Abstract
The voltage-gated sodium channel (VGSC) interacting peptide is of special interest for both basic research and pharmaceutical purposes. In this study, we established a yeast-two-hybrid based strategy to detect the interaction(s) between neurotoxic peptide and the extracellular region of VGSC. Using a previously reported neurotoxin JZTX-III as a model molecule, we demonstrated that the interactions between JZTX-III and the extracellular regions of its target hNav1.5 are detectable and the detected interactions are directly related to its activity. We further applied this strategy to the screening of VGSC interacting peptides. Using the extracellular region of hNav1.5 as the bait, we identified a novel sodium channel inhibitor SSCM-1 from a random peptide library. This peptide selectively inhibits hNav1.5 currents in the whole-cell patch clamp assays. This strategy might be used for the large scale screening for target-specific interacting peptides of VGSCs or other ion channels.
Among various types of ion channels, voltage-gated sodium channels (VGSCs) are responsible for the genesis and propagation of action potential, by which the responses of nociceptors to stimuli are encoded. The VGSCs are therefore considered ideal targets for the development of pain therapeutics1,2. For decades, small molecules such as lidocaine and mexiletine targeting VGSCs, although not specifically, have been widely used in the clinical treatment of pain3. Recently, biologicals such as peptides and antibodies were proposed to be the next generation of pain relief drug leads. Compared with small chemical molecules, peptides demonstrate a greater potential for ion channel targeting selectivity, whereas the lower selectivity over the sub-types of VGSCs or other ion channels of small chemical molecule might be responsible for their clinical side effects4.
Venom peptides are considered as an invaluable source of natural products for drug discovery. Several peptide toxins isolated from venoms of scorpion, spider, and cone snails were demonstrated to be able to interact with VGSCs and might be further developed into pain relief drugs1,5,6. Six venom peptides or protein derivatives became FDA-approved drugs and more are in clinical trials or various stages of preclinical development7. Although the pursuit of VGSC blockers is an exciting prospect, the progress of obtaining VGSCs blockers from venoms is somehow disappointing. For example, out of the conservatively estimated more than 10 million bioactive peptides that might be present in the spider venoms, only 62 peptides were identified to be VGSCs interacting peptides, and some of them target more than one type of channels8.
According to the previously published works and our own experiences, the isolation and characterization of a VGSC or other ion channel interacting peptide from venoms usually consists of four typical procedures: the collection of crude venoms, the isolation and purification of single component/peptide, the analysis of biochemical characterization of the peptide, and the physiological characterization of the peptide. The biochemical isolation and purification of single peptide from the crude venom requires not only a sufficient amount of crude venom, but also the equipment, reagents, skills and experiences, time, and sometimes even luck [some venoms might contain as many as 1,000 different kinds of peptides and proteins]9. The physiological activity of a purified peptide could be estimated by different in vivo assays by using animals, insects, or cultured cells. However, to determine its molecular target(s) could be a struggle. There are too many possible targets: receptors, various kinds of ion channels, and different sub-types of a certain ion channel. In summary, the current approaches for the isolation and characterization of ion channels interacting peptides from venoms are low throughput. Moreover, using the current approaches, we are isolating ion channel interacting peptide by chance or with luck, since it is hard to predict what kind of interacting peptide can be isolated from a venom mixture. We initiated our work reported here from two questions: For a given peptide or toxin, is there a high throughput approach to predict its potential interaction site(s)? Targeting a given voltage-gated sodium channel, is there a high throughput approach to screen for its interacting peptide(s)?
Results
Mapping the interaction sites of JZTX-III on VGSCs
It is commonly recognized that the peptide regulates the VGSC function through its direct or indirect interaction(s) with the target molecule. It is therefore reasonable to assume that by detecting the interaction(s) between peptides and VGSC proteins, we would be able to predict whether a peptide could be a VGSC inhibitor or activator. Yeast-two-hybrid approach developed in 1989 provides a powerful tool for the high throughput study of protein-protein interaction(s) in vivo and has been widely used in searching for protein-protein interactions in many research works, even in new drug discovery10,11. A major concern of using yeast-two-hybrid method to study the interaction(s) between peptides and VGSCs is the conformation issue. A functional voltage-gated sodium channel is composed of a large pore-forming α subunit (220 ~ 260 kDa) and one or more auxiliary β subunit(s) (33 ~ 36 kDa). Most of the previously reported VGSC interacting peptides bind to the α subunits. Structurally, a VGSC α subunit is comprised of four homologous domains (DI-DIV). In each domain, there are six transmembrane segments (S1–S6) connected by both intracellular and extracellular regions12,13. The maintenance of the natural conformation of a VGSC requires the interactions between the VGSC protein and the cell membrane. In a yeast-two-hybrid assay, the natural conformation of a full length VGSC could be completely disturbed when expressed in the cytoplasm of the yeast cells without the assistant of cell membrane. Although it is not difficult to imagine that an extraneous peptide could bind to the target VGSC at its extracellular regions, could this kind of interaction be maintained by the extracellular region taken out of the full-length context and detected by yeast-two-hybrid assay?
