogenous CD4+ T cells allowing a more comprehensive analysis of the cumulative polyclonal response (26). Using this approach, we have shown recombinant WT or the attenuated ΔACTA Lm mutant that retains cytoplasmic entry each primes selective expansion of Foxp3- CD4+ T cells with I-Ab:2W1S55-68 specificity (24). Reciprocally, pregnancy sired by 2W1S-expressing males stimulates induced Foxp3 expression and Treg accumulation among CD4+ T cells with the same I-Ab:2W1S55-68 specificity (19). Given the sharp discordance in Treg differentiation between Lm infection and fetal stimulation, we reasoned committed Th1 differentiation by recombinant Lm could be exploited to investigate the necessity for Foxp3 induction among maternal CD4+ T cells during pregnancy. As controls for infection and 2W1S55-68 stimulation, CD4+ T cells primed by Lm restricted from the cell cytoplasm due to combined defects in Listeriolysin-O (LLO) and phospholipase-C (PLC) (ΔLLOΔPLC Lm) with increased plasticity for differentiation into other effector lineages were evaluated in parallel (23, 25).
We found ΔACTA Lm and ΔLLOΔPLC Lm each engineered to express 2W1S55-68 as a secreted recombinant protein primed robust accumulation of Foxp3- CD4+ T cells with 2W1S specificity (Fig. 1A). In turn, the percentage of Foxp3+ Tregs among 2W1S+ CD4+ T cells declined precipitously after infection with each recombinant Lm compared with naive controls. These shifts were restricted to CD4+ T cells with Lm specificity because Foxp3 expression among 2W1S- CD4+ T cells remained similar in Lm infected and control mice (Fig. 1A). Furthermore, 2W1S+ CD4+ T cells primed by ΔACTA Lm selectively up-regulate T-bet and acquired the capacity to produce IFN-γ; whereas these shifts were markedly diminished for 2W1S+ cells stimulated by ΔLLOΔPLC Lm (Fig. 1B). Thus, Lm primes the selective expansion of antigen specific non-Treg CD4+ T cells regardless of cytoplasmic entry, while Th1 differentiation requires Lm cytoplasmic entry.
Figure 1.
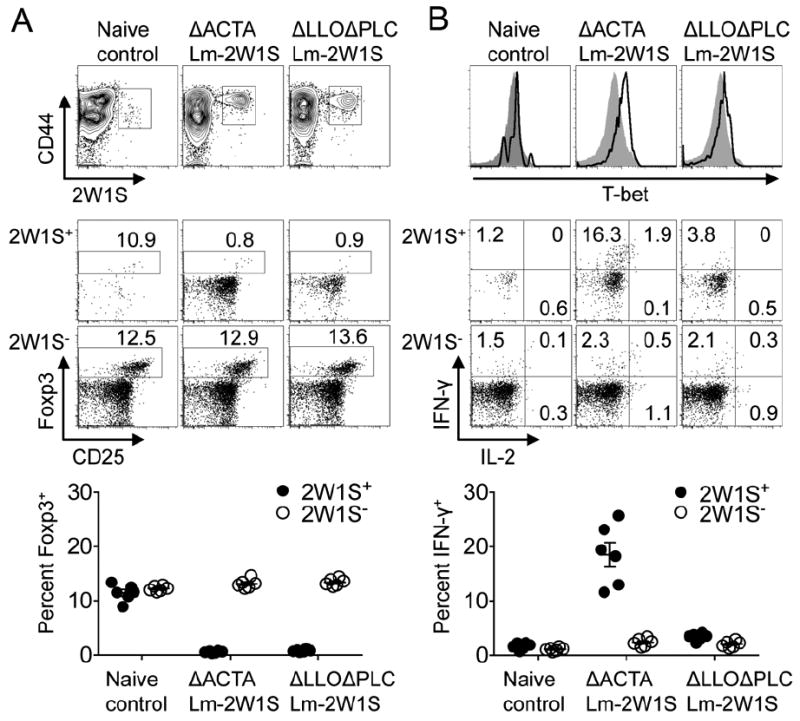
Recombinant Lm selectively primes Foxp3- CD4+ T cell expansion regardless of cytoplasmic entry. (A) Representative plots and cumulative data illustrating percent Foxp3+ among 2W1S+ and 2W1S- CD4+ T cells 60 days after infection with each recombinant Lm. (B) T-bet expression and IFN-γ production by 2W1S+ (line histogram) and 2W1S- (shaded histogram) for each group of mice described in panel A. Each point reflects the data from a single mouse, and results representative of three independent experiments each with similar results are shown. Bar, mean ± one standard error.
