Abstract
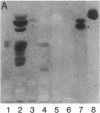
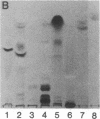
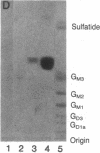
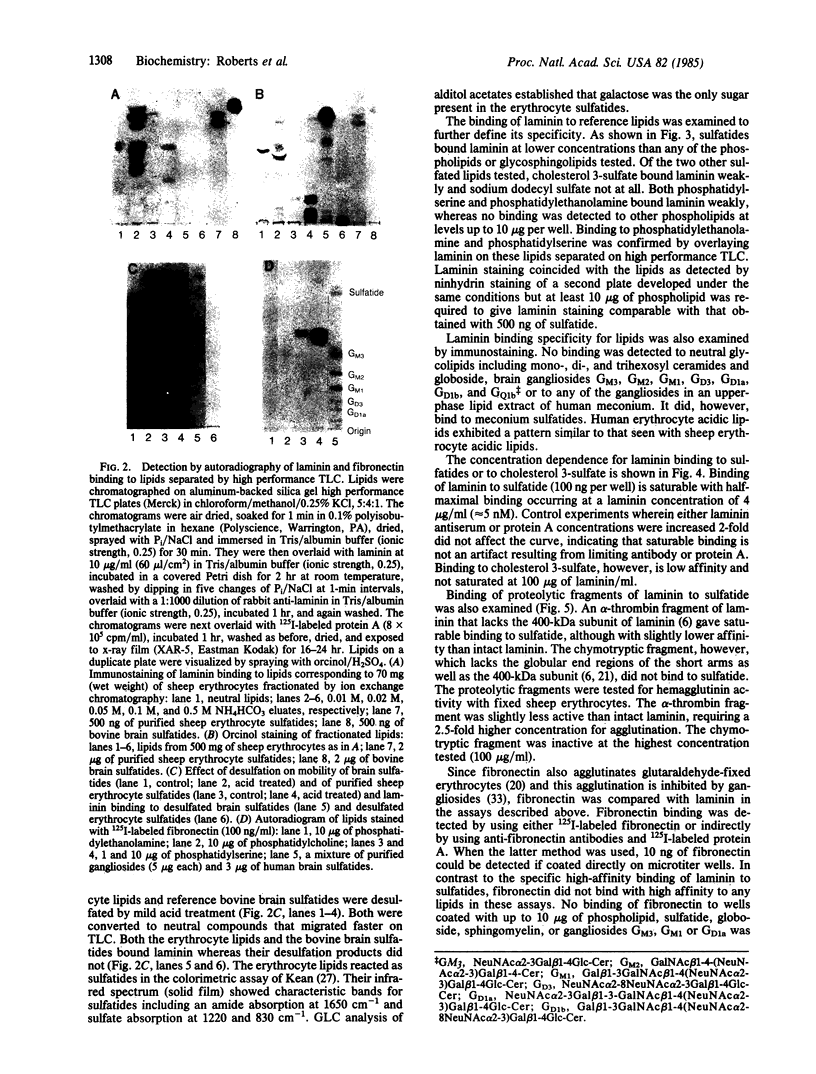
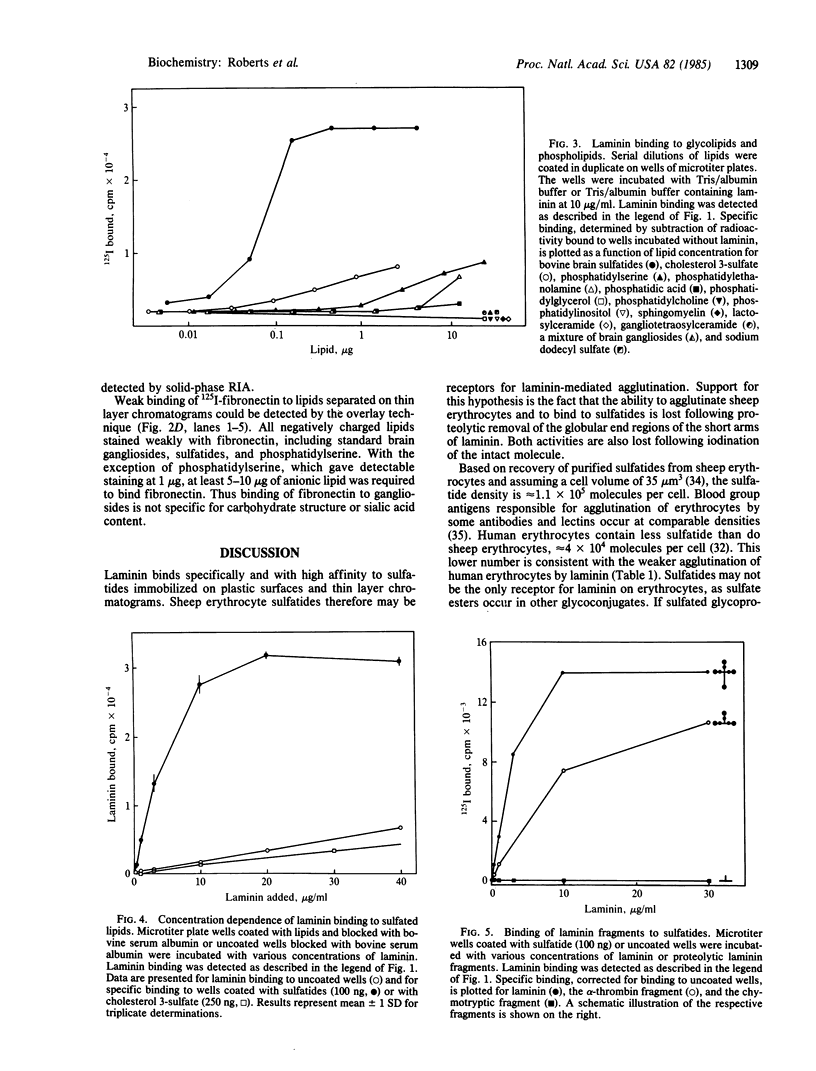
Previous studies of the agglutination of erythrocytes by the basement membrane glycoprotein laminin have suggested that laminin binds to gangliosides [Kennedy, D.W., Rohrbach, D.H., Martin, G.R., Momoi, T. & Yamada, K.M. (1983) J. Cell. Physiol. 114, 257-262]. Based on the following evidence, however, we find that laminin binds specifically to sulfatides, not gangliosides. Monogalactosyl sulfatides, purified from sheep erythrocytes with a yield of 4.3 mg/kg of packed cells, bound laminin with high affinity as did authentic bovine brain sulfatide (galactosylceramide-I3-sulfate). The binding activity of these lipids and of total erythrocyte lipids was stable to alkali and neuraminidase treatment but labile to dilute acid under conditions that destroy sulfatides but not gangliosides. Of various glycolipid and phospholipid standards tested, only sulfatides bound laminin with high affinity. Sulfatide binding and agglutinating activities of proteolytic fragments of laminin indicated that the globular end regions of the 200-kDa subunits are required for both activities. Thus, monogalactosylsulfatides, and possibly other more complex sulfated glycolipids, are probably involved in the agglutination of erythrocytes. These results also suggest a physiological function of sulfatides in cell adhesion. The agglutination of erythrocytes by fibronectin is also inhibited by gangliosides [Yamada, K.M., Kennedy, D.W., Grotendorst, G.R. & Momoi, T. (1981) J. Cell. Physiol. 