We chose jingzhaotoxin-III (JZTX-III), a neurotoxic peptide isolated from the spider venom, as a model molecule to address this question. JZTX-III specifically blocks human Nav1.5 (hNav1.5) and has almost no effect on hNav1.7 at 1 μM. In a previous report, it was demonstrated that the DII/S3–S4 extracellular region of hNav1.5 is critical to JZTX-III's inhibitory activity14. Is it possible that the DII/S3–S4 region alone interacts with JZTX-III and could such an interaction be detected in a yeast-two-hybrid assay? In our experiments, JZTX-III was expressed in fusion with the DNA binding domain (DBD) of Gal4 and the S3–S4 regions from the four homologous domains of hNav1.5 or hNav1.7 were expressed in fusion with the activation domain (AD) of Gal4 in yeast, respectively (Supplementary Table S1). As summarized in Table 1, the interactions between JZTX-III and the S3–S4 extracellular regions of hNav1.5 or hNav1.7 could be detected by yeast-two-hybrid assay. More interestingly, the interaction pattern of hNav1.5 with JZTX-III was different from that of the hNav1.7.
Table 1. Detected interactions between JZTX-III and the S3–S4 extracellular regions.
| Extracellular region | JZTX-III vs hNav1.5 | JZTX-III vs hNav1.7 |
|---|---|---|
| DI/S3–S4 | + | + |
| DII/S3–S4 | + | N.D. |
| DIII/S3–S4 | + | N.D. |
| DIV/S3–S4 | + | N.D. |
The symbol “+” represents a detectable interaction. N.D. (not detected).
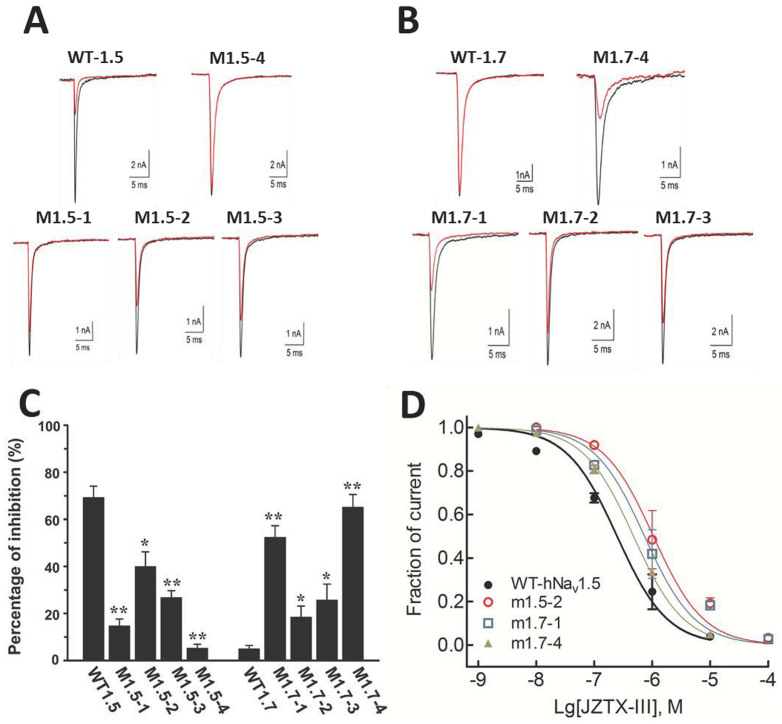
In addition to the expected interaction between JZTX-III and DII/S3–S4 region, interactions between JZTX-III and S3–S4 regions of other domains of hNav1.5 were also detected. Do these interactions contribute to JZTX-III's physiological activity? To address this question, we generated a series of hNav1.5 and hNav1.7 chimeras (Fig. 1; see also Supplementary Table S2). These chimeras were expressed in the cultured human embryonic kidney 293 (HEK293) cells and the corresponding sodium currents were detected by whole-cell patch clamp experiments. In a loss-of-function assay, as shown in panels A and C of Fig. 2, hNav1.5 chimeras lost its sensitivity to JZTX-III inhibition when its S3–S4 fragments were replaced by those from hNav1.7. On the other hand, when the S3–S4 fragments were replaced by those from hNav1.5, hNav1.7 chimeras gained partial sensitivity to JZTX-III (Fig. 2, panels B and C). Moreover, when all the three S3–S4 fragments were replaced, the affinity to JZTX-III of hNav1.5 chimera became almost identical to that of the wild type hNav1.7, and vice versa. The dose-response curves of JZTX-III inhibiting several chimeras were also shown in Fig. 2D, and the estimated IC50 values of JZTX-III were 0.297, 1.047, 0.736, and 0.476 μM for wild type hNav1.5, m1.5-2, m1.7-1, and m1.7-4 channels, respectively. The IC50 value for other chimeras could not be accurately calculated, but our data indicate that it is at least >10 μM (data not shown).