Discordant plasticity for Foxp3 expression among CD4+ T cells primed by recombinant Lm
Next, the capacity for Treg differentiation among 2W1S+ CD4+ T cells primed by each Lm strain was addressed. We found TGF-β stimulation, along with 2W1S55-68 peptide and IL-2, induced Foxp3 expression in a dose dependent fashion among 2W1S+ CD4+ T cells recovered from both groups of Lm infected mice. However, at each TGF-β concentration, Foxp3 expression was significantly reduced for cells from ΔACTA compared with ΔLLOΔPLC Lm-2W1S infected mice (Supplemental Fig. 1A). Similarly after stimulation under Th17 polarizing conditions, 2W1S+ CD4+ T cells from ΔACTA compared with ΔLLOΔPLC Lm-2W1S infected mice produced sharply reduced IL-17 while retaining robust IFN-γ levels (Supplemental Fig. 1B). Thus, CD4+ T cells primed by ΔACTA Lm maintain more stable Th1 commitment with resiliency against differentiation into Tregs or other effector lineages, whereas CD4+ T cells primed by ΔLLOΔPLC Lm have more differentiation plasticity.
To investigate how this discordance dictates Foxp3 expression in vivo, pregnancy induced Treg differentiation among 2W1S+ CD4+ T cells primed by ΔACTA Lm-2W1S compared with ΔLLOΔPLC Lm-2W1S was evaluated. Sixty days after infection with each Lm strain, virgin female mice were mated with allogeneic 2W1S-expressing males that transforms 2W1S55-68 into a surrogate fetal antigen (19, 22). Consistent with our recent studies using this mating scheme, ~30% of 2W1S+ CD4+ T cells in control mice without prior infection became Foxp3+ by E15.5 (Fig. 2A) (19). By contrast, only ~2% of 2W1S+ CD4+ T cells from mice previously infected with ΔACTA Lm-2W1S were Foxp3+, while a majority retained T-bet expression and IFN-γ production (Fig. 2). Comparatively, Foxp3 became more readily induced among 2W1S+ CD4+ T cells from mice previously infected with ΔLLOΔPLC Lm-2W1S, albeit at reduced levels compared with naive mice (Fig. 2). Thus, resiliency against Treg differentiation shown in vitro for CD4+ T cells primed by ΔACTA Lm is maintained, and becomes even more pronounced with pregnancy induced 2W1S stimulation.
Figure 2.
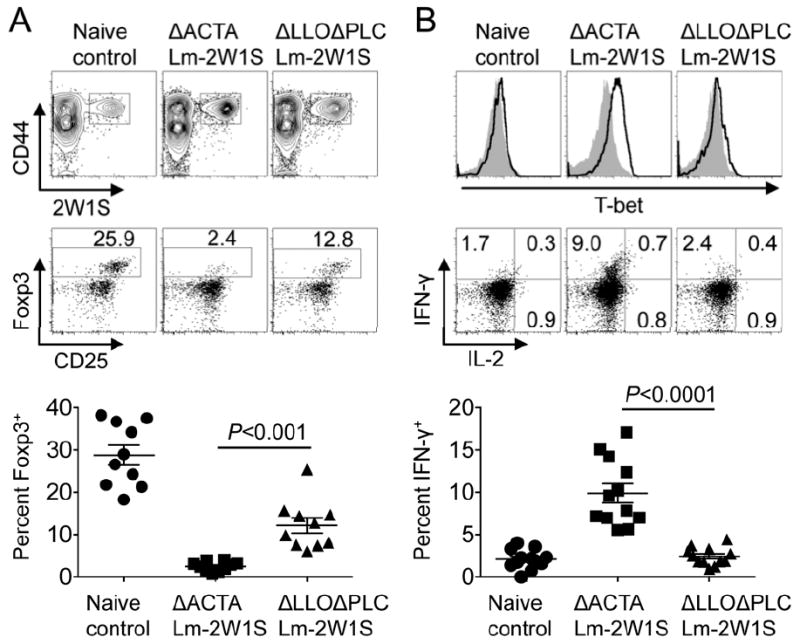
Cytoplasmic Lm mitigates accumulation of maternal Tregs with fetal specificity in subsequent pregnancy. (A) Representative plots and cumulative data illustrating percent Foxp3+ among maternal 2W1S+ CD4+ T cells E15.5 during pregnancy sired by 2W1S-expressing males among female mice primed by each recombinant Lm 60 days prior to mating. (B) T-bet expression and IFN-γ production by 2W1S+ (line histogram) and 2W1S- (shaded histogram) CD4+ T cells for each group of mice described in panel A. Each point reflects the data from a single mouse, and results representative of three independent experiments each with similar results are shown. Bar, mean ± one standard error.
