Abstract
Because TENS works by reducing central excitability and activating central inhibition pathways, we tested the hypothesis that TENS would reduce pain and fatigue and improve function and hyperalgesia in people with fibromyalgia who have enhanced central excitability and reduced inhibition. The current study used a double-blinded randomized, placebo controlled cross-over design to test effects of a single treatment of TENS in people with fibromyalgia. Three treatments were assessed in random order: active TENS, placebo TENS, no TENS. The following measures were assessed before and after each TENS treatment: pain and fatigue at rest and movement, pressure pain thresholds (PPTs), 6 minute walk test (6MWT), range of motion (ROM), five time sit to stand test (FTSTS), and single leg stance (SLS). Conditioned pain modulation (CPM) was completed at end of testing. There was a significant decrease in pain and fatigue with movement for active TENS compared to placebo and no TENS. PPTs increased at site of TENS (spine) and outside site of TENS (leg) when compared to placebo TENS or no TENS. During Active TENS CPM was significantly stronger compared to placebo TENS and no TENS. No changes in functional tasks were observed with TENS. Thus, the current study suggests TENS has short-term efficacy in relieving symptoms of fibromyalgia while the stimulator is active. Future clinical trials should examine the effects of repeated daily delivery of TENS, similar to how TENS is used clinically, on pain, fatigue, function and quality of life in individuals with fibromyalgia.
Keywords: electrical stimulation, hyperalgesia, pain, fatigue, fibromyalgia, chronic widespread pain, TENS, analgesia
1. Introduction
The American College of Rheumatology (ACR) classifies fibromyalgia as a clinical syndrome defined by chronic widespread muscle pain, fatigue and tenderness with hyperalgesia to pressure over tender points [68]. Pain and fatigue associated with fibromyalgia can interfere with daily function, work and social activities [1]. The etiology of fibromyalgia is unknown, but it is generally accepted that there is enhanced central excitability [47,59–61] and reduced pain inhibition [26,28,32,59,61]. Thus, one of the main treatments for patients with fibromyalgia must focus on pain relief to allow the person to function more independently both at home and at work. Research into the treatment of fibromyalgia has demonstrated strong evidence that aerobic cardiovascular exercise improves symptoms of fibromyalgia as well as improves quality of life [5]. However, exercise itself may be painful, and the increase in pain may potentially prevent a person from exercising [25,57,66]. Thus, treatments aimed at decreasing pain during movement should improve a person’s ability to participate in activities of daily living. Transcutaneous electrical nerve stimulation (TENS) is a non-pharmacological treatment modality that delivers electrical stimulation through the skin and is used for both acute and chronic pain control [2,9,10,15,46,49,51,67].
Transcutaneous electrical nerve stimulation (TENS) activates central inhibitory pathways [8,23,37,52] and decreases central excitability [23,34,36,53,55]. TENS activates descending inhibitory pathways from the midbrain and brainstem to inhibit excitability of nociceptive neurons in the spinal cord. Although TENS is shown to be effective for several pain conditions such as osteoarthritis, chronic musculoskeletal pain, and postoperative pain [4,20,45], its effectiveness in treatment of people with fibromyalgia is virtually unknown. The primary aim of the study was to test the effectiveness of TENS on pain, fatigue and function in a crossover design study for patients with fibromyalgia with random assignment to three treatments: no TENS control, placebo TENS and active high frequency TENS. A secondary aim was to test the effect of TENS on central inhibition and hyperalgesia as an indicator of central excitability. We hypothesized that the application of TENS to people with fibromyalgia (FM) would reduce pain and fatigue, reduce central excitability and restore conditioned pain modulation (CPM) which would be manifested as improved function.
2. Methods
The current study used a randomized, placebo controlled cross-over design to test the effects of a single treatment of TENS in people with primary fibromyalgia (NCT00932360, C9366). Three treatments were assigned in random order: active TENS, placebo TENS and no TENS with a washout period of one week between treatments. The outcomes assessor remained blinded to the all treatments: active TENS, placebo TENS, and no TENS. The subject was blinded to the active TENS and placebo TENS treatments. The study was approved by the Institutional Review Board at the University of Iowa.
2.1. Subjects
Sample size was calculated using a prior study comparing the effect of active TENS to placebo-TENS for chronic pain and effect sizes ranging between 1.56–1.93 [38]. In the Marchand study, the mean VAS pain intensity rating for each of 16 sessions of active TENS was 2.6 to 3.6 on a 0–10 cm scale and placebo TENS was 2.05 to 3.2. Using a significance level of .05 and power of .80, a sample size of 40 subjects was calculated for this current study. Subjects were recruited from the University of Iowa’s Rheumatology clinic, local rheumatologists and staff of the University of Iowa. The CONSORT diagram is shown in Figure 1. Subjects were screened by telephone with Inclusion criteria of 1) diagnosis of fibromyalgia by a physician and 2) history of cervical or lumbar pain. The 1990 ACR criteria for diagnosis of fibromyalgia were used for this study which includes axial pain above and below the waist as part of the diagnostic criteria [72]. Subjects were not required to restrict other treatments (pharmaceutical of non-pharmaceutical) but were required to be on a stable pharmaceutical management plan for 1 month prior to entering the study. Thus, effects of TENS will be tested as a supplement to standard care. Subjects were excluded if they had: 1) prior use of TENS in the last five years; 2) active inflammatory condition; 3) pacemaker; and 4) pregnancy 5) uncontrolled hypertension or 6) significant cognitive deficits. A total of 125 subjects were screened for eligibility with 82 people unable to participate and 43 people were eligible and agreed to participate with 2 withdrawing after the first session. Of the 82 people unable to participate, we were unable to contact 45 people, 15 declined and 22 were ineligible. The primary reason for ineligibility was prior TENS use. Forty three subjects with fibromyalgia (42F; 1M), aged 25–76 years (mean 49.2 ± 12 yr) participated in this study. Demographic information was gathered for age, gender, ethnicity, marital status, education, income, body mass index and length of diagnosis of fibromyalgia (see Table 1). The Fibromyalgia Impact Questionnaire (FIQ) was completed to help describe clinical presentation of the subjects. The FIQ was used to measure each subject’s ability to complete functional tasks at home, work and social areas of life. The reliability for the FIQ is r=0.72–0.92 and validity is 0.46–0.96 [3].
Figure 1.
