Abstract
Polynucleotide phosphorylase (polyribonucleotide:orthophosphate nucleotidyltransferase, EC 2.7.7.8) activity has been found in many prokaryotes and studied in detail since 1955. Such enzymes have been detected also in plants. We now describe the purification of polynucleotide phosphorylase from cucumber cotyledons and leaves. This enzyme is a complex of three subunits, possibly not identical, of about Mr 50,000. Its enzymatic properties are similar to those of the tobacco enzyme. Unlike the prokaryotic enzymes, the plant enzyme shows activity in the absence of primer but is to various extents stimulated by various ribopolynucleotides or RNAs. RNA-dependent RNA polymerase, not previously shown to exist in non-virus-infected cucumber, has been found to be present at a low level and was separated from the much greater amount of polynucleotide phosphorylase, although some of the physical properties of the two enzymes are rather similar.
Keywords: polynucleotide synthesis, RNA-dependent RNA polymerase, cucumber mosaic virus
Full text
PDF
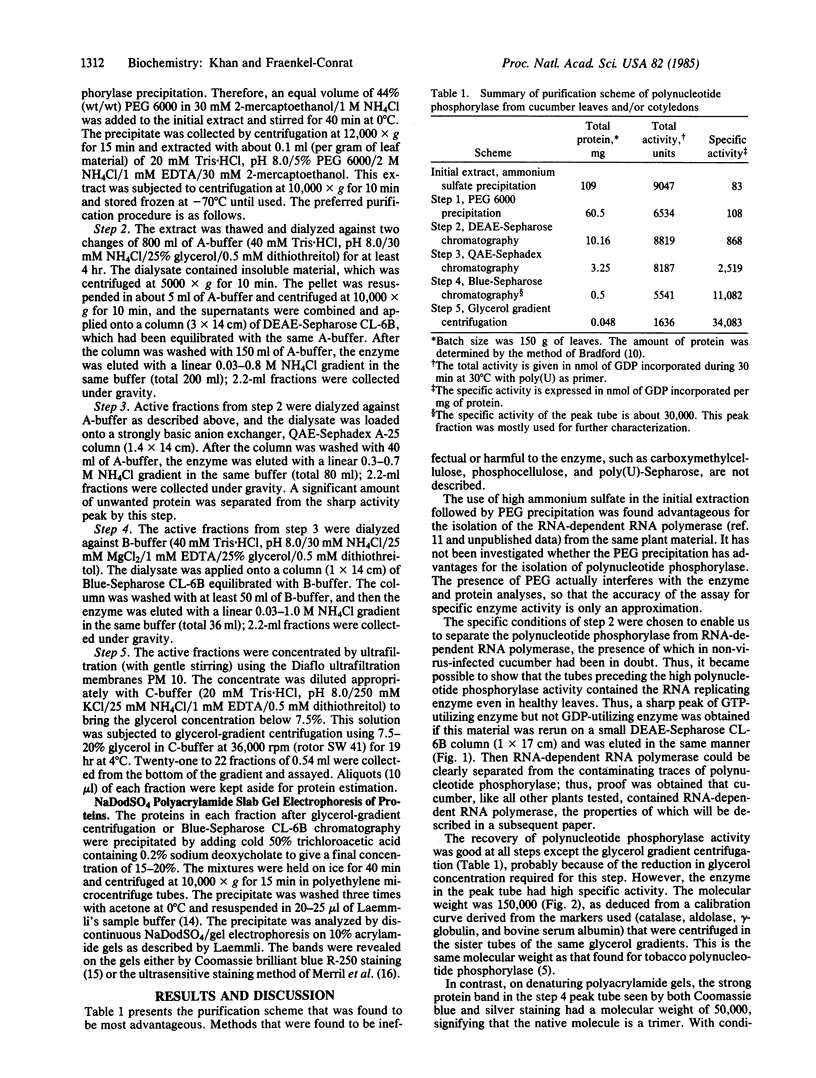
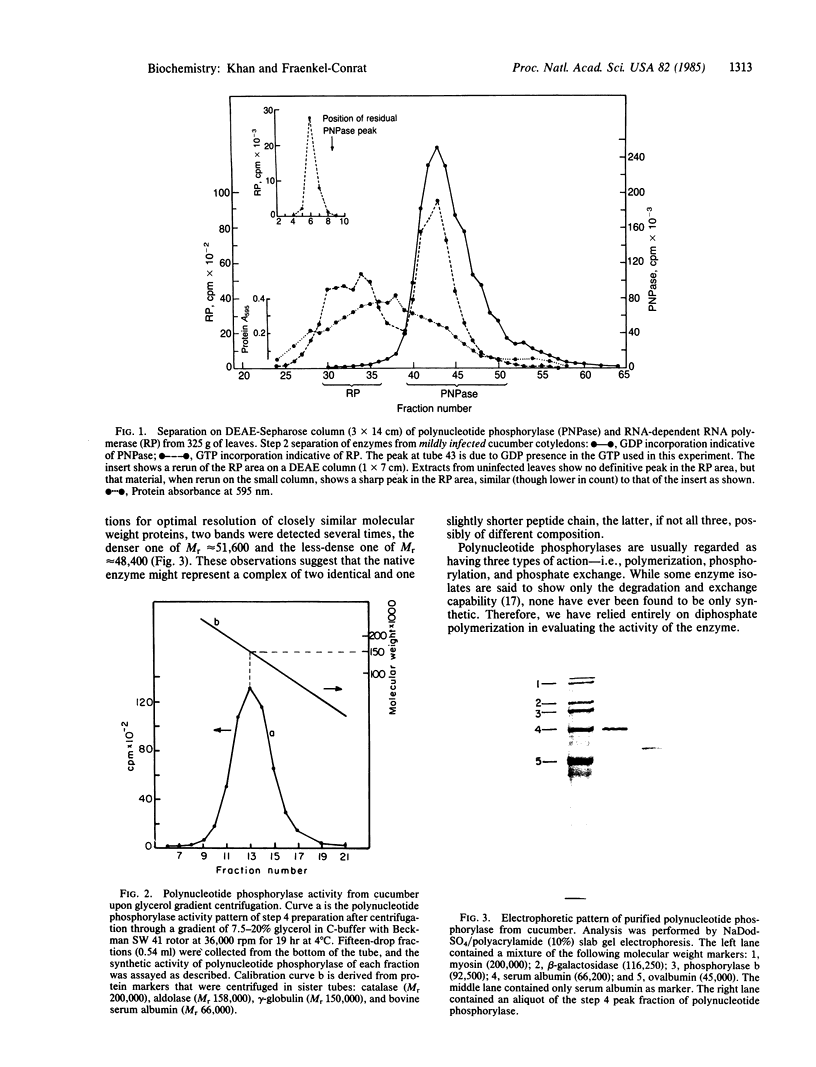
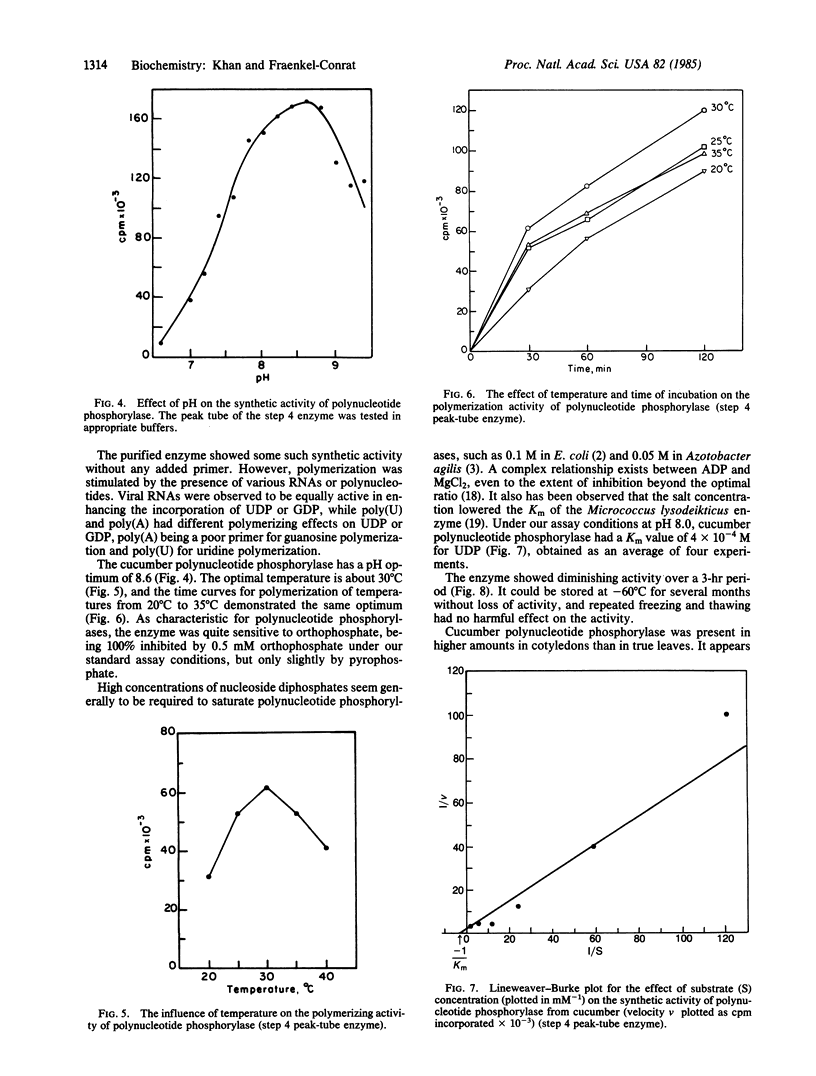
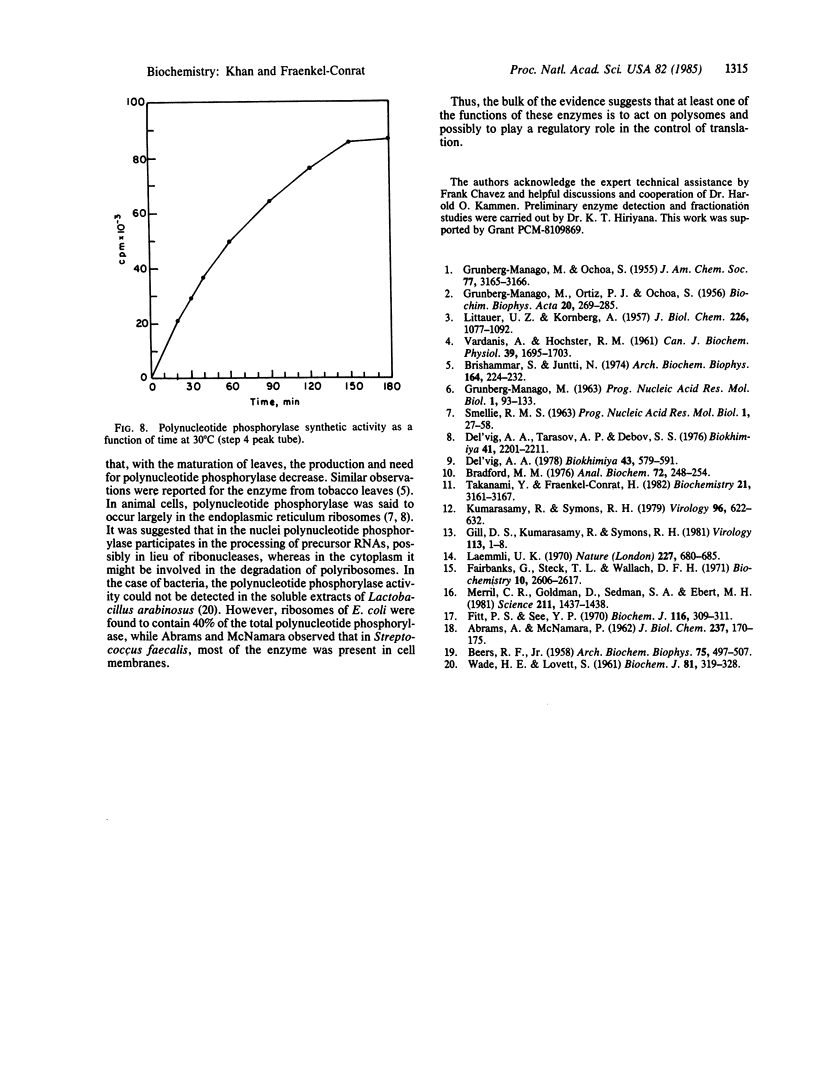
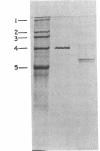
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P. Polynucleotide phosphorylase in isolated bacterial cell membranes. J Biol Chem. 1962 Jan;237:170–175. [PubMed] [Google Scholar]
- BEERS R. F., Jr Polynucleotides. IV. Role of salts and magnesium in the polymerization of ribonucleotides by polynucleotide phosphorylase. Arch Biochem Biophys. 1958 Jun;75(2):497–507. doi: 10.1016/0003-9861(58)90447-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. RNA-synthesizing enzymes in healthy and TMV-infected tobacco leaves. Partial purification and characterization to tobacco polynucleotide phosphorylase. Arch Biochem Biophys. 1974 Sep;164(1):224–232. doi: 10.1016/0003-9861(74)90026-5. [DOI] [PubMed] [Google Scholar]