Given the unique activation of immune components after ΔACTA compared with ΔLLOΔPLC Lm infection (23, 25), resiliency against subsequent Foxp3 induction among cells from ΔACTA Lm-2W1S infected mice could also reflect CD4+ T cell extrinsic differences, in addition to cell intrinsic shifts in differentiation stability. To discriminate between these possibilities, Foxp3 expression among CD4+ T cells from ΔACTA Lm-2W1S or non-recombinant ΔACTA Lm infected control mice were evaluated after adoptive transfer into naive recipients subsequently mated with 2W1S-expressing males. Here, mice with discordant expression of the CD45.1/2 and CD90.1/2 congenic markers were used so that each donor cell subset could be discriminated from each other and endogenous recipient cells (Fig. 3). We found resistance against pregnancy induced Foxp3 expression among CD4+ T cells from ΔACTA Lm-2W1S infected mice was sustained after adoptive transfer, whereas induced Foxp3 expression to levels indistinguishable from endogenous naive CD4+ T cells were found among donor cells from mice previously infected with non-recombinant ΔACTA Lm (Fig. 3). Thus despite considerable active debate on the relative stability of Foxp3+ Tregs (28), resiliency against pregnancy induced Foxp3 expression among Th1 effector CD4+ T cells primed by ΔACTA Lm-2W1S reflects differentiation stability that is both cell intrinsic and antigen specific.
Figure 3.
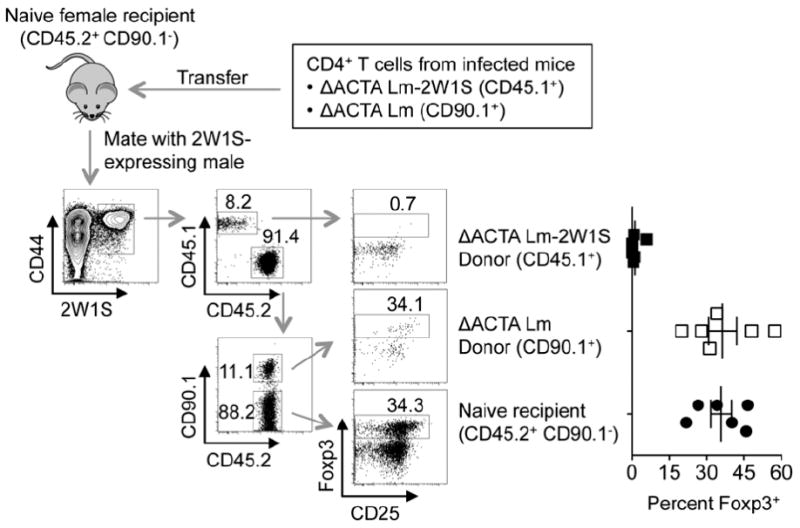
Resiliency against Foxp3 expression among CD4+ T cells after Lm infection is cell intrinsic and antigen specific. Schematic illustrating the strategy used to discriminate each donor and recipient cell subset, and percent Foxp3+ among each group of 2W1S+ CD4+ T cells E15.5 after mating with 2W1S-expressing males. Each point reflects the data from a single mouse, and results representative of two independent experiments each with similar results are shown. Bar, mean ± one standard error.