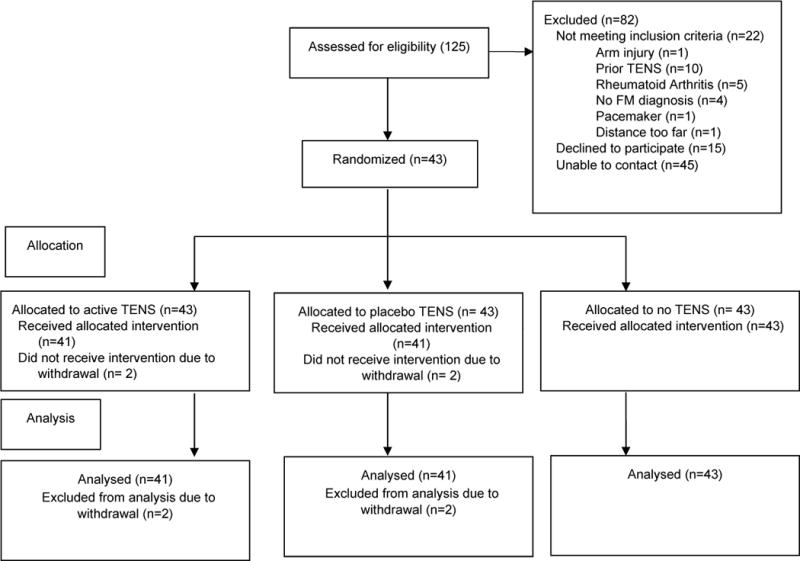
Consort diagram for the study
Table 1.
Demographics and clinical characteristics. * p-value < 0.05
| Total (N=41) | Cervical (n=17) | Lumbar (n=24) | |
|---|---|---|---|
| Age, years (mean ± S.E.M.) |
49.1±12.9 | 48.1±13.0 | 49.9±13.0 |
| Female (% sample) |
40 (97.6%) | 17 (100%) | 23 (95.8%) |
| Ethnicity (% sample) |
|||
| Caucasian | 36 (87.7%) | 14 (82.4%) | 22 (91.7%) |
| Others | 5 (12.2%) | 3 (17.7%) | 2 (8.3%) |
| Marital status (% sample) |
|||
| Married/co-habitating | 25 (61.0%) | 8 (47.1%) | 17 (70.8%) |
| Single/widowed/divorced | 16 (39.0%) | 9 (52.9%) | 7 (29.2%) |
| Education (% sample) |
|||
| High school or less | 10 (24.4%) | 4 (23.5%) | 6 (25.0%) |
| Some college or above | 31 (75.6%) | 13 (76.5%) | 18 (75.0%) |
| Income (% sample) |
|||
| < $ 60,000 | 26 (63.4%) | 13 (76.5%) | 13 (54.2%) |
| ≥ $60,000 | 15 (36.6%) | 4 (23.5%) | 11 (45.8%) |
| Body mass index* (mean ± S.E.M.) |
33.6 ± 9.4 | 37.3 ±11.7* | 31.0 ± 6.3 |
| Length of Fibromyalgia diagnosis, years (mean ± S.E.M.) |
7.4 ± 5.6 | 6.4 ± 5.0 | 8.1 ± 6.0 |
| FIQ (0–100) (mean ± S.E.M.) |
60.05 ± 2.3 | 60.04 ± 4.0 | 60.06 ± 2.6 |
| Pain at Rest (0–10cm scale) (mean ± S.E.M.) |
5.0 ± 0.5 | 4.8 ± 0.6 | 5.1 ± 0.5 |
| Pain with Movement (0–10cm scale) (mean ± S.E.M.) |
5.3 ± 0.4 | 5.6 ± 0.6 | 5.1 ± 0.7 |
| Fatigue at Rest (0–10cm scale) (mean ± S.E.M.) |
4.97 ± 0.4 | 5.3 ± 0.6 | 4.7 ± 0..6 |
| Fatigue with Movement (0–10cm scale) (mean ± S.E.M.) |
5.41 ± 0.4 | 6.0 ± 0.6 | 4.9 ± 0.6 |
| Pressure Pain Threshold Cervical (kPa) (mean ± S.E.M.) |
260.90 ± 22.81 | 265.88 ± 36.28 | 355.49 ± 47.89 |
| Pressure Pain Threshold Lumbar (kPa) (mean ± S.E.M.) |
365.98 ± 31.45 | 257.37 ± 29.92 | 373.42 ± 42.64 |
| Pressure Pain Threshold Anterior Tibialis (kPa) (mean ± S.E.M.) |
421.97 ± 29.45 | 423.91 ± 36.28 | 420.41 ± 44.50 |
Subjects were randomized to treatment group order after providing written consent. The order of active TENS, placebo TENS and no TENS was randomized in the sequentially numbered opaque sealed envelopes [12] that were not available to the outcomes assessor. The order of TENS treatment was randomized by drawing the order out of a hat and were stored in a secure area that was accessed only by the TENS allocator. The envelopes were signed, dated and opened by the TENS allocator before the TENS application and after the outcomes assessor had left the room.
2.2. Outcome Measures
We used a series of pain measures to examine resting and evoked pain as well as pain processing. We added a measure of fatigue since it is a common co-morbid symptom in fibromyalgia. Lastly, we examined several functional measures aimed at endurance, strength, flexibility and balance.
2.2.1. Pain Measures
Visual Analogue Scales (VAS)
A visual analogue scale was used for measurement of pain at rest, pain with movement, fatigue at rest and fatigue with movement. Resting pain and pain with movement (during the 6 minute walk test (6MWT)) was assessed using a 10 cm visual analogue scale. Pain VAS has good test-retest reliability (ICC 0.71–0.99) and convergent validity of 0.30 – 0.95 [22]. The subject was instructed to place a single mark through the line at the appropriate point on the scale. Each scale consisted of a 10-cm horizontal line with descriptors at the far left and far right as “no pain” and “worst pain imaginable”, respectively.
Pressure Pain Threshold (PPT)
PPTs measured deep tissue hyperalgesia using a digital pressure algometer (Somedic AB, Farsta, Sweden). Previous studies demonstrate that anesthetic blockade of the skin under the algometer has no effect on the PPT, thus this is a measure of deep tissue hyperalgesia. A 1cm2 algometer probe applied pressure at a rate of 40 kPa/sec. Subjects were instructed to activate a button when the sensation of pressure clearly became one of painful pressure and this value was recorded. PPTs were assessed over the cervical spine, lumbar spine to assess for effects of TENS at the site of stimulation and over the anterior tibialis muscle to assess for widespread effects of TENS outside the site of stimulation. Two bilateral cervical areas were tested changes at the site of treatment: 1) 2 cm lateral to the C3 spinous processes and 2) 2 cm lateral to C5 spinous processes. Two bilateral lumbar areas were tested: 1) 2 cm lateral to L3 spinous processes and 2) 2 cm lateral to the L5 spinous processes. The left leg was assessed at 5, 6, 7 cm below the inferior patellar pole, bisecting the anterior tibialis musculature. Each area was averaged for a composite PPT score at each site. Each subject had a practice trial on the non-testing forearm prior to data collection. PPT has excellent test-retest reliability (r=0.79–0.94) and is a valid measure of deep tissue hyperalgesia [58].