- Del vig A. A., Tarasov A. P., Debov S. S. Aktivnost' polinykleotidfosforilazy v polipibosomakh regeneriruiushchei pecheni krys, novorozhdennykh krysiat i nekotorykh perevivaemykh opukholei. Biokhimiia. 1976 Dec;41(12):2201–2211. [PubMed] [Google Scholar]
- Del'vig A. A. Polinukleotidfosforilaza tkanei zhivotnykh. Biokhimiia. 1978;43(4):579–591. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fitt P. S., See Y. P. Guinea-pig liver polynucleotide phosphorylase. Biochem J. 1970 Jan;116(2):309–311. doi: 10.1042/bj1160309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNBERG-MANAGO M., ORTIZ P. J., OCHOA S. Enzymic synthesis of polynucleotides. I. Polynucleotide phosphorylase of azotobacter vinelandii. Biochim Biophys Acta. 1956 Apr;20(1):269–285. doi: 10.1016/0006-3002(56)90286-4. [DOI] [PubMed] [Google Scholar]
- Kumarasamy R., Symons R. H. Extensive purification of the cucumber mosaic virus-induced RNA replicase. Virology. 1979 Jul 30;96(2):622–632. doi: 10.1016/0042-6822(79)90118-1. [DOI] [PubMed] [Google Scholar]
- LITTAUER U. Z., KORNBERG A. Reversible synthesis of polyribonucleotides with an enzyme from Escherichia coli. J Biol Chem. 1957 Jun;226(2):1077–1092. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Takanami Y., Fraenkel-Conrat H. Comparative studies on ribonucleic acid dependent RNA polymerases in cucumber mosaic virus infected cucumber and tobacco and uninfected tobacco plants. Biochemistry. 1982 Jun 22;21(13):3161–3167. doi: 10.1021/bi00256a020. [DOI] [PubMed] [Google Scholar]
- VARDANIS A., HOCHSTER R. M. On the nucleotide specificity of the polynucleotide phosphorylase of the crown-gall tumor-inducing organism Agrobacterium tumefaciens. Can J Biochem Physiol. 1961 Nov;39:1695–1704. doi: 10.1139/o61-188. [DOI] [PubMed] [Google Scholar]
- WADE H. E., LOVETT S. Polynucleotide phosphorylase in ribosomes from Escherichia coli. Biochem J. 1961 Nov;81:319–328. doi: 10.1042/bj0810319. [DOI] [PMC free article] [PubMed] [Google Scholar]