Abstract
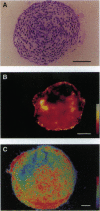
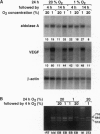
Blastocyst-derived pluripotent mouse embryonic stem cells can differentiate in vitro to form so-called embryoid bodies (EBs), which recapitulate several aspects of murine embryogenesis. We used this in vitro model to study oxygen supply and consumption as well as the response to reduced oxygenation during the earliest stages of development. EBs were found to grow equally well when cultured at 20% (normoxia) or 1% (hypoxia) oxygen during the first 5 days of differentiation. Microelectrode measurements of pericellular oxygen tension within 13- to 14-day-old EBs (diameter 510-890 micron) done at 20% oxygen revealed efficient oxygenation of the EBs' core region. Confocal laser scanning microscopy analysis of EBs incubated with fluorescent dyes that specifically stain living cells confirmed that the cells within an EB were viable. To determine the EBs' capability to sense low oxygen tension and to specifically respond to low ambient oxygen by modulating gene expression we quantified aldolase A and vascular endothelial growth factor (VEGF) mRNAs, since expression of these genes is upregulated by hypoxia in a variety of cells. Compared with the normoxic controls, we found increased aldolase A and VEGF mRNA levels after exposing 8- to 9-day-old EBs to 1% oxygen. We propose that EBs represent a powerful tool to study oxygen-regulated gene expression during the early steps of embryogenesis, where the preimplantation conceptus resides in a fluid environment with low oxygen tension until implantation and vascularization allow efficient oxygenation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bereiter-Hahn J., Seipel K. H., Vöth M., Ploem J. S. Fluorimetry of mitochondria in cells vitally stained with DASPMI or rhodamine 6 GO. Cell Biochem Funct. 1983 Oct;1(3):147–155. doi: 10.1002/cbf.290010306. [DOI] [PubMed] [Google Scholar]
- Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992 Feb;114(2):521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claffey K. P., Wilkison W. O., Spiegelman B. M. Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem. 1992 Aug 15;267(23):16317–16322. [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Doetschman T., Shull M., Kier A., Coffin J. D. Embryonic stem cell model systems for vascular morphogenesis and cardiac disorders. Hypertension. 1993 Oct;22(4):618–629. doi: 10.1161/01.hyp.22.4.618. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Kaufman M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981 Jul 9;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fandrey J., Bunn H. F. In vivo and in vitro regulation of erythropoietin mRNA: measurement by competitive polymerase chain reaction. Blood. 1993 Feb 1;81(3):617–623. [PubMed] [Google Scholar]
- Firth J. D., Ebert B. L., Pugh C. W., Ratcliffe P. J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6496–6500. doi: 10.1073/pnas.91.14.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J. D., Ebert B. L., Ratcliffe P. J. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem. 1995 Sep 8;270(36):21021–21027. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- Fischer B., Bavister B. D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993 Nov;99(2):673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Goldberg M. A., Schneider T. J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J Biol Chem. 1994 Feb 11;269(6):4355–4359. [PubMed] [Google Scholar]
- Grossmann U. Profiles of oxygen partial pressure and oxygen consumption inside multicellular spheroids. Recent Results Cancer Res. 1984;95:150–161. doi: 10.1007/978-3-642-82340-4_9. [DOI] [PubMed] [Google Scholar]
- Grote J. Die Sauerstoffdiffusionskonstanten im Lungengewebe und Wasser und ihre Temperaturabhängigkeit. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;295(3):245–254. [PubMed] [Google Scholar]
