Abstract
Many bacteria live only within animal cells and infect hosts through cytoplasmic inheritance. These endosymbiotic lineages show distinctive population structure, with small population size and effectively no recombination. As a result, endosymbionts are expected to accumulate mildly deleterious mutations. If these constitute a substantial proportion of new mutations, endosymbionts will show (i) faster sequence evolution and (ii) a possible shift in base composition reflecting mutational bias. Analyses of 16S rDNA of five independently derived endosymbiont clades show, in every case, faster evolution in endosymbionts than in free-living relatives. For aphid endosymbionts (genus Buchnera), coding genes exhibit accelerated evolution and unusually low ratios of synonymous to nonsynonymous substitutions compared to ratios for the same genes for enterics. This concentration of the rate increase in nonsynonymous substitutions is expected under the hypothesis of increased fixation of deleterious mutations. Polypeptides for all Buchnera genes analyzed have accumulated amino acids with codon families rich in A+T, supporting the hypothesis that substitutions are deleterious in terms of polypeptide function. These observations are best explained as the result of Muller's ratchet within small asexual populations, combined with mutational bias. In light of this explanation, two observations reported earlier for Buchnera, the apparent loss of a repair gene and the overproduction of a chaperonin, may reflect compensatory evolution. An alternative hypothesis, involving selection on genomic base composition, is contradicted by the observation that the speedup is concentrated at nonsynonymous sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S. Molecular analysis of the endosymbionts of tsetse flies: 16S rDNA locus and over-expression of a chaperonin. Insect Mol Biol. 1995 Feb;4(1):23–29. doi: 10.1111/j.1365-2583.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Aksoy S., Pourhosseini A. A., Chow A. Mycetome endosymbionts of tsetse flies constitute a distinct lineage related to Enterobacteriaceae. Insect Mol Biol. 1995 Feb;4(1):15–22. doi: 10.1111/j.1365-2583.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Andersson S. G., Kurland C. G. Codon preferences in free-living microorganisms. Microbiol Rev. 1990 Jun;54(2):198–210. doi: 10.1128/mr.54.2.198-210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Lai C. Y., Rouhbakhsh D., Moran N. A., Clark M. A. Genetics, physiology, and evolutionary relationships of the genus Buchnera: intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- Becker J., Craig E. A. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994 Jan 15;219(1-2):11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Breeuwer J. A., Stouthamer R., Barns S. M., Pelletier D. A., Weisburg W. G., Werren J. H. Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol Biol. 1992;1(1):25–36. doi: 10.1111/j.1365-2583.1993.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Baumann L., Baumann P. Sequence analysis of an aphid endosymbiont DNA fragment containing rpoB (beta-subunit of RNA polymerase) and portions of rplL and rpoC. Curr Microbiol. 1992 Nov;25(5):283–290. doi: 10.1007/BF01575863. [DOI] [PubMed] [Google Scholar]
- Dykhuizen D. E., Green L. Recombination in Escherichia coli and the definition of biological species. J Bacteriol. 1991 Nov;173(22):7257–7268. doi: 10.1128/jb.173.22.7257-7268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. The evolutionary advantage of recombination. Genetics. 1974 Oct;78(2):737–756. doi: 10.1093/genetics/78.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman D. S., Dykhuizen D. E. Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science. 1994 Nov 25;266(5189):1380–1383. doi: 10.1126/science.7973728. [DOI] [PubMed] [Google Scholar]
- Hartl F. U. Protein folding. Secrets of a double-doughnut. Nature. 1994 Oct 13;371(6498):557–559. doi: 10.1038/371557a0. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hartl F. U. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hughes A. L. Contrasting evolutionary rates in the duplicate chaperonin genes of Mycobacterium tuberculosis and M. leprae. Mol Biol Evol. 1993 Nov;10(6):1343–1359. doi: 10.1093/oxfordjournals.molbev.a040064. [DOI] [PubMed] [Google Scholar]
- Hughes A. L., Hughes M. K. Adaptive evolution in the rat olfactory receptor gene family. J Mol Evol. 1993 Mar;36(3):249–254. doi: 10.1007/BF00160480. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Biochemical and molecular aspects of endosymbiosis in insects. Int Rev Cytol. 1989;116:1–45. doi: 10.1016/s0074-7696(08)60637-3. [DOI] [PubMed] [Google Scholar]
- Jermiin L. S., Graur D., Lowe R. M., Crozier R. H. Analysis of directional mutation pressure and nucleotide content in mitochondrial cytochrome b genes. J Mol Evol. 1994 Aug;39(2):160–173. doi: 10.1007/BF00163805. [DOI] [PubMed] [Google Scholar]
- Jollès J., Jollès P., Bowman B. H., Prager E. M., Stewart C. B., Wilson A. C. Episodic evolution in the stomach lysozymes of ruminants. J Mol Evol. 1989 Jun;28(6):528–535. doi: 10.1007/BF02602933. [DOI] [PubMed] [Google Scholar]
- Kondrashov A. S. Deleterious mutations and the evolution of sexual reproduction. Nature. 1988 Dec 1;336(6198):435–440. doi: 10.1038/336435a0. [DOI] [PubMed] [Google Scholar]