Conditioned Pain Modulation (CPM)
The ability of subject’s to engage their descending inhibitory systems was tested using CPM. Assessment of CPM was completed using an ice water bath at 4°C as the conditioning stimulus. The subject’s left foot up to the ankle was placed into the cold water and pressure pain thresholds were measured proximal to the electrode in the cervical or lumbar area dependent on the area of TENS application: cervical or lumbar region.
2.2.2. Fatigue Measures
Visual Analogue Scales (VAS)
A visual analogue scale was used for measurement of fatigue at rest and fatigue with movement. Resting fatigue and fatigue with movement (6MWT) was assessed using a 10 cm visual analogue scale. Fatigue VAS has internal consistency (Cronbach’s alpha 0.91–0.96) and concurrent validity (Pearson’s correlation >0.30 with p<0.01). Each scale consisted of a 10-cm horizontal line with descriptors at the far left “no fatigue” and far right as “worst fatigue imaginable”.
2.2.3. Function Measures
6 Minute Walk Test (6MWT)
The 6MWT is a function test measures the maximum distance a person can walk as fast as comfortable in 6 minutes. The test measures time, distance, speed of walking and pain. The 6MWT is a sub maximal test of aerobic capacity with indications for endurance [50]. The subjects completed the walk test in a 200 foot lap turning around at 100 feet. The subjects rated pain and fatigue at the 3 minute time frame. The 6MWT has excellent test-retest reliability (ICC=0.95–0.97) and construct validity (r=0.63–0.79) [62].
Range of Motion (ROM)
Range of motion was completed using the two inclinometer method for cervical and lumbar flexion and extension. The measurement was taken using the method described by the American Medical Association (AMA) in The Guide to the Evaluation of Permanent Impairment [16]. ROM measures have good intrarater reliability for the cervical spine (r=0.864–0.866) and good intratester reliability lumbar spine (r=0.90)[27,43].
Sit to Stand Test (FTSTS)
The FTSTS is a test of strength for the lower body. The time it takes to complete five repetitions of sit to stand completed as quickly as possible is recorded. The FTSTS has good reliability (ICC >0.95) and validity (r=0.59–0.88)[44].
Single Leg Stance (SLS)
SLS is a measure of balance. It was measured with a three trial average to a 30 second maximum with alternating testing of lower extremities. The inter-rater reliability for the best of 3 trials is excellent (0.994 with 95% confidence interval 0.989–0.996 [56].
2.2.4. Transcutaneous Electrical Nerve Stimulation (TENS)
The study used a crossover design with random assignment to three treatments: active TENS, placebo TENS and no TENS (Figure 2). Testing was completed by two assessors: the outcomes assessor and the TENS assessor. The randomization of treatments was completed by the TENS assessor in order to maintain blinding of the outcomes assessor. Each treatment was completed once a week over a three week period. The skin was cleansed with an alcohol swab and a butterfly shaped electrode (Stimcare Premium Electrode, Empi Inc, St. Paul, MN) was applied to the skin (Figure 3). The placement of the electrode was based on patient preference for one of two locations: cervical thoracic junction (n=19) or lumbar-sacral junction (n=24). Instructions to the subject by the TENS assessor were: “You will receive one of three treatments: strong sensation TENS, no sensation TENS and no TENS. The strong sensation will feel like a twitching or tapping. The no sensation TENS will be subtle and you many not feel anything at all. The no TENS treatment means a TENS unit will be attached to the electrodes but not turned on. Neither you nor the outcomes assessor will know which study treatment you are receiving. Today, I will gradually increase the intensity until you feel a strong but tolerable, non-painful sensation. Every 5 minutes I will ask you if the sensation decreased and if needed I will make adjustments”. Previous studies show the greatest analgesic effect of TENS with the highest intensities including those described as maximal tolerable intensity [43,51].
Figure 2.
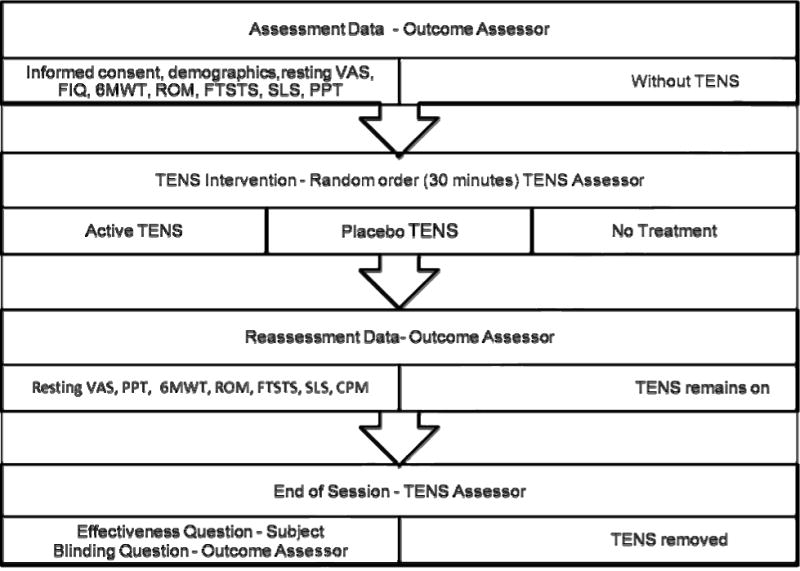
Session order with randomization of TENS treatment
Figure 3.

Picture of electrode used in the study (Stimcare Premium Electrode, Empi Inc, St. Paul, MN)
The TENS treatment consisted of a 30 minute session while the subject rested comfortably and continued to be worn throughout the last half of the testing session for a total of 60–75 minutes. Per standard procedure [33,49,65], the TENS unit was left on during the pain assessments to assess the effect of TENS during its maximally effective period. Once the TENS unit was applied, every 5 minutes, the TENS assessor asked the subject “Are you feeling comfortable?” Adjustments to intensity were made as needed. All TENS devices were Rehabilicare Maxima TENS units (Empi Inc., St Paul, MN). The active TENS settings were 100 Hz, 200 μs at maximal tolerable intensity. The peak amplitude used in the active TENS treatment group was 39.93 ± 13.79 mA. Maximal tolerable intensity was the highest intensity the subject tolerated that was not painful. With some subjects, this intensity produced a motor contraction. An adhesive cloth was placed over the TENS electrode to prevent visual inspection of motor contraction and maintain blinding of the outcome assessor. In addition, the TENS unit was placed in a pouch to maintain blinding for the subject and the outcomes assessor. The placebo TENS unit applied TENS at 100 Hz, 200 μs for 30 seconds and then the current ramped off over a 15 second time frame. Intensity was turned up until the subject reported feeling the stimulation. The average peak amplitude for the placebo unit was 7.81 ± 2.21 mA. The no TENS control was completed with the TENS unit turned off to blind the outcome assessor.
