Abstract
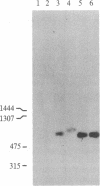
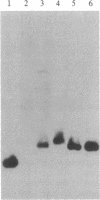
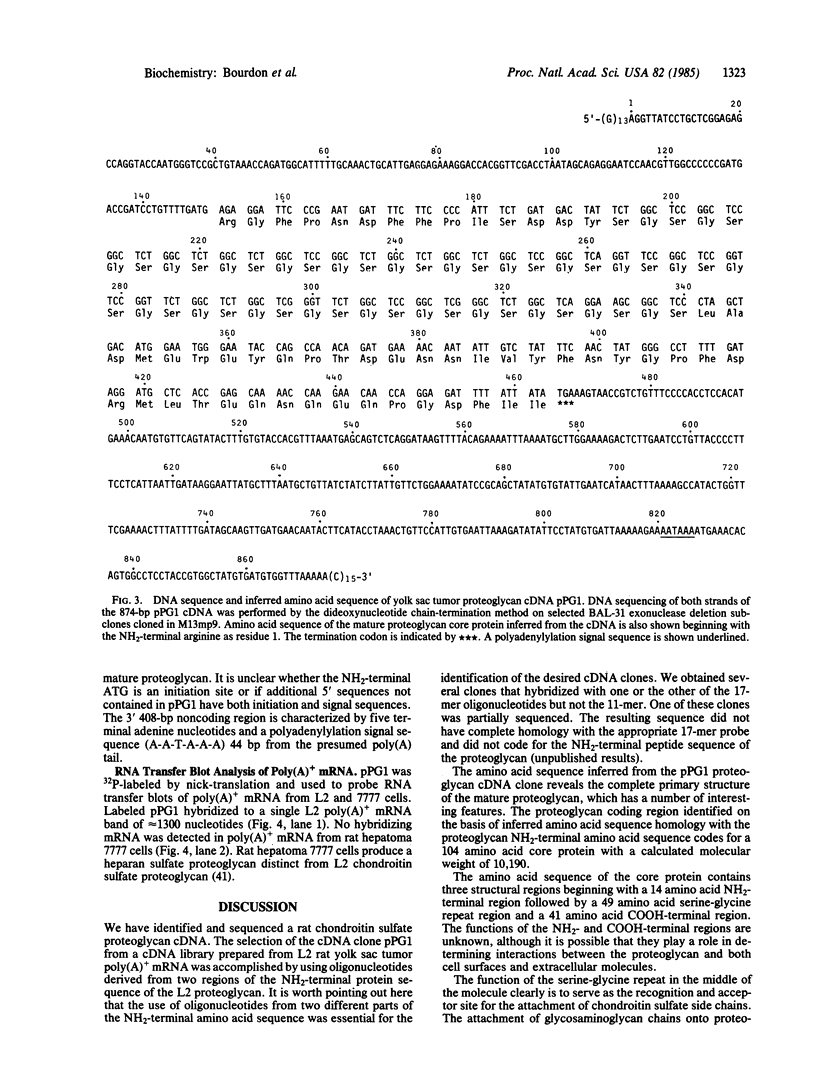
We report the identification and DNA sequence of a chondroitin sulfate proteoglycan core protein cDNA. A cDNA clone, pPG1, was selected from a rat yolk sac tumor poly(A)+RNA-derived cDNA library by using synthetic oligonucleotides predicted from the NH2-terminal peptide sequence of the mature chondroitin sulfate proteoglycan. The resulting sequence analysis demonstrated that the 874-base-pair pPG1 clone contained the complete coding region of the mature proteoglycan core protein as well as 5' and 3' flanking sequences. The 104 amino acid proteoglycan core protein sequence reveals that the core protein is composed of three regions, the most striking of which is the central 49 amino acid region composed of alternating serine and glycine residues. This region clearly functions as the acceptor site for the attachment of chondroitin sulfate side chains. The serine-glycine repeat region is flanked by a 14 amino acid NH2-terminal region identical to the NH2-terminal sequence of the proteoglycan obtained by amino acid sequencing and a 41 amino acid COOH-terminal region. RNA transfer blot hybridizations of poly(A)+ mRNA from rat yolk sac tumor cells with nick-translated pPG1 reveal a single mRNA of approximately equal to 1300 nucleotides. The possibility of detecting mRNAs and genomic sequences for other proteoglycans with a serine-glycine repeat by using this cDNA clone is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Hayman E. G., Ruoslahti E. Effect of a proteoglycan produced by rat tumor cells on their adhesion to fibronectin-collagen substrata. Cancer Res. 1983 Sep;43(9):4302–4307. [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Pierschbacher M. D., Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. J Biol Chem. 1984 Nov 25;259(22):13742–13750. [PubMed] [Google Scholar]
- Broka C., Hozumi T., Arentzen R., Itakura K. Simplications in the synthesis of short oligonucleotide blocks. Nucleic Acids Res. 1980 Nov 25;8(22):5461–5471. doi: 10.1093/nar/8.22.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimelius B., Norling B., Westermark B., Wasteson A. Composition and distribution of glycosaminoglycans in cultures of human normal and malignant glial cells. Biochem J. 1978 Jun 15;172(3):443–456. doi: 10.1042/bj1720443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Kasper C. B. Cloning of epoxide hydratase complementary DNA. J Biol Chem. 1981 May 25;256(10):4697–4700. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Schlesinger D. H., Kennedy D. W., Yamada K. M. Isolation and characterization of a heparin-binding domain of cellular fibronectin. J Biol Chem. 1980 Nov 10;255(21):10017–10020. [PubMed] [Google Scholar]
- Hayman E. G., Oldberg A., Martin G. R., Ruoslahti E. Codistribution of heparan sulfate proteoglycan, laminin, and fibronectin in the extracellular matrix of normal rat kidney cells and their coordinate absence in transformed cells. J Cell Biol. 1982 Jul;94(1):28–35. doi: 10.1083/jcb.94.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. Glycoproteins as components of cellular membranes. Prog Biophys Mol Biol. 1973;26:189–268. doi: 10.1016/0079-6107(73)90020-5. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Veis A., Kimura J. H., Jakubowski M. L. Characterization of heparan sulfate-proteoglycan of glomerular basement membranes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):762–766. doi: 10.1073/pnas.81.