Figure 1. Defining the motifs in chimeras.
The transplanted sequence in each chimera is show in colors.
Figure 2. Loss- or gain-of-function analysis of hNav1.5 and hNav1.7 chimeras.
The wild type (WT) or chimeric VGSC currents were recorded before (black trace) and after (red trace) the application of JZTX-III. Panel A, inhibition of 1 μM JZTX-III on WT-hNav1.5 and chimeric hNav1.5 currents with S3–S4 fragments replaced by those from hNav1.7. M1.5-1, M1.5-2, M1.5-3, and M1.5-4 represent chimeras with the S3–S4 fragments of DII, DIII, DIV, and all three domains (DII + DIII + DIV) of hNav1.5 replaced by those from hNav1.7, respectively. Panel B, inhibition of 1 μM JZTX-III on hNav1.7 currents, the naming of the chimeras followed the same rules as for the hNav1.5 chimeras. Panel C, quantitation of the inhibition percentage of JZTX-III (1 μM) on chimeras' currents. *, p < 0.05; **, p < 0.001 significance between WT and chimeric channels. Panel D, concentration-response inhibitory curves of JZTX-III. Data points (mean ± S.E., each from 3–5 cells).
These results demonstrated that in addition to the interaction between DII/S3–S4 region of hNav1.5 and JZTX-III, which was proposed in a previous study and confirmed here, the interactions of DIII and DIV S3–S4 regions of hNav1.5 with JZTX-III detected by the yeast-two-hybrid assay in this study also contribute to its sensitivity to JZTX-III. With these results, it would be safe to propose that the analysis of interaction profiles of a given peptide with the extracellular regions of the VGSCs might provide valuable clues to the defining of its target(s). The fact that the replacement of hNav1.7's extracellular regions with those from hNav1.5 alters its sensitivity to extraneous interacting peptide, at least in the JZTX-III case is also of potential applications. We have noticed that not all VGSCs currents could be functional reconstructed in cultured cells, for example, there is no reconstructed Nav1.9 current reported thus far. However, as we replaced the extracellular regions of hNav1.5 (using hNav1.5 as the scaffold) with those from hNav1.9 (i.e., the S1–S2 region or S3–S4 region, respectively), we were easily able to measure the sodium currents (0.5 ~ 3 nA) of some chimeric channels in cultured HEK293 cells in whole-cell patch clamp assays (Supplementary Table S3). We would suggest that these chimeric sodium channel be utilized in the finding of Nav1.9 interacting peptides, although voltage-gated potassium channel chimeras approach were proposed previously15.
Screening the random peptide library
After demonstrating that the interaction(s) between peptides and extracellular regions of VGSCs can be detected by this strategy and some the interaction(s) detected are physiologically relevant, we further carried out experiments using yeast-two-hybrid method to search for the interacting peptides of a given voltage-gated sodium channel.