Perceived effectiveness
We asked subjects to rate the relative effectiveness of the active TENS and the placebo treatment. Upon completing each session of testing, the subject was asked, “On a 0–10 scale, how effective was your treatment today?” Each scale consisted of a10-cm horizontal line with anchor points “not effective” and “very effective”.
Blinding
To assess blinding of the outcomes assessor, the outcomes assessor was asked “What treatment did the subject receive today? Choices for the outcome assessor were: active, placebo or no TENS”? The responses to these questions was recorded and compared to assess blinding of the subject and outcomes assessor.
2.2.5. Protocol
Each visit began with questionnaires followed by measurement of height and weight. The outcomes assessor completed testing in the order of: 6MWT, ROM, FTSTS, SLS, PPT, and TENS electrode application. A second assessor completed TENS application. Following the 30 minute resting TENS treatment, the outcomes assessor completed the final testing with the TENS unit on. Thus the TENS unit continued to be worn throughout the testing session for a total of 60–75 minutes. The order of testing following with the TENS on was: PPT, 6MWT, ROM, FTSTS, SLS and CPM.
2.2.5. Data Analyses
Descriptive statistics (mean, standard error, 95% confidence intervals) were determined for each study variable. Chi-square tests were used to make comparisons on categorical variables such as investigation for assessor blinding. The primary analysis was the comparisons between TENS treatments were completed using a mixed model approach which accounts for the repeated measures collected through the crossover design. When group differences were identified, comparisons between groups were made. To reduce the chance of a Type 1 error in multiple comparisons, Tukey’s adjusted p-values were used. Resulting least squares means are provided with 95% confidence intervals. Since CPM was given during TENS treatment we represented the PPT as the percent change in PPT during CPM+TENS divided by the PPT during TENS – thus, a positive number represents an increase in PPT above that produced by TENS, 0 represents no change, and a negative number represents a decrease in PPT below that produced by TENS. For CPM we removed one outlier where percent changes were over 1200%, and there were 3 instances where the subject did not complete the DNIC testing. For the CPM PPTs we therefore had 34 participants in the active TENS group, 35 participants in the placebo TENS group and 33 participants in the no TENS.
3. Results
Table 1 shows the demographic and clinical characteristics for all subjects with fibromyalgia as well as by area of TENS application (cervical or lumbar). There were not significant differences between cervical and lumbar treatment groups except for body mass indexes (p<0.05) which was incorporated in the mixed model analysis as a potential confounder. The peak amplitude used in the active TENS treatment group was 39.93 ± 13.79 mA. The peak amplitude computed from the initial 45 seconds of stimulation for the placebo treatment group was 7.81 ± 2.21 mA.
3.1 Pain at rest and with movement
The average pain intensity at rest (0–10 scale) before TENS was similar between treatments: active TENS was 5.0 ± 0.5, placebo TENS was 5.0 ± 0.4, no TENS was 5.2 ± 0.4. Pain at rest showed no significant difference between treatments: active TENS, placebo TENS or no TENS (Figure 4A). Average pain with movement before TENS is reported in Table 2. Pain with movement (during the 6MWT) was significantly less during active TENS (4.0 ± 0.4) when compared to placebo (4.7 ± 0.4) (p<0.05) or no TENS (5.0 ± 0.4)(p<0.05) (Figure 4A).
Figure 4.
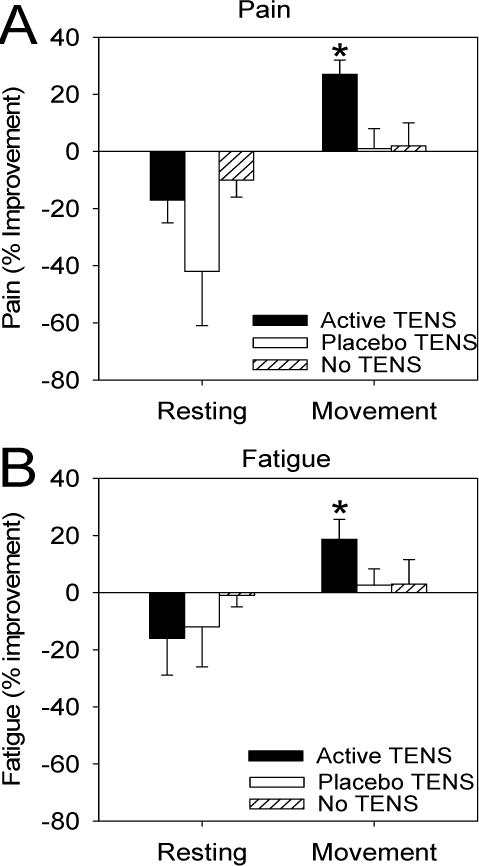
Graphs represent the percent improvement in pain (A) and fatigue (B) at rest and during movement (6MWT) during active TENS, placebo TENS, or no TENS. Significant differences were seen between active TENS and placebo TENS and no TENS for pain with movement and fatigue with movement. * p<0.05, significantly different from active and no TENS.
Table 2.