- Kondrashov A. S. Mutation load under vegetative reproduction and cytoplasmic inheritance. Genetics. 1994 May;137(1):311–318. doi: 10.1093/genetics/137.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. Y., Baumann L., Baumann P. Amplification of trpEG: adaptation of Buchnera aphidicola to an endosymbiotic association with aphids. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3819–3823. doi: 10.1073/pnas.91.9.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. Y., Baumann P. Genetic analysis of an aphid endosymbiont DNA fragment homologous to the rnpA-rpmH-dnaA-dnaN-gyrB region of eubacteria. Gene. 1992 Apr 15;113(2):175–181. doi: 10.1016/0378-1119(92)90393-4. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Baumann P., Moran N. A. Genetics of the tryptophan biosynthetic pathway of the prokaryotic endosymbiont (Buchnera) of the aphid Schlechtendalia chinensis. Insect Mol Biol. 1995 Feb;4(1):47–59. doi: 10.1111/j.1365-2583.1995.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Baumann P. Sequence analysis of a DNA fragment from Buchnera aphidicola (an endosymbiont of aphids) containing genes homologous to dnaG, rpoD, cysE, and secB. Gene. 1992 Sep 21;119(1):113–118. doi: 10.1016/0378-1119(92)90074-y. [DOI] [PubMed] [Google Scholar]
- Larsen N., Olsen G. J., Maidak B. L., McCaughey M. J., Overbeek R., Macke T. J., Marsh T. L., Woese C. R. The ribosomal database project. Nucleic Acids Res. 1993 Jul 1;21(13):3021–3023. doi: 10.1093/nar/21.13.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. H. Unbiased estimation of the rates of synonymous and nonsynonymous substitution. J Mol Evol. 1993 Jan;36(1):96–99. doi: 10.1007/BF02407308. [DOI] [PubMed] [Google Scholar]
- Lynch M., Bürger R., Butcher D., Gabriel W. The mutational meltdown in asexual populations. J Hered. 1993 Sep-Oct;84(5):339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- McKane M., Milkman R. Transduction, restriction and recombination patterns in Escherichia coli. Genetics. 1995 Jan;139(1):35–43. doi: 10.1093/genetics/139.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman R., Bridges M. M. Molecular evolution of the Escherichia coli chromosome. IV. Sequence comparisons. Genetics. 1993 Mar;133(3):455–468. doi: 10.1093/genetics/133.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M. A., Baumann L., Baumann P. Buchnera aphidicola (a prokaryotic endosymbiont of aphids) contains a putative 16S rRNA operon unlinked to the 23S rRNA-encoding gene: sequence determination, and promoter and terminator analysis. Gene. 1993 Dec 31;137(2):171–178. doi: 10.1016/0378-1119(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Munson M. A., Baumann P., Clark M. A., Baumann L., Moran N. A., Voegtlin D. J., Campbell B. C. Evidence for the establishment of aphid-eubacterium endosymbiosis in an ancestor of four aphid families. J Bacteriol. 1991 Oct;173(20):6321–6324. doi: 10.1128/jb.173.20.6321-6324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M. A., Baumann P., Moran N. A. Phylogenetic relationships of the endosymbionts of mealybugs (Homoptera: Pseudococcidae) based on 16S rDNA sequences. Mol Phylogenet Evol. 1992 Mar;1(1):26–30. doi: 10.1016/1055-7903(92)90032-c. [DOI] [PubMed] [Google Scholar]