In a typical screening of cofactors of a given protein using yeast-two-hybrid approach, a DNA library or pool is usually required. At least three types of DNA libraries are optional for the screening for VGSC interacting peptides, including a cDNA library constructed using the tissues of venomous animals, a saturated-mutation library based on a known toxic peptide, or a random peptide library from “nowhere”. The cDNA library of venomous animals seemed to be a good choice since many researchers considered the natural venoms might be the valuable source of ion channel blockers. However, previous study demonstrated that the coding region of a piece of cDNA presented in a venomous animal tissue cDNA library usually consist of not only the matured peptide, but also the signal peptide and the pro-peptide16,17,18. The existence of such non-matured peptide fragments might interfere with the yeast-two-hybrid assay and yield the false-positive or false-negative results. Obtaining several bioactive peptides by either saturated or site-directed mutating some residues of a known toxin is almost guaranteed. Therefore a saturated-mutation library based on a known toxin backbone is a safer choice yet may be too conservative in our view. We generated a random peptide library by inserting 30 continuous random nucleotides following the DBD coding sequence in the pGADT7 plasmid by PCR. In a 105 scale screening using the DII/S3–S4 region of hNav1.5 as the bait, we identified a DNA sequence that encodes a peptide containing 26 amino acid residues which was able to interact with hNav1.5 in vivo. The deduced amino acid sequence of this peptide is NH2-MKYKNSTRVGIDTGSIELELQMNRRY-COOH.
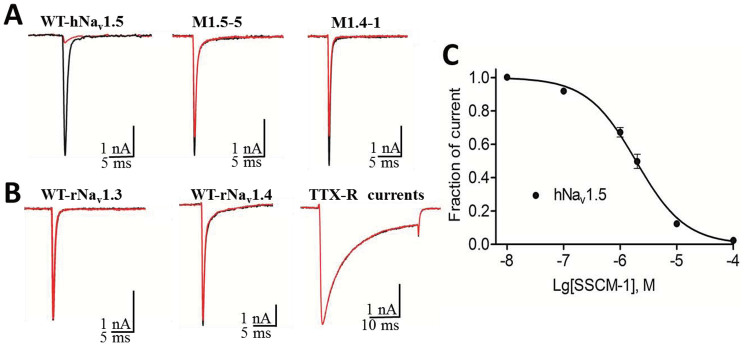
This peptide was then chemically synthesized and applied to the electrophysiological assay of its bioactivity on hNav1.5. As shown in panel A of Fig. 3, in a whole-cell patch-clamp assay, it inhibited wild type hNav1.5 currents over 90% at 10 μM. It was therefore named Screened-Sodium-Channel-Interacting peptide-1 (SSCM-1). The selectivity of VGSC isoforms was also observed since SSCM-1 has subtle inhibitory activity on rNav1.3, rNav1.4, or TTX-R currents as shown in Fig. 3B. To confirm that its inhibitory effect on hNav1.5 is due to its interaction with the DII/S3–S4 region of hNav1.5, the loss or gain-of-function assays were performed (Fig. 1 and Supplementary Table S2). As shown in Fig. 3A, in a loss-of-function assay, it was demonstrated that SSCM-1 had almost no effect on the chimeric hNav1.5 currents with its DII/S3–S4 region replaced by that of rNav1.4 (M1.5-5). On the other hand, when the DII/S3–S4 region of rNav1.4 region was replaced by that of hNav1.5, a detectable inhibitory effect of SSCM-1 over the resulted chimeric rNav1.4 (M1.4-1) currents was observed. In Fig. 3C, Hill logistic equation fits of the dose-response curves indicated that the IC50 values yielded approximately was 2.88 μM on wild-type hNav1.5.
Figure 3. Effects of peptide SSCM-1 on VGSCs.
The wild type (WT) or chimeric VGSC currents were recorded before (black trace) and after (red trace) the application of SSCM-1. Panel A, WT-hNav1.5 currents were inhibited over 90% at 10 μM SSCM-1, but SSCM-1 could only inhibit about 15% chimera M1.5-5 currents at 50 μM. SSCM-1 could not inhibit TTX-R currents expressed on DRG cells (containing 200 μM TTX) at 10 μM. Panel B, WT-rNav1.3 and WT-rNav1.4 currents almost no detectable inhibitory effect was observed at 50 μM SSCM-1. Chimera M1.4-1 currents could be inhibited 15% by SSCM-1 at 50 μM. Panel C, concentration-response inhibitory curves of SSCM-1 on hNav1.5 currents. Data points (mean ± S.E., each from 3–5 cells).