109, 343-351]. Fibronectin, however, did not bind to sulfatides with high affinity but rather bound with low affinity to all anionic lipids tested, including phospholipids, gangliosides, and sulfatides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron-Van Evercooren A., Kleinman H. K., Ohno S., Marangos P., Schwartz J. P., Dubois-Dalcq M. E. Nerve growth factor, laminin, and fibronectin promote neurite growth in human fetal sensory ganglia cultures. J Neurosci Res. 1982;8(2-3):179–193. doi: 10.1002/jnr.490080208. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hansson C. G., Karlsson K. A., Samuelsson B. E. The identification of sulphatides in human erythrocyte membrane and their relation to sodium-potassium dependent adenosine triphosphatase. J Biochem. 1978 Mar;83(3):813–819. doi: 10.1093/oxfordjournals.jbchem.a131977. [DOI] [PubMed] [Google Scholar]
- Ishizuka I., Tadano K. The sulfoglycolipid, highly acidic amphiphiles of mammalian renal tubules. Adv Exp Med Biol. 1982;152:195–214. [PubMed] [Google Scholar]
- Johansson S., Kjellén L., Hök M., Timpl R. Substrate adhesion of rat hepatocytes: a comparison of laminin and fibronectin as attachment proteins. J Cell Biol. 1981 Jul;90(1):260–264. doi: 10.1083/jcb.90.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L. Rapid, sensitive spectrophotometric method for quantitative determination of sulfatides. J Lipid Res. 1968 May;9(3):319–327. [PubMed] [Google Scholar]
- Kennedy D. W., Rohrbach D. H., Martin G. R., Momoi T., Yamada K. M. The adhesive glycoprotein laminin is an agglutinin. J Cell Physiol. 1983 Mar;114(3):257–262. doi: 10.1002/jcp.1041140302. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Horan Hand P., Rao C. N., Bryant G., Barsky S. H., Schlom J. Monoclonal antibodies to the human laminin receptor recognize structurally distinct sites. Exp Cell Res. 1985 Jan;156(1):117–126. doi: 10.1016/0014-4827(85)90266-6. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Ott U., Odermatt E., Engel J., Furthmayr H., Timpl R. Protease resistance and conformation of laminin. Eur J Biochem. 