Data for all outcome measures are represented as a difference score before and during TENS except for PPT with CPM. CPM is represented as the percent change in PPT during DNIC + TENS testing when compared to TENS alone. Data are mean ± S.E.M. and 95% confidence intervals. TENS=Transcutaneous electrical nerve stimulation, VAS=visual analogue scale, PPT=pressure pain threshold, CPM=conditioned pain modulation, 6MWT=six minute walk test, SLS=single leg stance, ROM=range of motion, aSignificant difference between active and placebo; bSignificant difference between active and no TENS; cSignificant difference between placebo and no TENS, p<0.05 considered significant
| Variable | |||
|---|---|---|---|
| Active TENS | Placebo TENS | No TENS | |
| Pain at rest (0–10cm) |
−0.38 ± 0.26 (−0.9 to 0.13) |
−0.74 ± 0.25 (−1.25 to −0.25) |
−0.47 ± .26 (−0.98 to 0.04) |
| Pain with movement (0–10cm) |
1.11 ± 0.26 (0.59–1.63) |
0.23 ± .26a (0.24 to 0.77) |
0.26 ± 0.25b (−0.28 to 0.75) |
| Fatigue at rest (0–10cm) |
−0.09 ± 0.21 (−0.52 to 0.33) |
−0.14 ± 0.21 (−0.56 to 0.28) |
0.12 ± .22 (−0.31 to 0.54) |
| Fatigue with movement (0–10cm) |
0.94 ± 0.23 (0.47 to 1.41) |
0.39 ± .24a (−0.43 to 0.5) |
0.04 ± 0.23b,c (−0.41 to 0.49) |
| PPT cervical (kPa) | 53.15 ±10.09 (73.17 to 33.13) |
26.42 ±10.02 (46.29 to 6.55) |
19.39 ±10.06b (39.34 to −0.57) |
| PPT lumbar (kPa) | 86.97 ± 15.97 (118.76 to 55.18) |
34.89 ± 15.91a (66.58 to 3.2) |
33.23 ± 15.95b (64.97 to 1.48) |
| PPT anterior tibialis (kPa) n=36 |
62.86 ± 18.34 (100.09 to 25.63) |
38.98 ± 21.85 (83.39 to −5.43) |
4.4 ± 15.47b (35.8 to −27.0) |
| PPT with CPM (% change) n=33–34 |
29.99 ± 6.69 (143.6 to 116.37) |
11.21 ± 4.27a (119.88 to 102.53) |
13.59 ± 4.3b (122.38 to 104.82) |
| 6MWT average change (feet) |
41.85 ± 36.7 (116.02 to −32.3) |
−40.48 ± 36.31 (32.89 to −113.87) |
−.97 ± 36.01 (71.81 to −73.76) |
| FTSTS (seconds) | −.07 ± −0.46 (−1.01 to 0.86) |
−0.46± 0.46 (−1.39 to 0.47) |
−0.10 ± 0.46 (−1.04 to 0.83) |
| SLS left (seconds) | −0.48 ± 0.73 (−1.93 to 0.97) |
−1.02 ± 0.72 (−0.41 to 2.44) |
−0.68 ± 0.73 (−2.13 to 0.78) |
| SLS right (seconds) | −0.45 ± 0.74 (−1.91 to 1.02) |
−1.24 ± 0.73 (−2.69 to 0.21) |
1.83 ± 0.74 (−3.3 to −0.36) |
| ROM cervical (degrees) | 1.99 ± 2.37 (−2.66 to 6.64) |
−0.39 ± 2.75 (−5.78 to 5.0) |
−3.12 ± 2.6 (−8.22 to 1.98) |
| ROM lumbar (degrees) | −0.54 to 2.6 (−5.64 to 4.56) |
2.94 ± 2.15 (−1.27 to 7.15) |
−0.41 to 2.04 (−4.41 to 3.59) |
3.2 Fatigue at rest and with movement
The average fatigue intensity at rest before TENS was similar between treatments: active TENS was 5.0 + 0.4, placebo TENS was 5.3 + 0.6, no TENS was 4.7 + 0.6 and were not significantly different (Figure 4B). Average fatigue with movement before TENS is reported in Table 2. Fatigue with movement (during the 6MWT) was significantly different after treatment between active TENS (4.4 ± 2.3) and placebo TENS (5.5 ± 2.6)(p<0.05), and between active TENS and no TENS (5.0 ± 2.7)(p<0.01)(Figure 4A).
3.3 Pressure Pain Thresholds
Average PPT in the cervical, lumbar and leg prior to TENS are reported in Table 2. In the cervical region (n=41), PPTs were significantly increased during active TENS when compared to no TENS (p<0.05) but not placebo TENS (Figure 5A). In the lumbar region (n=41), PPTs were significantly increased in the active TENS when compared to either placebo TENS or no TENS (p<0.05)(Figure 5A). Outside the site of TENS, PPTs (n=36) over the anterior tibialis muscle were significantly increased during active TENS when compared to no TENS (p<0.05) (Figure 5A).
Figure 5.
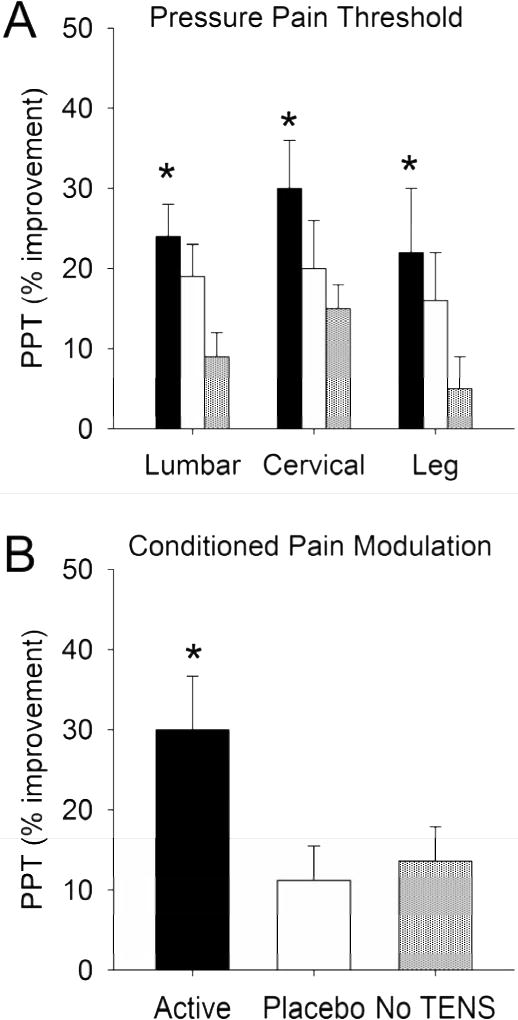
A. Graphs represent percent improvement in pressure pain thresholds (PPTs) in the lumbar region, cervical region, and leg after active TENS, placebo TENS, or no TENS. Significant increases in PPTs were observed in all areas for active TENS (*p<0.05). B. Changes in PPTs during conditioned pain modulation (CPM) during active TENS, placebo TENS or no TENS when compared to the TENS condition alone. A significantly greater change in PPTs occurred in the treatment group that received active TENS when compared to placebo TENS or no TENS treatment group (* p<0.05).
3.4 Conditioned Pain Modulation
To test if TENS modified central inhibition we examined the effects of TENS on CPM. During active TENS PPTs in response to CPM were significantly greater than during placebo TENS (p<0.05) or no TENS (p<0.01) (Figure 5B).
3.5 Function
There were no significant changes in function when examining the ROM, SLS, FTSTS and 6MWTduring active TENS when compared to the placebo TENS or no TENS treatment group (Table 2). While there was a difference in the distance walked during active TENS this measure showed significant variability and did not reach statistical significance.
3.6 Blinding
The outcomes assessor was able to state the treatment (active, placebo, no TENS) correctly between 34% and 58% of the time. For the cervical region, correct treatment was active 54%, placebo 50% and no TENS 58%. For the lumbar region, correct treatment was active 53%, placebo 34% and no TENS 50%. No significant difference was noted between treatments when assessed with a chi squared test (p=0.75).