Discussion
The primary objective of the present study was to establish a high-throughput method to screen the given VGSC interacting peptide(s). Using a random peptide library and yeast-two-hybrid assays, we have succeeded in identifying a specific hNav1.5 inhibitor SSCM-1. A homologous search showed that SSCM-1 has no significant similarity with any previously known peptides. Compared with other toxins isolated from natural venoms targeting VGSCs, SSCM-1 has no cysteine residue in its primary sequence and forms no disulfide bridge. It was claimed that after thousands of millions of years of evolution, the animal venoms contain most optimized toxins that can be used as drug model molecules for pharmaceutical purposes. We have no doubt of the power of evolution yet have noticed that in the nature, venomous animals developed their venoms in a most effective way for survival, they are preferably multifunctional-targeting more targets to adapt for the evolution of prey19. However, one of the primary considerations of whether a peptide could be developed to a potential drug is its selectivity, and highly selectivity is preferred for drug development. After decades of struggle, less than 1% of the peptides or proteins were isolated and partially characterized from natural developed venoms, even less were demonstrated to have potential pharmaceutical values8,9,20. Besides the lack of highly effective research tools, could the nature of the natural venoms be an intrinsic problem?
Theoretically, a peptide composed of 26 amino acid residues has 2026 different possible sequences. The number of possible existed peptides or proteins would increases in an exponential way if a peptide or protein contains more amino acid residues. A random peptide library obviously provides much more candidates for drug discovery or other purposes, as long as a high throughput screening tool is available. Practically, the yeast-two-hybrid approach could be used to screen a library with a complexity up to 107, more efficient screening tools need to be developed to make full use of the random peptide libraries. Nevertheless, the finding of SSCM-1 provides a practical example for the screening of bioactive peptides targeting a given VGSC protein.
In conclusion, using the extracellular region of a given voltage-gated sodium channel protein as a bait, the yeast-two-hybrid technology could be used to screen for specific interacting peptide(s) of the target protein from a laboratory constructed proper peptide library. Based on the fact that many voltage-gated ion channels including potassium channels, sodium channels, and calcium channels possessing similar structures13,21,22, our strategy may provide a high throughput solution to the identification of novel interacting peptides at these ion channels, which would facilitate the in depth study of ion channel function and the development of new bioactive drugs targeting on these ion channels.
Methods
Materials
Used for yeast two hybrid assays, MATCHMAKER GAL4 Two-Hybrid System 3 was purchased from Clontech (Mountain View, CA, USA). KOD-Plus-DNA Polymerase which was employed to site-directed mutation or chimera assays was purchased from TOYOBO (Osaka, Japan). All the restriction enzymes used for molecular cloning assays were purchased from Fermentas (Burlington, ON, Canada) if not otherwise noted. The synthesis of non-random primers and DNA sequencing were performed by Sangon (Shanghai, China). The synthesis of random primers was performed by sbsgene (Shanghai, China). Peptide JZTX-III was purified as previously described23. Peptide SSCM-1 was obtained by chemical synthesis. If no specially noted, all the chemicals were purchased from Sigma (St. Louis, MO, USA).
Yeast-two-hybrid assay
All the data of toxin and VGSCs are downloaded from UniProtKB/Swiss-Prot database [accession P08104 for rNav1.3, P15390 for rNav1.4, Q14524 for hNav1.5, Q15858 for hNav1.7, Q9UI33 for hNav1.9, P62520 for JZTX-III]. For JZTX-III related yeast-two -hybrid assays, the coding sequences of the extracellular regions (S1–S2 or S3–S4) of VGSCs were inserted to vector pGADT7 as the Preys, respectively (Supplementary Table S1). The coding sequence of JZTX-III was inserted to another vector pGBKT7 as Bait, using upper primer (5′-GGAATTCCATATGGTGTGCATGACCCGAACTGAT-3′, Nde I) and lower primer (5′-GGAATTCTTAGACGTACTCGACTTTCTCTGG-3′, EcoR I). For screening random-10-library assay, the coding sequences of extracellular regions of VGSCs were inserted to vector pGBKT7 as the bait.
Autonomous activation and interaction detecting assays were performed according to Yeast Protocols Handbook of Clontech. For JZTX-III related yeast-two-hybrid assays, interactions were detected using Colony-lift Filter Assay. For screening the random-10-library, interactions were identified using medium stringency selection media (-Leu/-Trp/-His).