- Muse S. V., Weir B. S. Testing for equality of evolutionary rates. Genetics. 1992 Sep;132(1):269–276. doi: 10.1093/genetics/132.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Osawa S. The guanine and cytosine content of genomic DNA and bacterial evolution. Proc Natl Acad Sci U S A. 1987 Jan;84(1):166–169. doi: 10.1073/pnas.84.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill S. L., Giordano R., Colbert A. M., Karr T. L., Robertson H. M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Wilson A. C. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J Mol Evol. 1987;26(1-2):74–86. doi: 10.1007/BF02111283. [DOI] [PubMed] [Google Scholar]
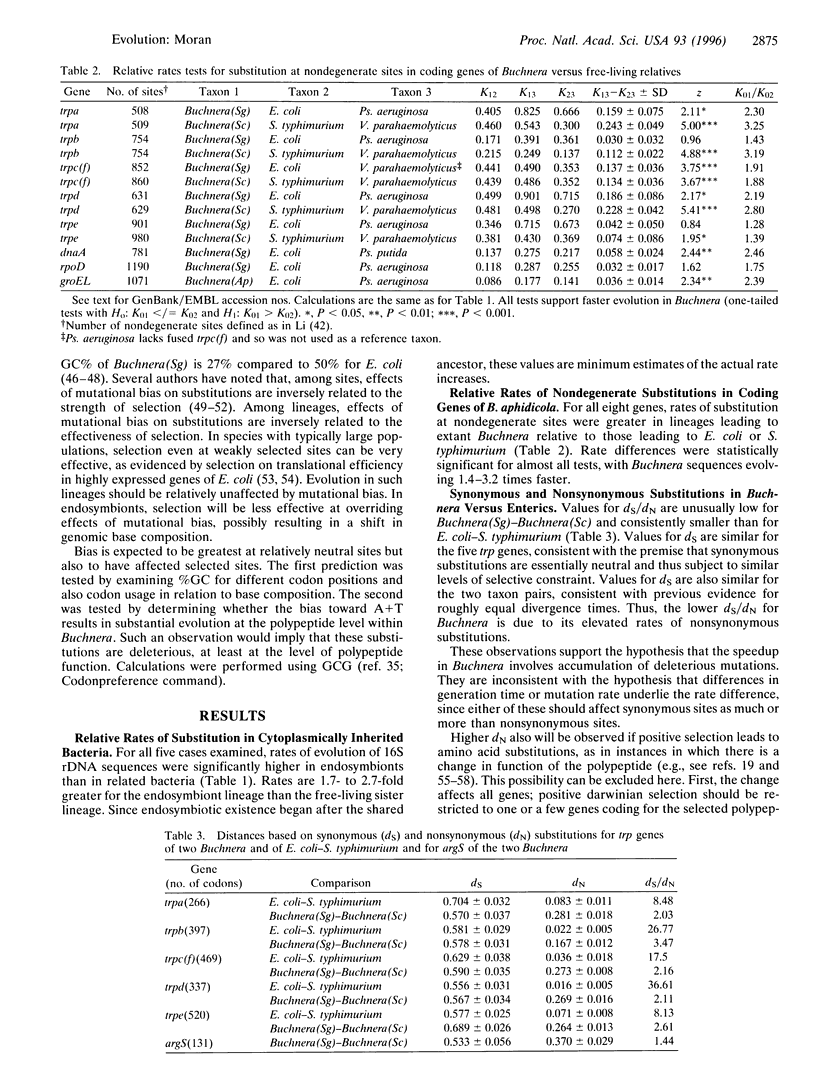
- Ohtaka C., Ishikawa H. Accumulation of adenine and thymine in a groE-homologous operon of an intracellular symbiont. J Mol Evol. 1993 Feb;36(2):121–126. doi: 10.1007/BF00166247. [DOI] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24(1-2):28–38. doi: 10.1007/BF02099948. [DOI] [PubMed] [Google Scholar]
- Stephan W., Chao L., Smale J. G. The advance of Muller's ratchet in a haploid asexual population: approximate solutions based on diffusion theory. Genet Res. 1993 Jun;61(3):225–231. doi: 10.1017/s0016672300031384. [DOI] [PubMed] [Google Scholar]
- Sueoka N. CORRELATION BETWEEN BASE COMPOSITION OF DEOXYRIBONUCLEIC ACID AND AMINO ACID COMPOSITION OF PROTEIN. Proc Natl Acad Sci U S A. 1961 Aug;47(8):1141–1149. doi: 10.1073/pnas.47.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
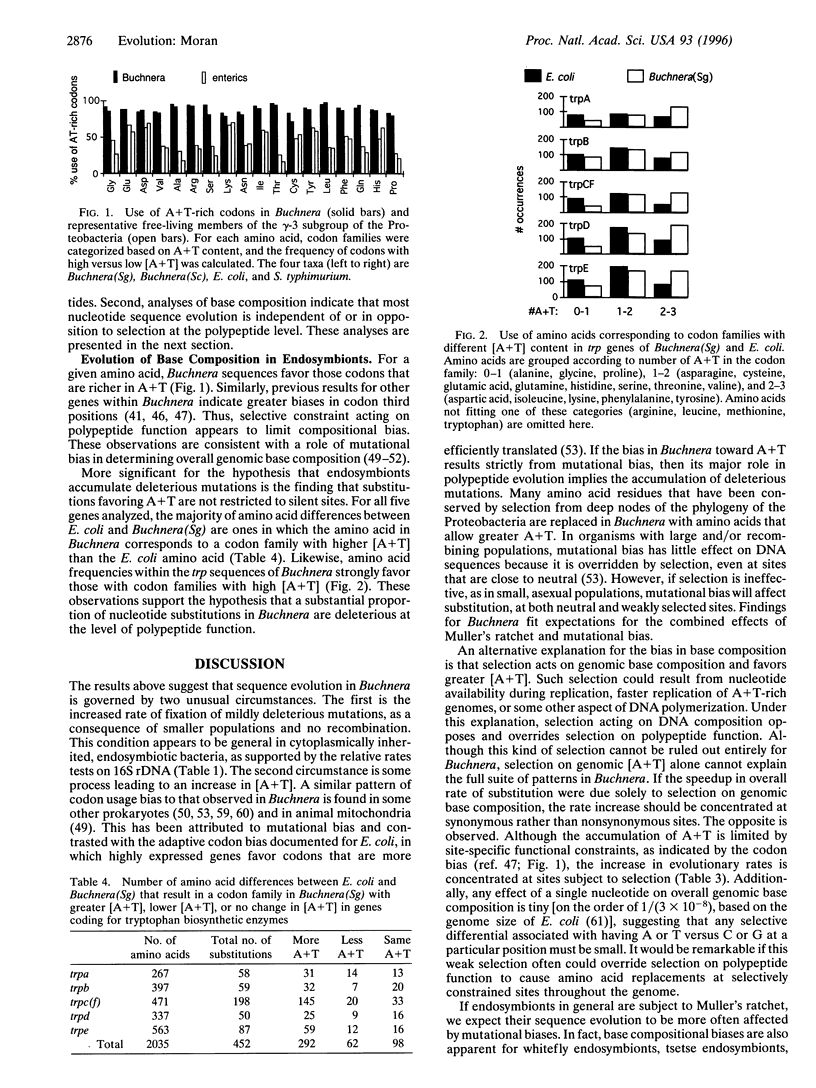
- Sueoka N. Directional mutation pressure and neutral molecular evolution. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2653–2657. doi: 10.1073/pnas.85.8.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W. J., Vacquier V. D. Extraordinary divergence and positive Darwinian selection in a fusagenic protein coating the acrosomal process of abalone spermatozoa. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4957–4961. doi: 10.1073/pnas.92.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam T. S. Sex in the soil. Curr Biol. 1992 Dec;2(12):676–678. doi: 10.1016/0960-9822(92)90140-6. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wood D. O. Codon usage in selected AT-rich bacteria. Biochimie. 1988 Aug;70(8):977–986. doi: 10.1016/0300-9084(88)90262-3. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. I., Li W. H. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1741–1745. doi: 10.1073/pnas.82.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]