3.7 Perceived Effectiveness
Effectiveness ratings (0–10 scale) by the subject after treatment was significantly different between active TENS (6.31 ± 0.34) and placebo TENS (3.96 ± 0.39)(p<0.01).
4. Discussion
The current study suggests that TENS improves certain indices of fibromyalgia during or directly after application. Specifically we show that both pain and fatigue during movement, but not at rest, are reduced by a one-time 30 minute treatment with active TENS in individuals with fibromyalgia. Pain thresholds increased not only at the location of TENS application (spine) but also outside the site of TENS (leg) suggesting widespread effects of TENS. Further, we showed increased CPM in the active TENS treatment group suggesting that TENS restores central inhibition. The results of the current pilot study therefore show decreases in pain and increases in inhibition in people with fibromyalgia during a single TENS treatment delivered in a clinical setting. However, TENS is typically given over more prolonged periods of time, weeks to months, and patients are generally given a unit for home use. Thus, future clinical trials should test the effects of TENS over a prolonged treatment period in people with fibromyalgia in which the units are sent home with the subject for daily use. We would suggest future studies deliver TENS during physical activity and exercise since TENS reduces movement pain. We further suggest the use of multiple outcomes including pain at rest and with movement, function and quality of life and those that are in alliance with measures proposed by IMMPACT (Initiative on Methods, Measurement and Pain Assessment in Clinical Trials) which focus on core outcome domains: pain, physical function, emotional function, global improvement, symptoms and adverse events [13,64]
Four randomized controlled-trials have investigated the effectiveness of TENS on pain in people with fibromyalgia with mixed results. One compared effectiveness to s-adenosyl-l-methionin (SAMe), one to warmth therapy, one to massage therapy or sham TENS, and one to placebo. When compared to SAMe, TENS was not effective; however, TENS application was being applied at minimal intensities that caused a tingling sensation over 4 tender points [11]. We, and others, have previously shown that TENS applied at inadequate intensities does not reduce pain or increase pressure pain thresholds (PPTs) [4,48,49]. When compared to warmth therapy TENS effectiveness was similar, with approximately a 1/10 decrease in pain for both treatments [35]. The treatments in this case were not compared to a placebo or a no-treatment control and thus specific effects could not be concluded [35]. Subjects were also simultaneously enrolled in a multidisciplinary treatment program consisting of exercise and cognitive behavioral therapies [35], both effective treatments for fibromyalgia [19]. Thus “effectiveness” of these interventions could be related to the multidisciplinary treatment and not to TENS or warmth therapy. Another study showed that both TENS and massage therapy were better than sham TENS for resting pain [63] which is in direct contrast to the current study showing no effect on resting pain. It is possible that repeated TENS used in the prior study had a cumulative effect as compared to the study that used a one-time treatment [63]. In contrast, a fourth study [41] used TENS in combination with an exercise program. However TENS was given in the morning 5 times per week for 3 weeks, for 30 minutes, and exercise was done in the afternoon and not during TENS. If TENS reduces pain during movement, the use of TENS during exercise should be more beneficial. Further, effectiveness of TENS is greater during the stimulation versus after it has been removed [9,49]. Like most pharmaceutical agents, TENS has a limited duration of action and thus studies should be designed to test effectiveness during peak response. Lastly, all the above studies used resting pain as their primary measure for pain. In the current study, resting pain was unaffected by TENS while movement pain was significantly reduced. Thus, measurement of resting pain in this population may provide conflicting results on effectiveness of the treatment.
Our study extended the prior findings by examining pain during movement, fatigue at rest and during movement, and function. We show that pain and fatigue during movement, but not at rest, are significantly reduced during TENS. This change in movement pain, but not resting pain, was also shown in a prior study by Rakel and Frantz [49] in people with postoperative pain. Further in people with osteoarthritis, pain during movement is also significantly reduced [29,30]. Movement pain in people with fibromyalgia is a significant barrier to exercise and leads to a sedentary lifestyle [21,24,39]. A reduction in pain with movement might be expected to increase physical activity levels and improve quality of life.
Surprisingly, the current study showed that TENS reduced fatigue during movement. Fatigue is a significant symptom in fibromyalgia and is associated with decreased physical activity [7,14,42]. Prior work in animal shows that fatiguing exercise can enhance pain through central mechanisms including those classically involved in inhibition of pain [54,60,69]. Future studies should examine the relationship between pain and fatigue both at rest and with movement to further understand this interaction.
It has become clear that fibromyalgia is associated with enhanced central excitability [47,60] in the pain pathways and loss of pain inhibition [26,28,32,59]. Basic science studies show that TENS reduces enhanced excitability of neurons in the pain pathways [17,18,23,31,55] and activates pain inhibitory mechanisms to reduce hyperalgesia [9,23,53]. The current study showed that TENS increased PPTs not only at the site of TENS application (spine) but also outside the area of TENS (leg) - consistent with reduced central excitability. We further show that PPTs during CPM were greater during active TENS when compared to placebo TENS or no TENS – consistent with an increase in inhibition. Thus the current study validates animal studies by suggesting TENS decreases central excitability and activates central inhibition mechanisms.
The current study showed a lack of change in function during a single TENS treatment. While there was a trend towards an increase in distance walked on the 6MWT this was not significant. As a secondary measure, we may not have had significant power to observe changes in the 6MWT and further repetitive treatment may be necessary to observe a change in function. Prior work shows that repetitive treatment with TENS reduces pain in a cumulative manner in people with chronic musculoskeletal pain [6,38]. Thus, future studies should examine if longer term use of TENS decreases pain with movement, increases physical activity and increases function in fibromyalgia.
Intensity of TENS is critical for producing a reduction in pain [43,52] in healthy controls, and in postoperative pain [5]. In fact, Moran et al. [40] show dose-dependent increase in pressure pain thresholds with increasing intensity from no change at sensory perception thresholds to significant increases at a “strong, but comfortable intensity”. The current study used a maximal tolerable intensity which was above sensory threshold and below pain thresholds, and described to subjects as ‘strong, tolerable and non-painful” to promote the greatest potential relief of pain during active TENS. It should also be noted that we tested effectiveness of the TENS with the unit still on as this is the time when TENS is most effective.
We have previously validated the transient placebo used in the current study [65] showing complete blinding of the outcome and TENS applicator, and adequate blinding of the subject (approx. 50% guessed correctly) allowing us to deliver a true placebo treatment [48][65]. Similar to our prior studies, the assessor was adequately blinded to treatment group in the current study. However, in prior studies we show that subjects identify the active TENS correctly the majority of the time (close to 100%) [48]. This lack of ability to blind the active treatment is an inherent limitation in TENS trials - when TENS is applied at adequate intensities to produce analgesia the subject is aware of receiving an active treatment. To improve blinding, the current study modified the instructions so that subjects thought all 3 treatments were “real” and subjects rated their perceived effectiveness. As such, the current study was unable to directly test blinding of the subject with method of delivery of the intervention in the current study and is a limitation of the study.