Chimeras for electrophysiological assay, Random peptide library
Chimeras (Supplementary Table S2 and Table S3) for electrophysiological assays were obtained using the overlapped PCR mutation method described previously24. Random-10-peptide library was constructed using insertion of a pair of random primers to vector pGADT7, including upper primer (5′-CGTACCAGATTACGCTNNKNNKNNKNNKNNKNNKNNKNNKNNKNNKTAATAGCCGGGTGGGCATCGATACGGGATCCAT-3′) and lower primer (5′-ATGCCCACCCGGCTATTAMNNMNNMNNMNNMNNMNNMNNMNNMNNMNNAGCGTAATCTGGTACGTCGTATGGGTACTC-3′), according to a method reported previously25. However, in order to harvest enough library plasmids, the cycles of PCR were 25–30 times which was much more than the method mentioned. (N = A:T:G:C = 25%:25%:25%:25%; K = A:T:G:C = 0:50%:50%:0; M = A:T:G:C = 50%:0:0:50%).
Whole-cell patch clamp assay
The whole-cell patch clamp assay was performed as previously described26,27. JZTX-III or SSCM-1 was dissolved to 1 mM as stock solution using double distilled water, and aliquots were stored at −20°C. HEK293 cells were grown under standard tissue culture conditions (5% CO2; 37°C) in DMEM supplemented with 10% FBS. The β1 and β2 subunits were cotransfected with the hNav1.7 channel to increase the current density. For Nav experiments on HEK293 cells, the pipette solution contained 140 mM CsF, 10 mM NaCl, 1 mM EGTA and 10 mM HEPES, pH 7.3; the bathing solution was 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES, pH 7.3. For Nav experiments on DRG neurons, the pipette solution contained 145 mM CsCl, 4 mM MgCl2·6H2O, 10 mM HEPES, 10 mM EGTA, 10 mM Glucose, 2 mM ATP (pH 7.2); the bathing solution contained 145 mM NaCl, 2.5 mM KCl, 1.5 mM CaCl2, 1.2 mM MgCl2·6H2O, 10 mM HEPES, 10 mM D-Glucose (pH 7.4)27. The whole-cell patch clamp assays were performed at room temperature (25 ± 2) using an EPC-10 amplifier (HEKA, Lambrecht, Germany). Data were analyzed using Pulsefit (HEKA) and Sigmaplot 10.0 (Systat Software Inc.) programs.
Author Contributions
D.-Y.Z. and E.M. designed the appropriate technology routes. E.M. and T.-F.C. conducted main experiments including cloning, random-10-peptie library, yeast-two-hybrid, and whole-cell patch clamp assays. H.Z., S.T. and M.-J.L. participated in random-10-peptide library. W.-Y.L., L.L., L.W. and L.-Y.Z. participated in building constructs. P.-F.H., K.L. and K.P. participated in whole-cell patch clamp. X.-D.D., H.J. and X.-Z.Z. performed the mass spectrometry and contributed to chemical synthesis and purification of SSCM-1. D.-Y.Z. and S.-P.L. supervised the project and wrote the paper. All authors read and approved the final manuscript.
Supplementary Material
Screening for Voltage-Gated Sodium Channel Interacting Peptide
Acknowledgments
This work was supported by the Ministry of Science and Technology of China “973” Project (No. 2010CB529800), National Natural Science Foundation of China (No. 3097142, 11004246, and 31100609), New Century Excellent Talents in University (No. NCET-05-0715), and NUDT University Project (No. JC2006-02-01).