1982 Mar;123(1):63–72. doi: 10.1111/j.1432-1033.1982.tb06499.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Sato M., Muramatsu T. Basement membrane glycoprotein laminin is an agglutinin. J Biochem. 1983 Aug;94(2):479–485. doi: 10.1093/oxfordjournals.jbchem.a134378. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Goldfarb R. H., Madri J. A., Woodley D. T., Liotta L. A. Differential proteolytic susceptibility of laminin alpha and beta subunits. Arch Biochem Biophys. 1982 Nov;219(1):65–70. doi: 10.1016/0003-9861(82)90134-5. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rossi J. D., Wallace B. A. Binding of fibronectin to phospholipid vesicles. J Biol Chem. 1983 Mar 10;258(5):3327–3331. [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Engvall E., Cothran W. C., Butler W. T. Alignment of biologically active domains in the fibronectin molecule. J Biol Chem. 1981 Jul 25;256(14):7277–7281. [PubMed] [Google Scholar]
- STOFFYN P., STOFFYN A. Structure of sulfatides. Biochim Biophys Acta. 1963 Apr 23;70:218–220. doi: 10.1016/0006-3002(63)90746-7. [DOI] [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Furcht L. T. Localization of two unique heparin binding domains of human plasma fibronectin with monoclonal antibodies. J Biol Chem. 1982 Jun 10;257(11):6518–6523. [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Watanabe K., Laine R. A., Hakomori S. I. On neutral fucoglycolipids having long, branched carbohydrate chains: H-active and I-active glycosphingolipids of human erythrocyte membranes. Biochemistry. 1975 Jun 17;14(12):2725–2733. doi: 10.1021/bi00683a026. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Grotendorst G. R., Momoi T. Glycolipids: receptors for fibronectin? J Cell Physiol. 1981 Nov;109(2):343–351. doi: 10.1002/jcp.1041090218. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]