Resiliency against Treg differentiation causes antigen specific fetal loss
Having established resiliency against Foxp3 induction among 2W1S+ CD4+ T cells after ΔACTA Lm-2W1S infection and the efficiency whereby pregnancy induced 2W1S stimulation primes accumulation of maternal Tregs with the same specificity, these conditions were sequentially combined to address the necessity for Treg differentiation among maternal CD4+ T cells with fetal specificity during pregnancy. Using males heterozygous for the 2W1S transgene for mating, a reduction in the expected 1:1 ratio of 2W1S+ to 2W1S- pups would illustrate antigen specific fetal loss. Remarkably, the percentage and number of 2W1S+ pups were each significantly reduced in mice previously infected with ΔACTA Lm-2W1S compared with ΔLLOΔPLC Lm-2W1S or no infection controls (Fig. 4A). In particular, while the expected 50% 2W1S+ pups did not deviate in pregnancies among either naive (49% [43-55]) (mean [95% confidence interval]) or mice with prior ΔLLOΔPLC Lm-2W1S infection (50% [44-56]), 2W1S+ pups were significantly reduced in pregnancies among mice with prior ΔACTA Lm-2W1S infection (38% [30-46]); and this decline paralleled a 26% reduction in number of live 2W1S+ pups (3.3 per litter in ΔACTA Lm-2W1S infected, compared with 4.6 per litter in ΔLLOΔPLC Lm-2W1S infected or 4.6 per litter in naive control mice) (Fig. 4A). Additionally, no differences in fetal resorption (each group background levels under 10%) were found at these later pregnancy time points, consistent with the necessity of maternal Tregs for implantation or beginning early during allogeneic pregnancy (17).
Figure 4.
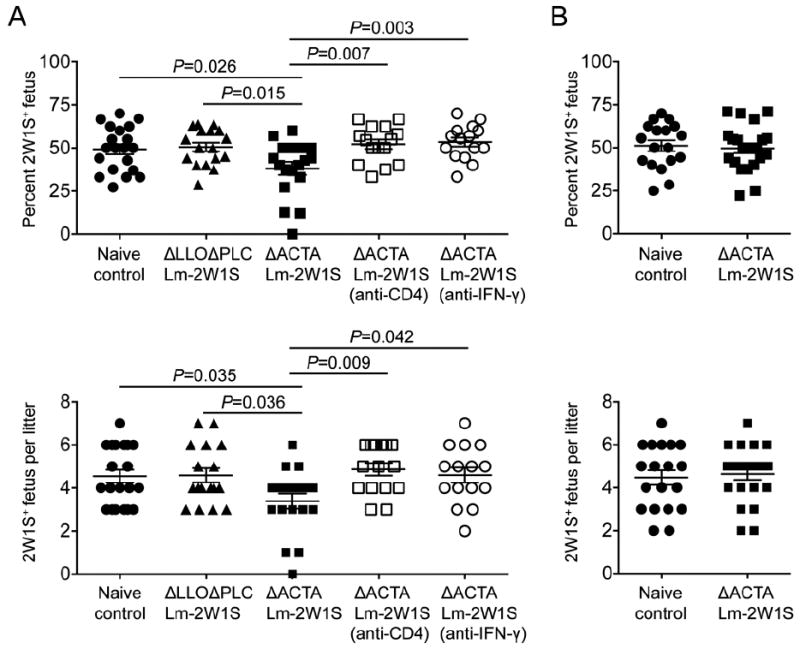
Resistance against Foxp3 expression among maternal CD4+ T cells causes antigen specific fetal loss in allogeneic pregnancy. Percent and number of 2W1S+ offspring per litter with (A) allogeneic pregnancy sired by 2W1S-expressing males on the Balb/c (H-2d) background or (B) syngeneic pregnancy sired by 2W1S-expressing males on the C57Bl/6 (H-2b) background among virgin C57Bl/6 females infected with the indicated recombinant Lm 60 days prior to mating. For some mice, CD4+ cells were depleted (anti-CD4) or IFN-γ neutralized (anti-IFN-γ) prior to mating. Each point reflects the data from a single mouse, and results combined from at least three independent experiments each with similar results are shown. Bar, mean ± one standard error.