In summary, TENS improved movement pain and fatigue, increased pain thresholds both at and outside of the site of stimulation, and increased conditioned pain modulation. Importantly, the current study examined only a single treatment of TENS. Whether longer duration or repeated TENS applications will provide more effective and sustained pain management in fibromyalgia patients remains to be determined, ideally in a large-scale clinical trial. TENS is certainly not a ‘cure’ for fibromyalgia, but should be considered as an additional non-pharmacological treatment option in an existing treatment plan.
Summary.
Pain and fatigue during movement, but not at rest, are reduced by a one-time 30 minute treatment with active TENS in individuals with fibromyalgia.
Acknowledgments
Supported by a grant from the Orthopedic Section of the American Physical Therapy Association, the Carver College of Medicine at the University of Iowa, College of Nursing at the University of Iowa, NIH R34 AR060378. TENS units were donated by DJO, Inc. Additional assistance provided by Shannon Lehman for coordination of the study and Ann Lawler for secretarial and manuscript assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.Gov: NCT 00932360, C9366, Transcutaneous Electrical Nerve Stimulation (TENS) and Fibromyalgia (FM)
Conflict of Interest: Dr. Sluka is a consultant for DJO, Inc. Dr. Rakel has received research support from DJO, Inc.
References
- 1.Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP, Jr, Sharma U, Martin SA, Barrett JA, Haig G. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008;9:792–805. doi: 10.1016/j.jpain.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Aubin M, Marks R. The Efficacy of Short-term Treatment with Transcutaneous Electrical Nerve Stimulation for Osteo-arthritic Knee Pain. Physiotherapy. 1995;81:661. [Google Scholar]
- 3.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–62. [PubMed] [Google Scholar]
- 4.Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. doi: 10.1016/S1090-3801(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 5.Busch AJ, Thille P, Barber KA, Schachter CL, Bidonde J, Collacott BK. Best practice: E-Model–prescribing physical activity and exercise for individuals with fibromyalgia. Physiother Theory Pract. 2008;24:151–166. doi: 10.1080/09593980701686872. [DOI] [PubMed] [Google Scholar]
- 6.Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil. 1999;80:305–312. doi: 10.1016/s0003-9993(99)90142-9. [DOI] [PubMed] [Google Scholar]
- 7.Cook DB, Stegner AJ, Nagelkirk PR, Meyer JD, Togo F, Natelson BH. Responses to exercise differ for chronic fatigue syndrome patients with fibromyalgia. Med Sci Sports Exerc. 2012;44:1186–1193. doi: 10.1249/MSS.0b013e3182417b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantana JM, Da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163:1233–1241. doi: 10.1016/j.neuroscience.2009.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–760. doi: 10.1016/j.apmr.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiBenedetto P, Iona LF, Zidarich V. Clinical Evaluation of S-adnosyl-L-methionine versus transcutaneous electrical nerve stimulaiton in primary fibromyalgia. Current Therapeutic Research. 1993;53:222. [Google Scholar]
- 12.Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20:187–91. doi: 10.1016/j.jcrc.2005.04.005. discussion 191–3. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–1158. doi: 10.1016/j.pain.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13:195–206. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmiler M, Solak O, Kocogullari C, Dundar U, Ayva E, Ela Y, Cekirdekci A, Kavuncu V. Control of acute postoperative pain by transcutaneous electrical nerve stimulation after open cardiac operations: a randomized placebo-controlled prospective study. Heart Surg Forum. 2008;11:E300–3. doi: 10.1532/HSF98.20081083. [DOI] [PubMed] [Google Scholar]
- 16.Engelberg AL, American MA. In: Guides to the evaluation of permanent impairment. Engelberg Alan L., editor. Chicago, Ill: American Medical Association; 1988. [Google Scholar]
- 17.Garrison DW, Foreman RD. Decreased activity of spontaneous and noxiously evoked dorsal horn cells during transcutaneous electrical nerve stimulation (TENS) Pain. 1994;58:309–315. doi: 10.1016/0304-3959(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 18.Garrison DW, Foreman RD. Effects of transcutaneous electrical nerve stimulation (TENS) on spontaneous and noxiously evoked dorsal horn cell activity in cats with transected spinal cords. Neurosci Lett. 1996;216:125–128. doi: 10.1016/0304-3940(96)13023-8. [DOI] [PubMed] [Google Scholar]
- 19.Hauser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, Busch A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79. doi: 10.1186/ar3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007;130:157–165. doi: 10.1016/j.pain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Jones J, Rutledge DN, Jones KD, Matallana L, Rooks DS. Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Health Issues. 2008;18:406–412. doi: 10.1016/j.whi.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Kahl C, Cleland J. Visual Analogue Scale, Numeric Rating Scale and the McGill Pain Quesitonniare: An Overview of Psychometric Properties. Phys Ther. 2005;10:123. [Google Scholar]
- 23.Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS) J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 24.Kop WJ, Lyden A, Berlin AA, Ambrose K, Olsen C, Gracely RH, Williams DA, Clauw DJ. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 25.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 26.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 27.Lantz CA, Chen J, Buch D. Clinical validity and stability of active and passive cervical range of motion with regard to total and unilateral uniplanar motion. Spine (Phila Pa 1976) 1999;24:1082–1089. doi: 10.1097/00007632-199906010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Law PP, Cheing GL. Optimal stimulation frequency of transcutaneous electrical nerve stimulation on people with knee osteoarthritis. J Rehabil Med. 2004;36:220–225. doi: 10.1080/16501970410029834. [DOI] [PubMed] [Google Scholar]
- 30.Law PP, Cheing GL, Tsui AY. Does transcutaneous electrical nerve stimulation improve the physical performance of people with knee osteoarthritis? J Clin Rheumatol. 2004;10:295–299. doi: 10.1097/01.rhu.0000147047.77460.b0. [DOI] [PubMed] [Google Scholar]
- 31.Lee KH, Chung JM, Willis WD., Jr Inhibition of primate spinothalamic tract cells by TENS. J Neurosurg. 1985;62:276–287. doi: 10.3171/jns.1985.62.2.0276. [DOI] [PubMed] [Google Scholar]
- 32.Leffler AS, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain. 2002;6:149–159. doi: 10.1053/eujp.2001.0312. [DOI] [PubMed] [Google Scholar]