References
- Dib-Hajj S. D. et al. Voltage-gated sodium channels in pain states: role in pathophysiology and targets for treatment. Brain Res Rev 60, 65–83, 10.1016/j.brainresrev.2008.12.005 (2009). [DOI] [PubMed] [Google Scholar]
- Krafte D. S. & Bannon A. W. Sodium channels and nociception: recent concepts and therapeutic opportunities. Curr Opin Pharmacol 8, 50–56, S1471-4892(07)00163 -4 (2008). [DOI] [PubMed] [Google Scholar]
- Eijkelkamp N. et al. Neurological perspectives on voltage-gated sodium channels. Brain 135, 2585–2612, 10.1093/brain/aws225 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffet A. Peptides as drugs: Is there a market? J. Peptide Sci. 8, 1–7, 10.1002/psc.366 (2002). [DOI] [PubMed] [Google Scholar]
- Mouhat S., Andreotti N., Jouirou B. & Sabatier J.-M. Animal toxins acting on voltage-gated potassium channels. Curr Pharm Des. 14, 2503–2518, 10.2174/138161208785777441 (2008). [DOI] [PubMed] [Google Scholar]
- Estrada G., Villegas E. & Corzo G. Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat. Prod. Rep. 24, 145–161, 10.1039/b603083c (2007). [DOI] [PubMed] [Google Scholar]
- King G. F. Venoms as a platform for human drugs: translating toxins into therapeutics. Expert Opin Biol Ther 11, 1469–1484, 10.1517/14712598.2011.621940 (2011). [DOI] [PubMed] [Google Scholar]
- Klint J. K. et al. Spider-venom peptides that target voltage-gated sodium channels: Pharmacological tools and potential therapeutic leads. Toxicon, 10.1016/j.toxicon.2012.04.337 (2012). [DOI] [PubMed] [Google Scholar]
- Escoubas P., Sollod B. & King G. F. Venom landscapes: mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 47, 650–663, S0041-0101(06)00038-9 (2006). [DOI] [PubMed] [Google Scholar]
- Fields S. & Song O. A novel genetic system to detect protein-protein interactions. Nature 340, 245–246, 10.1038/340245a0 (1989). [DOI] [PubMed] [Google Scholar]
- Lentze N. & Auerbach D. The yeast two-hybrid system and its role in drug discovery. Expert Opin Ther Targets 12, 505–515, 10.1517/14728222.12.4.505 (2008). [DOI] [PubMed] [Google Scholar]
- Catterall W. A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26, 13–25, S0896-6273(00)81133-2 (2000). [DOI] [PubMed] [Google Scholar]
- Payandeh J., Scheuer T., Zheng N. & Catterall W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358, 10.1038/nature10238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong M. et al. Molecular basis of the tarantula toxin jingzhaotoxin-III ({beta}-TRTX-Cj1{alpha}) interacting with voltage sensors in sodium channel subtype Nav1.5. FASEB J 25, 3177–3185, fj.10-178848 (2011). [DOI] [PubMed] [Google Scholar]
- Bosmans F., Puopolo M., Martin-Eauclaire M. F., Bean B. P. & Swartz K. J. Functional properties and toxin pharmacology of a dorsal root ganglion sodium channel viewed through its voltage sensors. J Gen Physiol 138, 59–72, 10.1085/jgp.201110614 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. Molecular diversity and evolution of cystine knot toxins of the tarantula Chilobrachys jingzhao. Cell Mol Life Sci 65, 2431–2444, 10.1007/s00018-008-8135-x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliger C. A. et al. Diversity of conotoxin types from Conus californicus reflects a diversity of prey types and a novel evolutionary history. Toxicon 57, 311–322, 10.1016/j.toxicon.2010.12.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero-Hernandez V. et al. Scorpion and spider venom peptides: gene cloning and peptide expression. Toxicon 58, 644–663, 10.1016/j.toxicon.2011.09.015 (2011). [DOI] [PubMed] [Google Scholar]
- Soong T. W. & Venkatesh B. Adaptive evolution of tetrodotoxin resistance in animals. Trends Genet 22, 621–626, S0168-9525(06)00276-9 (2006). [DOI] [PubMed] [Google Scholar]
- Bosmans F. & Tytgat J. Voltage-gated sodium channel modulation by scorpion alpha-toxins. Toxicon 49, 142–158, 10.1016/j.toxicon.2006.09.023 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998). [DOI] [PubMed] [Google Scholar]
- King G. F. Modulation of insect Ca(v) channels by peptidic spider toxins. Toxicon 49, 513–530, 10.1016/j.toxicon.2006.11.012 (2007). [DOI] [PubMed] [Google Scholar]
- Xiao Y. et al. Jingzhaotoxin-III, a novel spider toxin inhibiting activation of voltage-gated sodium channel in rat cardiac myocytes. JBC 279, 26220–26226, 10.1074/jbc.M401387200 (2004). [DOI] [PubMed] [Google Scholar]
- Liu H. & Naismith J. H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol 8, 91, 1472-6750-8-91 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Baumann U. & Reymond J. L. An efficient one-step site-directed and site-saturation mutagenesis protocol. NAR 32, e115, 10.1093/nar/gnh110 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. et al. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain ii voltage sensor in the closed configuration. JBC 283, 27300–27313, 10.1074/jbc.M708447200 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Guan X. & Liang S. The cross channel activities of spider neurotoxin huwentoxin-I on rat dorsal root ganglion neurons. BBRC 357, 579–583, S0006-291X(07)00373-7 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Screening for Voltage-Gated Sodium Channel Interacting Peptide