To investigate if the selective loss of 2W1S+ offspring in ΔACTA Lm-2W1S infected mice was caused by impaired CD4+ T cell differentiation, the impacts of CD4 cell depletion prior to mating on subsequent pregnancy were evaluated. We found the reduction in 2W1S+ pups among ΔACTA Lm-2W1S infected mice became overturned with CD4 cell depletion as the percent and number of 2W1S+ offspring (52% [46-58]; 4.8 pups per litter) each rebounded to levels indistinguishable from naive controls (Fig. 4A). Likewise, neutralizing the Th1 effector cytokine, IFN-γ, prior to mating also abolished the loss of 2W1S+ offspring in ΔACTA Lm-2W1S infected mice (54% [48-60] and 4.6 2W1S+ pups per litter) (Fig. 4A). Thus, CD4+ T cells and IFN-γ are each essential for the protective contribution of maternal Tregs with fetal specificity; and these findings in mice reinforce the protective role of human decidual Tregs that suppress effector T cell IFN-γ production (9-12). Together, these results suggest maternal Tregs with fetal specificity confer protection by mitigating activation of Th1 effector cells that are harmful to pregnancy. In this regard, detrimental effector CD4+ T cells appear to be less constrained by decidual chemokine gene silencing compared with CD8+ T cells (29).
On the other hand, the degree of fetal loss triggered by prior ΔACTA Lm-2W1S infection can also be viewed as being somewhat marginal since 38% pups remained 2W1S+, and the reduction in 2W1S+ offspring was on average only slightly more than one pup per litter despite overwhelming resiliency against Foxp3 induction among maternal 2W1S+ CD4+ T cells with this specificity. Here, it is important to highlight this significant loss of offspring reflects circumvented Foxp3 induction among maternal CD4+ T cells of only a single surrogate fetal specificity that may be compensated by Tregs with specificity to other maternal-paternal mismatch antigens in allogeneic pregnancy or alternatively Tregs with self-specificity (20). To discriminate between these possibilities, outcomes after syngeneic pregnancy in mice previously infected with ΔACTA Lm-2W1S sired by 2W1S-expressing males on the C57Bl/6 background were evaluated. Interestingly, the loss of 2W1S+ pups observed allogeneic pregnancy did not occur in syngeneic pregnancy where the percent and number of 2W1S+ pups were indistinguishable among ΔACTA Lm-2W1S infected and control mice (49% [42-55] and 4.4 2W1S+ pups per litter after ΔACTA Lm-2W1S infection compared with 51% [44-57] and 4.5 2W1S+ pups per litter for naive no infection controls) (Fig. 4B). Considering the muted protective role of maternal Tregs in maintaining syngeneic compared with allogeneic pregnancy (13, 17, 19), these findings are perhaps not unexpected and may be explained by Treg-independent immune silencing mechanisms recently described during syngeneic pregnancy (30). However, for allogeneic pregnancy that more closely recapitulates the mismatch between MHC haplotype antigens in human pregnancy, the immune-activating properties of fetal MHC allo-antigens appear to be needed for uncovering the protective contribution of maternal Tregs with specificity to the surrogate fetal-2W1S minor histocompatibility antigen (1, 2). Applied to the reproductive process in humans and other outbred species where there is considerably more variability in MHC haplotype and minor histocompatibility antigens between individuals, maternal tolerance likely needs to expand even more to accommodate the enhanced repertoire of foreign paternal-fetal antigens. However, increased antigenic mismatch that stimulates maternal Treg expansion for a broader assortment of foreign fetal antigens may also offset fetal loss shown here with resistance against Treg differentiation among maternal CD4+ T cells of a single specificity. These additional questions will require more comprehensive immunological tools capable of tracking CD4+ T cells with specificity for a significantly broader array of fetal and non-fetal specificities. Nevertheless, by experimentally establishing overlap between a single Lm expressed and surrogate fetal antigen, the protective benefits of Treg differentiation among maternal CD4+ T cells with fetal specificity in optimal pregnancy outcomes is revealed.