- 33.Liebano RE, Rakel B, Vance CG, Walsh DM, Sluka KA. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 2011;152:335–342. doi: 10.1016/j.pain.2010.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lisi TL, Sluka KA. A new electrochemical HPLC method for analysis of enkephalins and endomorphins. J Neurosci Methods. 2006;150:74–79. doi: 10.1016/j.jneumeth.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Lofgren M, Norrbrink C. Pain relief in women with fibromyalgia: a cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J Rehabil Med. 2009;41:557–562. doi: 10.2340/16501977-0371. [DOI] [PubMed] [Google Scholar]
- 36.Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137:94–102. doi: 10.1007/s002210000629. [DOI] [PubMed] [Google Scholar]
- 37.Maeda Y, Lisi TL, Vance CG, Sluka KA. Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res. 2007;1136:43–50. doi: 10.1016/j.brainres.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marchand S, Charest J, Li J, Chenard JR, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain. 1993;54:99–106. doi: 10.1016/0304-3959(93)90104-W. [DOI] [PubMed] [Google Scholar]
- 39.McLoughlin MJ, Colbert LH, Stegner AJ, Cook DB. Are women with fibromyalgia less physically active than healthy women? Med Sci Sports Exerc. 2011;43:905–912. doi: 10.1249/MSS.0b013e3181fca1ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, Rakel BA, Sluka KA, Walsh DM. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12:929–935. doi: 10.1016/j.jpain.2011.02.352. [DOI] [PubMed] [Google Scholar]
- 41.Mutlu B, Paker N, Bugdayci D, Tekdos D, Kesiktas N. Efficacy of supervised exercise combined with transcutaneous electrical nerve stimulation in women with fibromyalgia: a prospective controlled study. Rheumatol Int. 2012 doi: 10.1007/s00296-012-2390-8. [DOI] [PubMed] [Google Scholar]
- 42.Newcomb LW, Koltyn KF, Morgan WP, Cook DB. Influence of preferred versus prescribed exercise on pain in fibromyalgia. Med Sci Sports Exerc. 2011;43:1106–1113. doi: 10.1249/MSS.0b013e3182061b49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng JK, Kippers V, Richardson CA, Parnianpour M. Range of motion and lordosis of the lumbar spine: reliability of measurement and normative values. Spine (Phila Pa 1976) 2001;26:53–60. doi: 10.1097/00007632-200101010-00011. [DOI] [PubMed] [Google Scholar]
- 44.Novy DM, Simmonds MJ, Lee CE. Physical performance tasks: what are the underlying constructs? Arch Phys Med Rehabil. 2002;83:44–47. doi: 10.1053/apmr.2002.27397. [DOI] [PubMed] [Google Scholar]
- 45.Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell P, Wells G. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database Syst Rev. 2000;(4):CD002823. doi: 10.1002/14651858.CD002823. [DOI] [PubMed] [Google Scholar]
- 46.Platon B, Andrell P, Raner C, Rudolph M, Dvoretsky A, Mannheimer C. High-frequency, high-intensity transcutaneous electrical nerve stimulation as treatment of pain after surgical abortion. Pain. 2010;148:114–119. doi: 10.1016/j.pain.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 47.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ. Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 48.Rakel B, Cooper N, Adams HJ, Messer BR, Frey Law LA, Dannen DR, Miller CA, Polehna AC, Ruggle RC, Vance CG, Walsh DM, Sluka KA. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230–238. doi: 10.1016/j.jpain.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. doi: 10.1067/s1526-5900(03)00780-6. [DOI] [PubMed] [Google Scholar]
- 50.Rikli R, Jones C. The Reliability and Validity of a 6-Minute Walk Test as a Measure of Physical Endurance in Older Adults. Journal of Aging and Physical Activity. 1998;6:363–375. [Google Scholar]
- 51.Rutjes AW, Nuesch E, Reichenbach S, Juni P. S-Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;(4):CD007321. doi: 10.1002/14651858.CD007321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sluka KA. Blockade of N- and P/Q-type calcium channels reduces the secondary heat hyperalgesia induced by acute inflammation. J Pharmacol Exp Ther. 1998;287:232–237. [PubMed] [Google Scholar]
- 53.Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 54.Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain. 2010;148:188–197. doi: 10.1016/j.pain.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sluka KA, Vance CG, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J Neurochem. 2005;95:1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- 56.Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30:8–15. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Staud R. Predictors of clinical pain intensity in patients with fibromyalgia syndrome. Curr Pain Headache Rep. 2005;9:316–321. doi: 10.1007/s11916-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 58.Staud R. Treatment of fibromyalgia and its symptoms. Expert Opin Pharmacother. 2007;8:1629–1642. doi: 10.1517/14656566.8.11.1629. [DOI] [PubMed] [Google Scholar]
- 59.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. doi: 10.1016/s0304-3959(02)00344-5. [DOI] [PubMed] [Google Scholar]
- 60.Staud R, Domingo M. Evidence for abnormal pain processing in fibromyalgia syndrome. Pain Med. 2001;2:208–215. doi: 10.1046/j.1526-4637.2001.01030.x. [DOI] [PubMed] [Google Scholar]
- 61.Staud R, Smitherman ML. Peripheral and central sensitization in fibromyalgia: pathogenetic role. Curr Pain Headache Rep. 2002;6:259–266. doi: 10.1007/s11916-002-0046-1. [DOI] [PubMed] [Google Scholar]
- 62.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82:128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 63.Sunshine W, Field TM, Quintino O, Fierro K, Kuhn C, Burman I, Schanberg S. Fibromyalgia benefits from massage therapy and transcutaneous electrical stimulation. J Clin Rheumatol. 1996;2:18–22. doi: 10.1097/00124743-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Turk DC, O’Connor AB, Dworkin RH, Chaudhry A, Katz NP, Adams EH, Brownstein JS, Comer SD, Dart R, Dasgupta N, Denisco RA, Klein M, Leiderman DB, Lubran R, Rappaport BA, Zacny JP, Ahdieh H, Burke LB, Cowan P, Jacobs P, Malamut R, Markman J, Michna E, Palmer P, Peirce-Sandner S, Potter JS, Raja SN, Rauschkolb C, Roland CL, Webster LR, Weiss RD, Wolf K. Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain. 2012;153:1997–2008. doi: 10.1016/j.pain.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vance CG, Rakel BA, Blodgett NP, DeSantana JM, Amendola A, Zimmerman MB, Walsh DM, Sluka KA. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92:898–910. doi: 10.2522/ptj.20110183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 67.Walsh DM, Howe TE, Johnson MI, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst Rev. 2009;(2):CD006142. doi: 10.1002/14651858.CD006142.pub2. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe F. Fibromyalgia. Rheum Dis Clin North Am. 1990;16:681–698. [PubMed] [Google Scholar]
- 69.Yokoyama T, Maeda Y, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]