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima J., Nakamura N., Kanatani M., Akiyama M. Glycosaminoglycans in 3'-methyl-4-dimethylaminoazobenzene-induced rat hepatic cancer. Cancer Res. 1982 Jul;42(7):2857–2860. [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Laterra J., Silbert J. E., Culp L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983 Jan;96(1):112–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenberger J. A. A program for reading DNA sequence gels using a small computer equipped with a graphics tablet. Nucleic Acids Res. 1982 Jan 11;10(1):27–30. doi: 10.1093/nar/10.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Hayman E. G., Ruoslahti E. Isolation of a chondroitin sulfate proteoglycan from a rat yolk sac tumor and immunochemical demonstration of its cell surface localization. J Biol Chem. 1981 Nov 10;256(21):10847–10852. [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Oldberg A., Linney E., Ruoslahti E. Molecular cloning and nucleotide sequence of a cDNA clone coding for the cell attachment domain in human fibronectin. J Biol Chem. 1983 Sep 10;258(17):10193–10196. [PubMed] [Google Scholar]
- Oldberg A., Ruoslahti E. Interactions between chondroitin sulfate proteoglycan, fibronectin, and collagen. J Biol Chem. 1982 May 10;257(9):4859–4863. [PubMed] [Google Scholar]
- Oldberg A., Schwartz C., Ruoslahti E. Isolation and partial characterization of a rat hepatoma heparan sulfate proteoglycan. Arch Biochem Biophys. 1982 Jul;216(2):400–406. doi: 10.1016/0003-9861(82)90228-4. [DOI] [PubMed] [Google Scholar]
- Oohira A., Wight T. N., McPherson J., Bornstein P. Biochemical and ultrastructural studies of proteoheparan sulfates synthesized by PYS-2, a basement membrane-producing cell line. J Cell Biol. 1982 Feb;92(2):357–367. doi: 10.1083/jcb.92.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins M. E., Ji T. H., Hynes R. O. Cross-linking of fibronectin to sulfated proteoglycans at the cell surface. Cell. 1979 Apr;16(4):941–952. doi: 10.1016/0092-8674(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Robinson J., Viti M., Hök M. Structure and properties of an under-sulfated heparan sulfate proteoglycan synthesized by a rat hepatoma cell line. J Cell Biol. 1984 Mar;98(3):946–953. doi: 10.1083/jcb.98.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodén L., Smith R. Structure of the neutral trisaccharide of the chondroitin 4-sulfate-protein linkage region. J Biol Chem. 1966 Dec 25;241(24):5949–5954. [PubMed] [Google Scholar]
- Sakashita S., Ruoslahti E. Laminin-like glycoproteins in extracellular matrix of endodermal cells. Arch Biochem Biophys. 1980 Dec;205(2):283–290. doi: 10.1016/0003-9861(80)90109-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Pierschbacher M. D., Hayman E. G., Nguyen K., Ohgren Y., Ruoslahti E. Domain structure of vitronectin. Alignment of active sites. J Biol Chem. 1984 Dec 25;259(24):15307–15314. [PubMed] [Google Scholar]
- Tan Z. K., Ikuta S., Huang T., Dugaiczyk A., Itakura K. Solid-phase synthesis of polynucleotides. VIII: A simplified synthesis of oligodeoxyribonucleotides. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):383–391. doi: 10.1101/sqb.1983.047.01.045. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Binding and precipitation of soluble collagens by chick embryo cartilage proteoglycan. J Biol Chem. 1976 Feb 10;251(3):895–897. [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wewer U. Characterization of a rat yolk sac carcinoma cell line. Dev Biol. 1982 Oct;93(2):416–421. doi: 10.1016/0012-1606(82)90128-2. [DOI] [PubMed] [Google Scholar]