Supplementary Material
Acknowledgments
This research was supported by the NIAID, R01-AI087830 and R01-AI100934 (SSW); and NIDDK, F30DK084674 (JHR). SSW holds an Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund.
References
- 1.Munoz-Suano A, Hamilton AB, Betz AG. Gimme shelter: the immune system during pregnancy. Immunological reviews. 2011;241:20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- 2.Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nature reviews. 2013;13:23–33. doi: 10.1038/nri3361. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Molecular human reproduction. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- 4.Yang H, Qiu L, Chen G, Ye Z, Lu C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertility and sterility. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 6.Winger EE, Reed JL. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am J Reprod Immunol. 2011;66:320–328. doi: 10.1111/j.1600-0897.2011.00992.x. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Sasaki Y, Sakai M. CD4(+)CD25high regulatory T cells in human pregnancy. Journal of reproductive immunology. 2005;65:111–120. doi: 10.1016/j.jri.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38–43. doi: 10.1111/j.1365-2567.2004.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilburgs T, Roelen DL, van der Mast BJ, de Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 10.Tilburgs T, Scherjon SA, van der Mast BJ, Haasnoot GW, Versteeg VDV-MM, Roelen DL, van Rood JJ, Claas FH. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. Journal of reproductive immunology. 2009;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Mjosberg J, Berg G, Jenmalm MC, Ernerudh J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biology of reproduction. 2010;82:698–705. doi: 10.1095/biolreprod.109.081208. [DOI] [PubMed] [Google Scholar]
- 12.Mjosberg J, Svensson J, Johansson E, Hellstrom L, Casas R, Jenmalm MC, Boij R, Matthiesen L, Jonsson JI, Berg G, Ernerudh J. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J Immunol. 2009;183:759–769. doi: 10.4049/jimmunol.0803654. [DOI] [PubMed] [Google Scholar]
- 13.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nature immunology. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 14.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, Way SS. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe. 2011;10:54–64. doi: 10.1016/j.chom.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA. Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19- mediated recruitment. Biology of reproduction. 2011;85:397–408. doi: 10.1095/biolreprod.110.088591. [DOI] [PubMed] [Google Scholar]
- 16.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biology of reproduction. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, Saito S. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. Journal of reproductive immunology. 2010;85:121–129. doi: 10.1016/j.jri.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29–38. doi: 10.1016/j.cell.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Darrasse-Jeze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, Klatzmann D. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol. 2013;191:2273–2281. doi: 10.4049/jimmunol.1202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perchellet AL, Jasti S, Petroff MG. Maternal CD4+ and CD8+ T Cell Tolerance Towards a Fetal Minor Histocompatibility Antigen in T Cell Receptor Transgenic Mice. Biology of reproduction. 2013 doi: 10.1095/biolreprod.113.110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis MM, Rowell E, Shafiani S, Negash A, Urdahl KB, Wilson CB, Way SS. Fidelity of pathogen-specific CD4+ T cells to the Th1 lineage is controlled by exogenous cytokines, interferon-gamma expression, and pathogen lifestyle. Cell Host Microbe. 2010;8:163–173. doi: 10.1016/j.chom.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertelt JM, Rowe JH, Johanns TM, Lai JC, McLachlan JB, Way SS. Selective priming and expansion of antigen-specific Foxp3- CD4+ T cells during Listeria monocytogenes infection. J Immunol. 2009;182:3032–3038. doi: 10.4049/jimmunol.0803402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature immunology. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nature reviews. 2013;13:461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 29.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science New York, N Y. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tay CS, Tagliani E, Collins MK, Erlebacher A. -Acting Pathways Selectively Enforce the Non-Immunogenicity of Shed Placental Antigen for Maternal CD8 T Cells. PloS one. 2013;8:e84064. doi: 10.1371/journal.pone.0084064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