- Görlach A., Acker H. pO2- and pH-gradients in multicellular spheroids and their relationship to cellular metabolism and radiation sensitivity of malignant human tumor cells. Biochim Biophys Acta. 1994 Nov 29;1227(3):105–112. doi: 10.1016/0925-4439(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Görlach A., Holtermann G., Jelkmann W., Hancock J. T., Jones S. A., Jones O. T., Acker H. Photometric characteristics of haem proteins in erythropoietin-producing hepatoma cells (HepG2). Biochem J. 1993 Mar 15;290(Pt 3):771–776. doi: 10.1042/bj2900771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Johansson B. M., Wiles M. V. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995 Jan;15(1):141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakurum M., Shreeniwas R., Chen J., Pinsky D., Yan S. D., Anderson M., Sunouchi K., Major J., Hamilton T., Kuwabara K. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. J Clin Invest. 1994 Apr;93(4):1564–1570. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. L., Mitchell J. A. Intrauterine oxygen tension during the oestrous cycle in the hamster: patterns of change. Comp Biochem Physiol Comp Physiol. 1994 Apr;107(4):673–678. doi: 10.1016/0300-9629(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kuwabara K., Ogawa S., Matsumoto M., Koga S., Clauss M., Pinsky D. J., Lyn P., Leavy J., Witte L., Joseph-Silverstein J. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4606–4610. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levy A. P., Levy N. S., Wegner S., Goldberg M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995 Jun 2;270(22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Marti H. H., Jung H. H., Pfeilschifter J., Bauer C. Hypoxia and cobalt stimulate lactate dehydrogenase (LDH) activity in vascular smooth muscle cells. Pflugers Arch. 1994 Dec;429(2):216–222. doi: 10.1007/BF00374315. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B., Shawver L. K., Plate K. H., Risau W., Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994 Feb 10;367(6463):576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- Minchenko A., Bauer T., Salceda S., Caro J. Hypoxic stimulation of vascular endothelial growth factor expression in vitro and in vivo. Lab Invest. 1994 Sep;71(3):374–379. [PubMed] [Google Scholar]
- Mitchell J. A., Yochim J. M. Intrauterine oxygen tension during the estrous cycle in the rat: its relation to uterine respiration and vascular activity. Endocrinology. 1968 Oct;83(4):701–705. doi: 10.1210/endo-83-4-701. [DOI] [PubMed] [Google Scholar]
- Morris S. J. Real-time multi-wavelength fluorescence imaging of living cells. Biotechniques. 1990 Mar;8(3):296–308. [PubMed] [Google Scholar]
- Plate K. H., Breier G., Risau W. Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol. 1994 Jul;4(3):207–218. doi: 10.1111/j.1750-3639.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Potocnik A. J., Nielsen P. J., Eichmann K. In vitro generation of lymphoid precursors from embryonic stem cells. EMBO J. 1994 Nov 15;13(22):5274–5283. doi: 10.1002/j.1460-2075.1994.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W. Angiogenic growth factors. Prog Growth Factor Res. 1990;2(1):71–79. doi: 10.1016/0955-2235(90)90010-h. [DOI] [PubMed] [Google Scholar]
- Risau W., Sariola H., Zerwes H. G., Sasse J., Ekblom P., Kemler R., Doetschman T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development. 1988 Mar;102(3):471–478. doi: 10.1242/dev.102.3.471. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Schmitt R. M., Bruyns E., Snodgrass H. R. Hematopoietic development of embryonic stem cells in vitro: cytokine and receptor gene expression. Genes Dev. 1991 May;5(5):728–740. doi: 10.1101/gad.5.5.728. [DOI] [PubMed] [Google Scholar]
- Semenza G. L., Roth P. H., Fang H. M., Wang G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994 Sep 23;269(38):23757–23763. [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Siebert P. D., Larrick J. W. Competitive PCR. Nature. 1992 Oct 8;359(6395):557–558. doi: 10.1038/359557a0. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- Wang R., Clark R., Bautch V. L. Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development. 1992 Feb;114(2):303–316. doi: 10.1242/dev.114.2.303. [DOI] [PubMed] [Google Scholar]
- Webster K. A. Regulation of glycolytic enzyme RNA transcriptional rates by oxygen availability in skeletal muscle cells. Mol Cell Biochem. 1987 Sep;77(1):19–28. doi: 10.1007/BF00230147. [DOI] [PubMed] [Google Scholar]
- Wiles M. V. Embryonic stem cell differentiation in vitro. Methods Enzymol. 1993;225:900–918. doi: 10.1016/0076-6879(93)25057-9. [DOI] [PubMed] [Google Scholar]
- Wiles M. V., Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991 Feb;111(2):259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- Yan S. F., Tritto I., Pinsky D., Liao H., Huang J., Fuller G., Brett J., May L., Stern D. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995 May 12;270(19):11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- Young P. E., Baumhueter S., Lasky L. A. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995 Jan 1;85(1):96–105. [PubMed] [Google Scholar]